Abstract
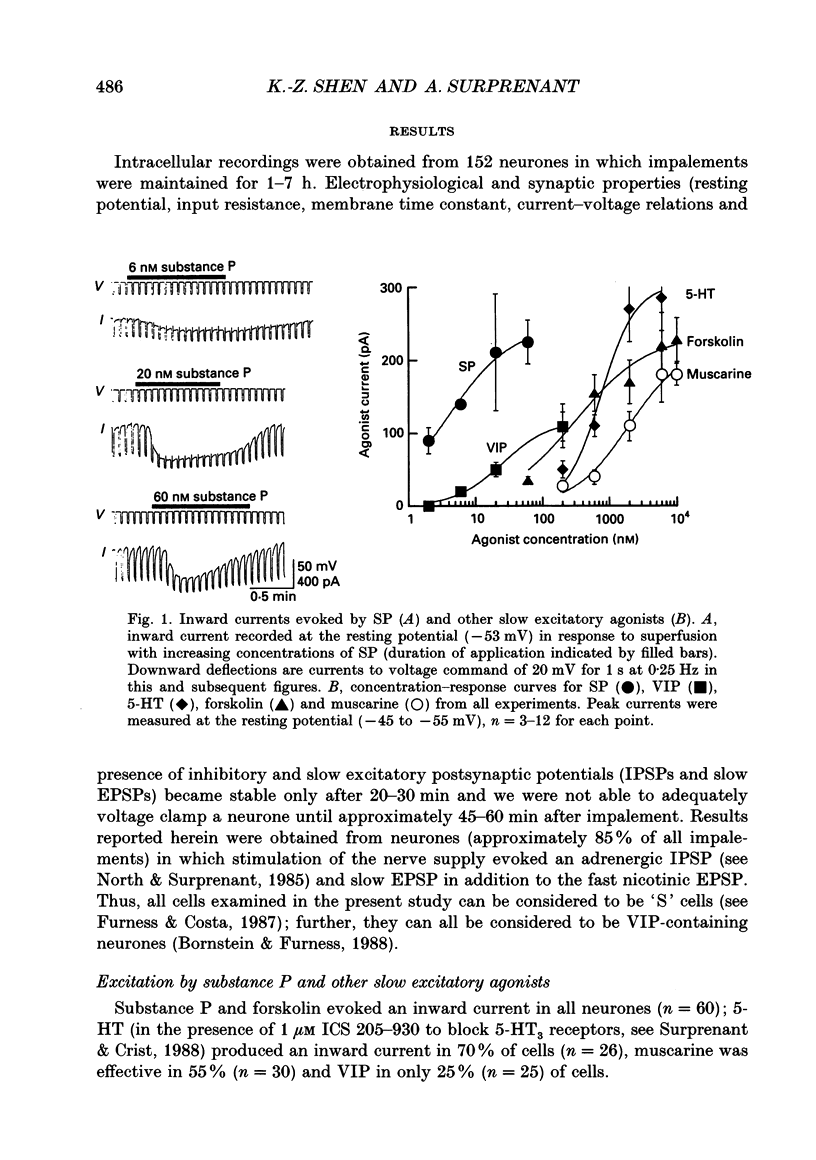
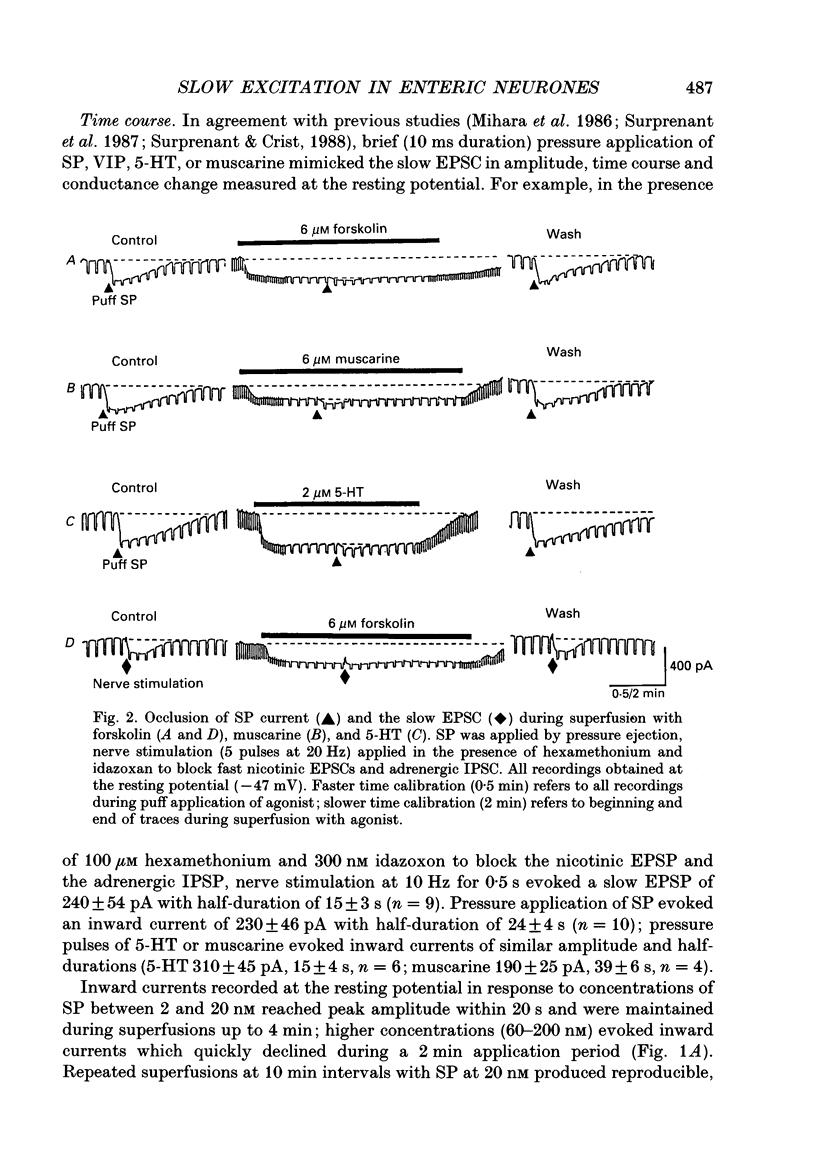
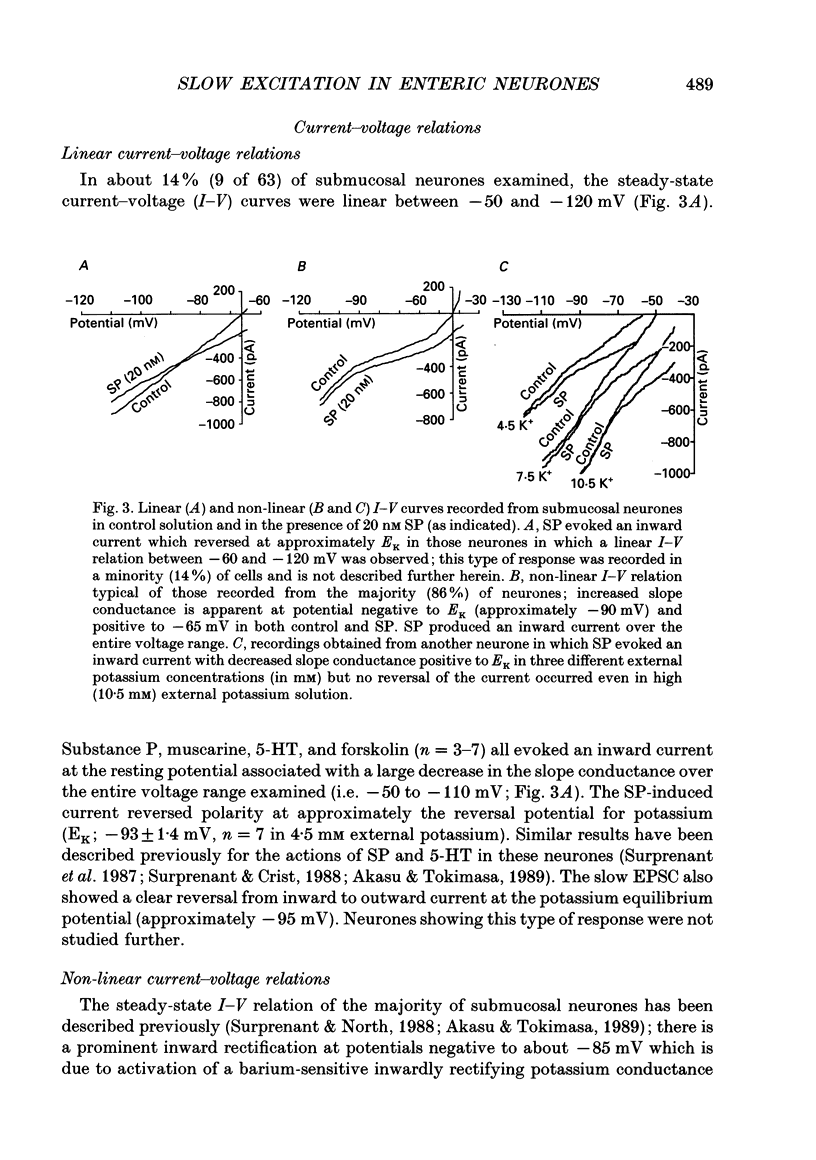
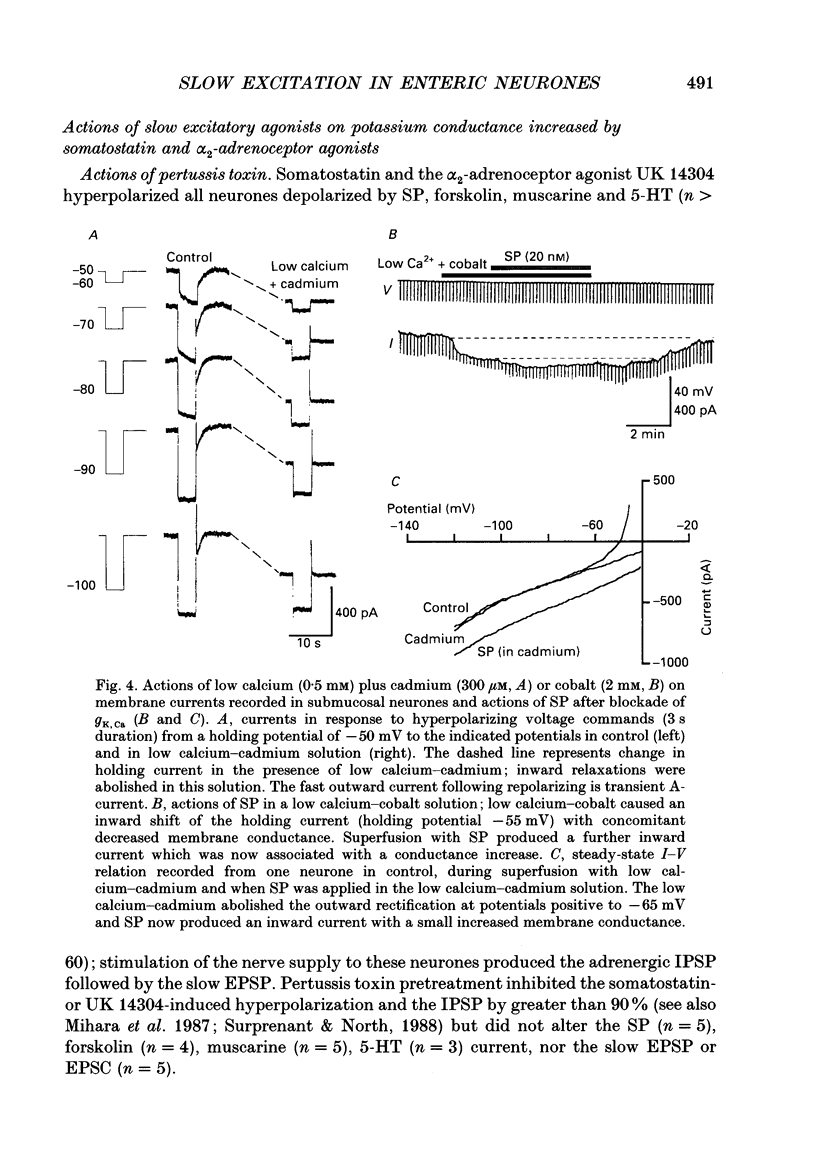
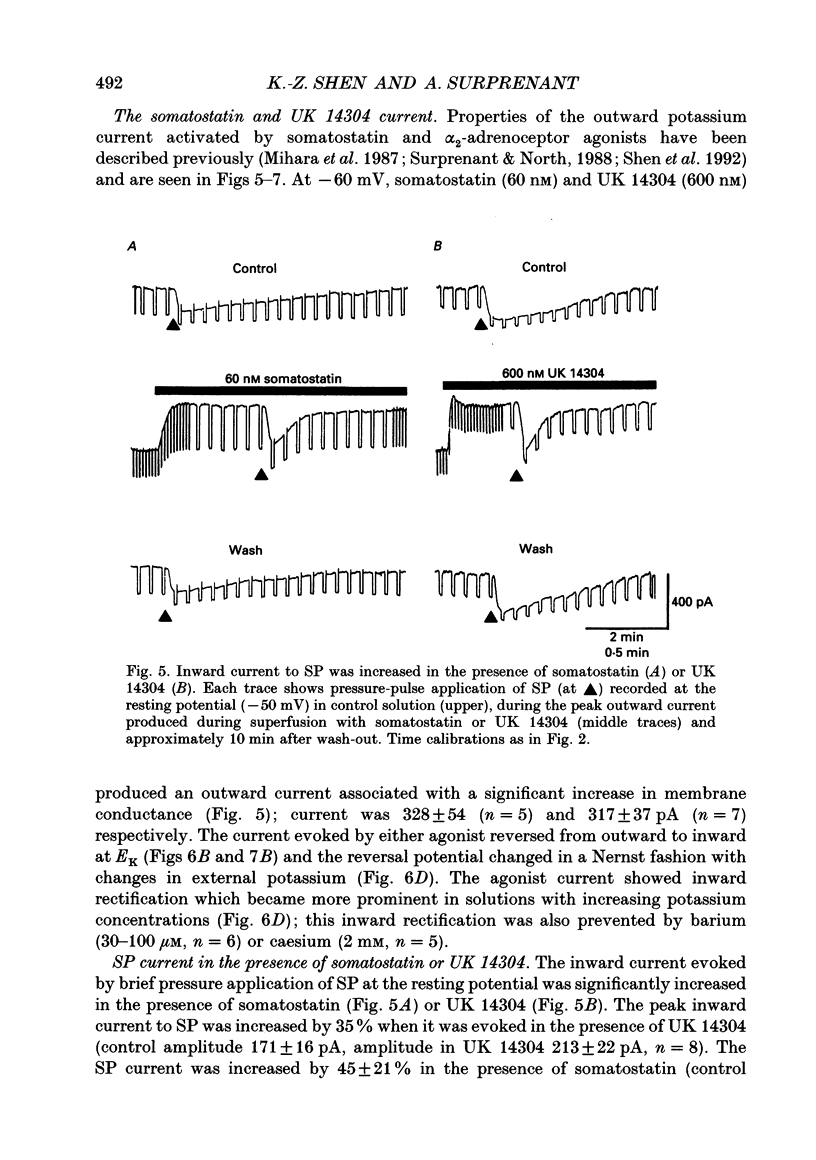
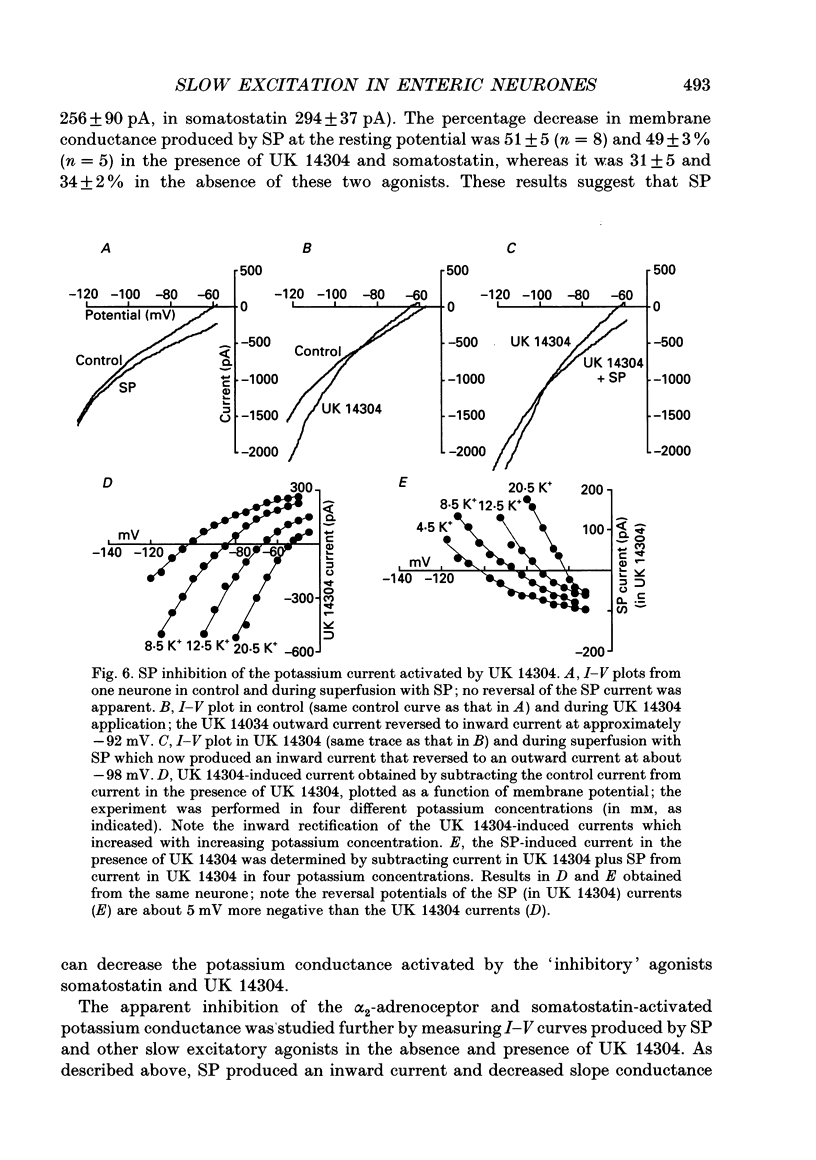
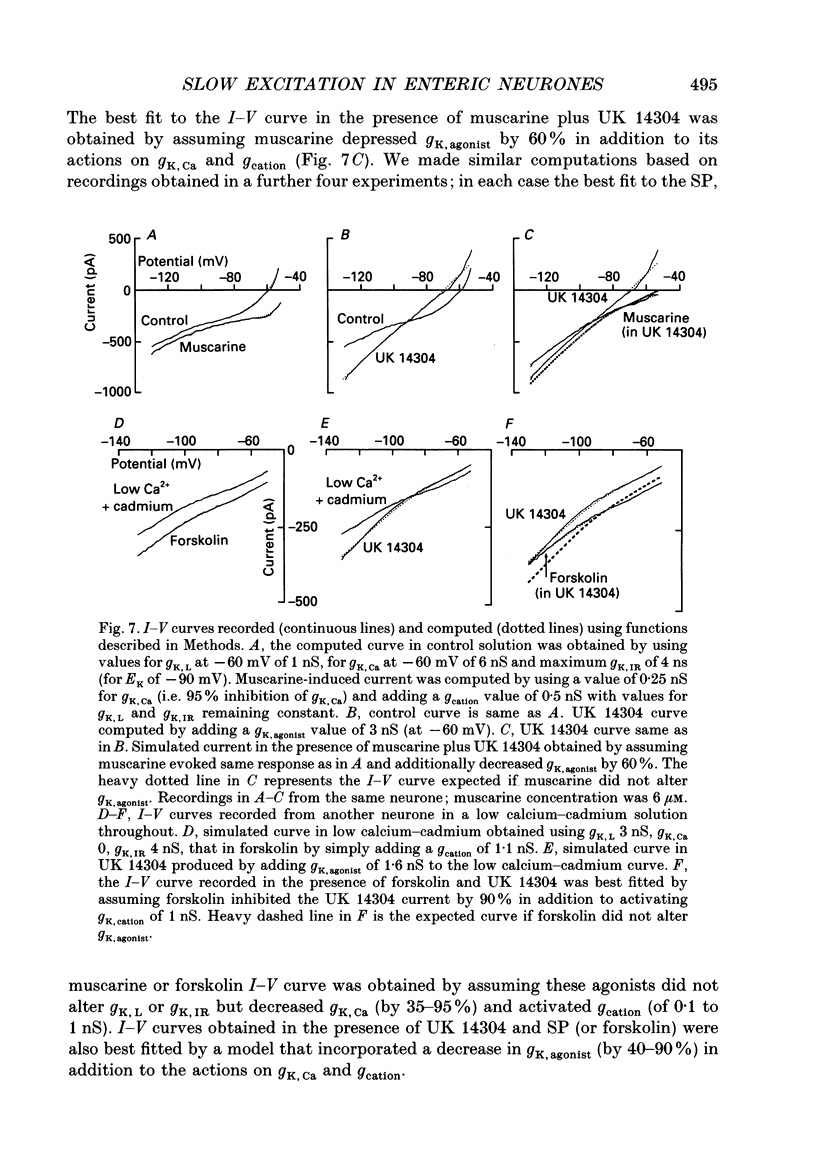
1. Intracellular recordings were made from submucosal neurones and single-electrode voltage-clamp methods were used to record membrane currents. The actions of substance P (SP), 5-hydroxytryptamine (5-HT), muscarine, vasoactive intestinal polypeptide (VIP), forskolin and nerve stimulation were studied. 2. Substance P, 5-HT (in the presence of 5-HT3 receptor antagonists), muscarine, VIP, forskolin and slow excitatory synaptic transmission all produced identical responses: an inward current associated with a membrane conductance decrease at the resting potential. The actions of any one occluded the actions of any other and all responses were pertussis-toxin insensitive. 3. These agonists produced a voltage-independent decrease in a 'leak' potassium conductance between -40 and -120 mV in 14% of neurones. 