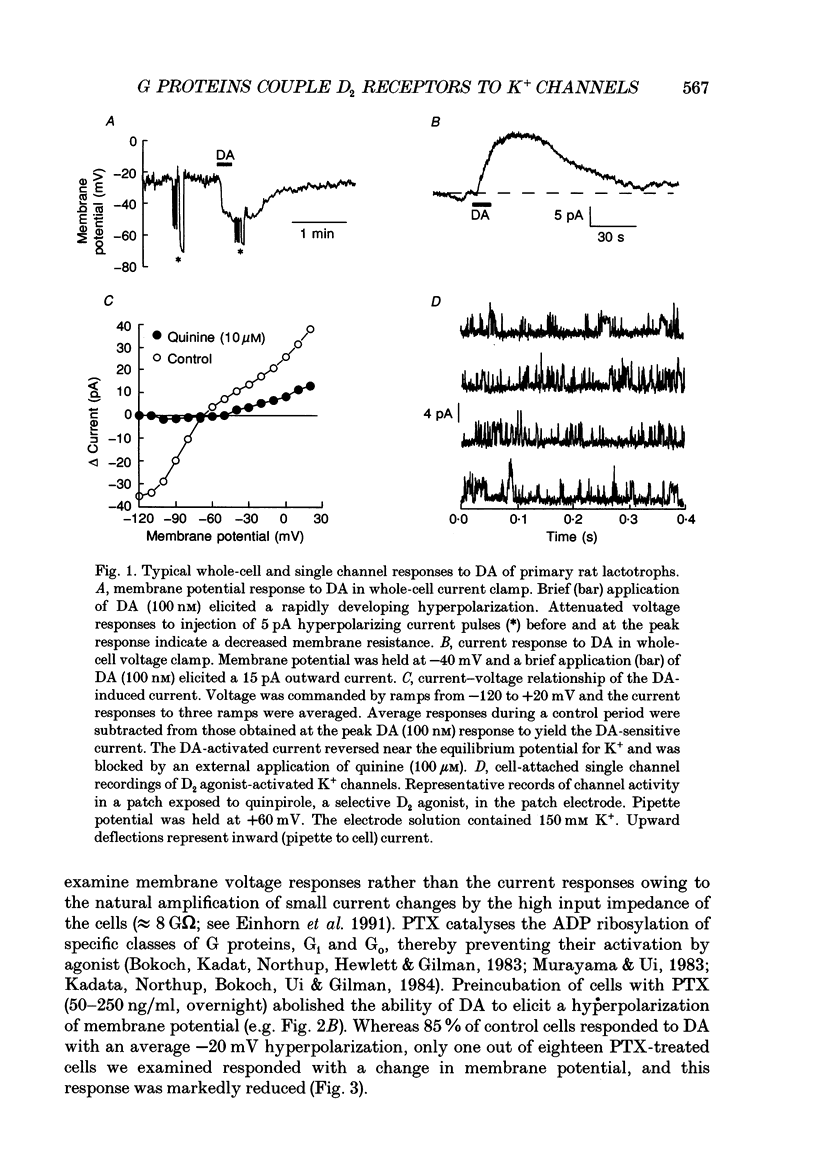
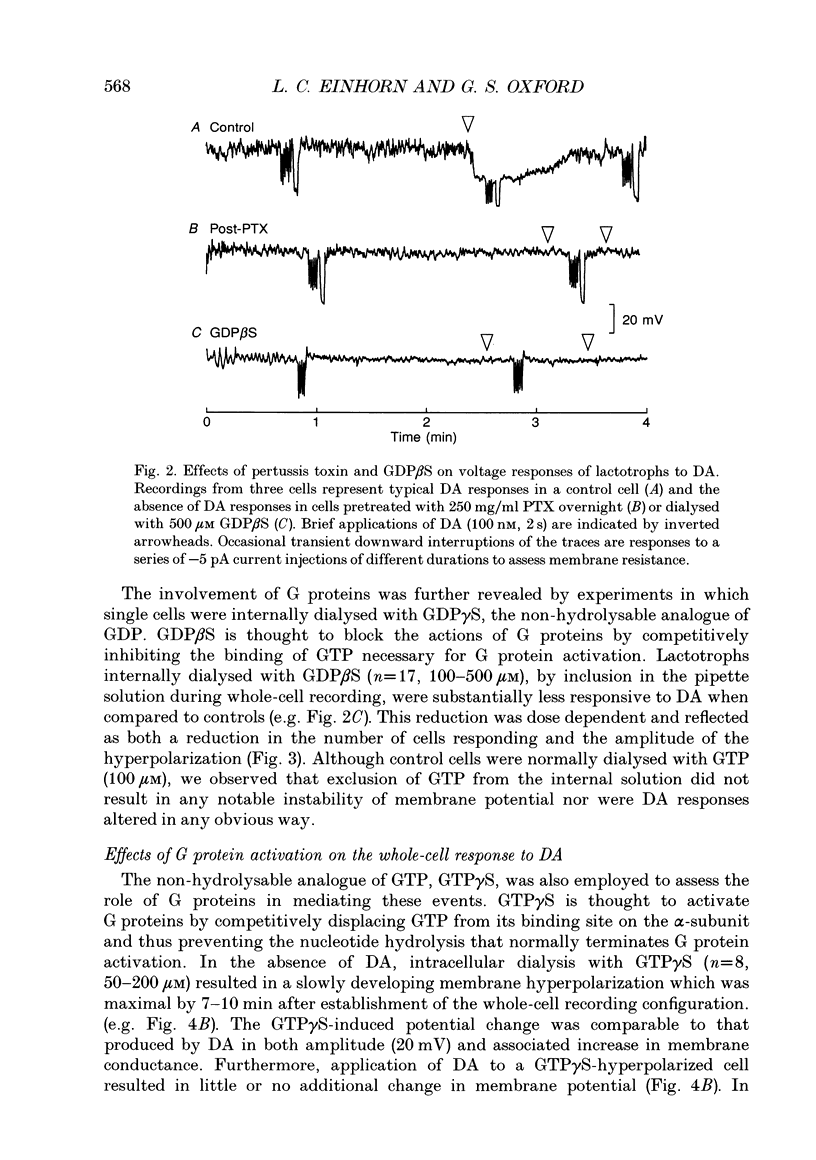