4. These agonists decreased a voltage-dependent, calcium-activated potassium conductance between -40 and -80 mV in all other (86%) neurones. The agonists still evoked an inward current without apparent conductance change at potentials between -90 and -130 mV. 5. In a low calcium solution containing cobalt or cadmium, the agonists produced an inward current associated with a conductance increase from -40 to -120 mV. Ion replacement studies indicated this current was due to an increase in a cation-selective (mainly sodium) conductance. 6. The agonists also reduced the inwardly rectifying potassium current that is activated by somatostatin and alpha 2-adrenoceptor agonists in these neurones. The agonists did not alter the inwardly rectifying potassium current that is present in these neurones in the absence of somatostatin or alpha 2-agonists. 7. Thus, SP, 5-HT, muscarine, VIP and the release of slow excitatory transmitters all appear to act through a common intracellular transduction pathway, an increase in adenylate cyclase. This results in an activation of a sodium-selective cation current and an inhibition of three distinct potassium conductances: the background potassium conductance, the calcium-activated potassium conductance and the inwardly rectifying potassium conductance activated by somatostatin and alpha 2-adrenoceptor agonists.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Tokimasa T. Potassium currents in submucous neurones of guinea-pig caecum and their synaptic modification. J Physiol. 1989 Sep;416:571–588. doi: 10.1113/jphysiol.1989.sp017778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985 Jul 25;316(6026):345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Bornstein J. C., Furness J. B. Correlated electrophysiological and histochemical studies of submucous neurons and their contribution to understanding enteric neural circuits. J Auton Nerv Syst. 1988 Nov;25(1):1–13. doi: 10.1016/0165-1838(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Brown D. A. M currents. Ion Channels. 1988;1:55–94. doi: 10.1007/978-1-4615-7302-9_2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980 Aug;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Acetylcholine activates single sodium channels in smooth muscle cells. Pflugers Arch. 1987 Sep;410(1-2):69–74. doi: 10.1007/BF00581898. [DOI] [PubMed] [Google Scholar]
- Katayama Y., North R. A. Does substance P mediate slow synaptic excitation within the myenteric plexus? Nature. 1978 Jul 27;274(5669):387–388. doi: 10.1038/274387a0. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Pappano A. J. Sodium-dependent membrane current induced by carbachol in single guinea-pig ventricular myocytes. J Physiol. 1989 Aug;415:487–502. doi: 10.1113/jphysiol.1989.sp017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara S., Katayama Y., Nishi S. Slow postsynaptic potentials in neurones of submucous plexus of guinea-pig caecum and their mimicry by noradrenaline and various peptides. Neuroscience. 1985 Dec;16(4):1057–1068. doi: 10.1016/0306-4522(85)90116-2. [DOI] [PubMed] [Google Scholar]
- Mihara S., Katayama Y., Nishi S. Slow postsynaptic potentials in neurones of submucous plexus of guinea-pig caecum and their mimicry by noradrenaline and various peptides. Neuroscience. 1985 Dec;16(4):1057–1068. doi: 10.1016/0306-4522(85)90116-2. [DOI] [PubMed] [Google Scholar]
- Mihara S., North R. A., Surprenant A. Somatostatin increases an inwardly rectifying potassium conductance in guinea-pig submucous plexus neurones. J Physiol. 1987 Sep;390:335–355. doi: 10.1113/jphysiol.1987.sp016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Katayama Y. Substance P inhibits activation of calcium-dependent potassium conductances in guinea-pig myenteric neurones. J Physiol. 1992 Feb;447:293–308. doi: 10.1113/jphysiol.1992.sp019003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. Muscarinic agonists inactivate potassium conductance of guinea-pig myenteric neurones. J Physiol. 1982 Dec;333:125–139. doi: 10.1113/jphysiol.1982.sp014443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. ADP-ribosylation of guanyl nucleotide-binding regulatory proteins by bacterial toxins. Adv Enzymol Relat Areas Mol Biol. 1988;61:303–379. doi: 10.1002/9780470123072.ch6. [DOI] [PubMed] [Google Scholar]
- Nemeth P. R., Palmer J. M., Wood J. D., Zafirov D. H. Effects of forskolin on electrical behaviour of myenteric neurones in guinea-pig small intestine. J Physiol. 1986 Jul;376:439–450. doi: 10.1113/jphysiol.1986.sp016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- North R. A. Electrophysiology of the enteric nervous system. Neuroscience. 1982 Feb;7(2):315–325. doi: 10.1016/0306-4522(82)90269-x. [DOI] [PubMed] [Google Scholar]
- North R. A., Slack B. E., Surprenant A. Muscarinic M1 and M2 receptors mediate depolarization and presynaptic inhibition in guinea-pig enteric nervous system. J Physiol. 1985 Nov;368:435–452. doi: 10.1113/jphysiol.1985.sp015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Surprenant A. Inhibitory synaptic potentials resulting from alpha 2-adrenoceptor activation in guinea-pig submucous plexus neurones. J Physiol. 1985 Jan;358:17–33. doi: 10.1113/jphysiol.1985.sp015537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Depression of calcium-dependent potassium conductance of guinea-pig myenteric neurones by muscarinic agonists. J Physiol. 1983 Sep;342:253–266. doi: 10.1113/jphysiol.1983.sp014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Persistent calcium-sensitive potassium current and the resting properties of guinea-pig myenteric neurones. J Physiol. 1987 May;386:333–353. doi: 10.1113/jphysiol.1987.sp016537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. M., Wood J. D., Zafirov D. H. Elevation of adenosine 3',5'-phosphate mimics slow synaptic excitation in myenteric neurones of the guinea-pig. J Physiol. 1986 Jul;376:451–460. doi: 10.1113/jphysiol.1986.sp016163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. M., Wood J. D., Zafirov D. H. Transduction of aminergic and peptidergic signals in enteric neurones of the guinea-pig. J Physiol. 1987 Jun;387:371–383. doi: 10.1113/jphysiol.1987.sp016578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt P. C., Spain W. J., Foehring R. C., Chubb M. C., Crill W. E. Slow conductances in neurons from cat sensorimotor cortex in vitro and their role in slow excitability changes. J Neurophysiol. 1988 Feb;59(2):450–467. doi: 10.1152/jn.1988.59.2.450. [DOI] [PubMed] [Google Scholar]
- Shen K. Z., North R. A. Muscarine increases cation conductance and decreases potassium conductance in rat locus coeruleus neurones. J Physiol. 1992 Sep;455:471–485. doi: 10.1113/jphysiol.1992.sp019312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K. Z., North R. A., Surprenant A. Potassium channels opened by noradrenaline and other transmitters in excised membrane patches of guinea-pig submucosal neurones. J Physiol. 1992 Jan;445:581–599. doi: 10.1113/jphysiol.1992.sp018941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K. Z., Surprenant A. Mechanisms underlying presynaptic inhibition through alpha 2-adrenoceptors in guinea-pig submucosal neurones. J Physiol. 1990 Dec;431:609–628. doi: 10.1113/jphysiol.1990.sp018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A., Crist J. Electrophysiological characterization of functionally distinct 5-hydroxytryptamine receptors on guinea-pig submucous plexus. Neuroscience. 1988 Jan;24(1):283–295. doi: 10.1016/0306-4522(88)90331-4. [DOI] [PubMed] [Google Scholar]
- Surprenant A., North R. A., Katayama Y. Observations on the actions of substance P and [D-Arg1,D-Pro2,D-Trp7,9,Leu11)substance P on single neurons of the guinea pig submucous plexus. Neuroscience. 1987 Jan;20(1):189–199. doi: 10.1016/0306-4522(87)90011-x. [DOI] [PubMed] [Google Scholar]
- Surprenant A., North R. A. Mechanism of synaptic inhibition by noradrenaline acting at alpha 2-adrenoceptors. Proc R Soc Lond B Biol Sci. 1988 Jun 22;234(1274):85–114. doi: 10.1098/rspb.1988.0039. [DOI] [PubMed] [Google Scholar]
- Surprenant A. Slow excitatory synaptic potentials recorded from neurones of guinea-pig submucous plexus. J Physiol. 1984 Jun;351:343–361. doi: 10.1113/jphysiol.1984.sp015249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji S., Kuba K. Muscarinic regulation of two ionic currents in the bullfrog sympathetic neurone. Pflugers Arch. 1988 Apr;411(4):361–370. doi: 10.1007/BF00587714. [DOI] [PubMed] [Google Scholar]


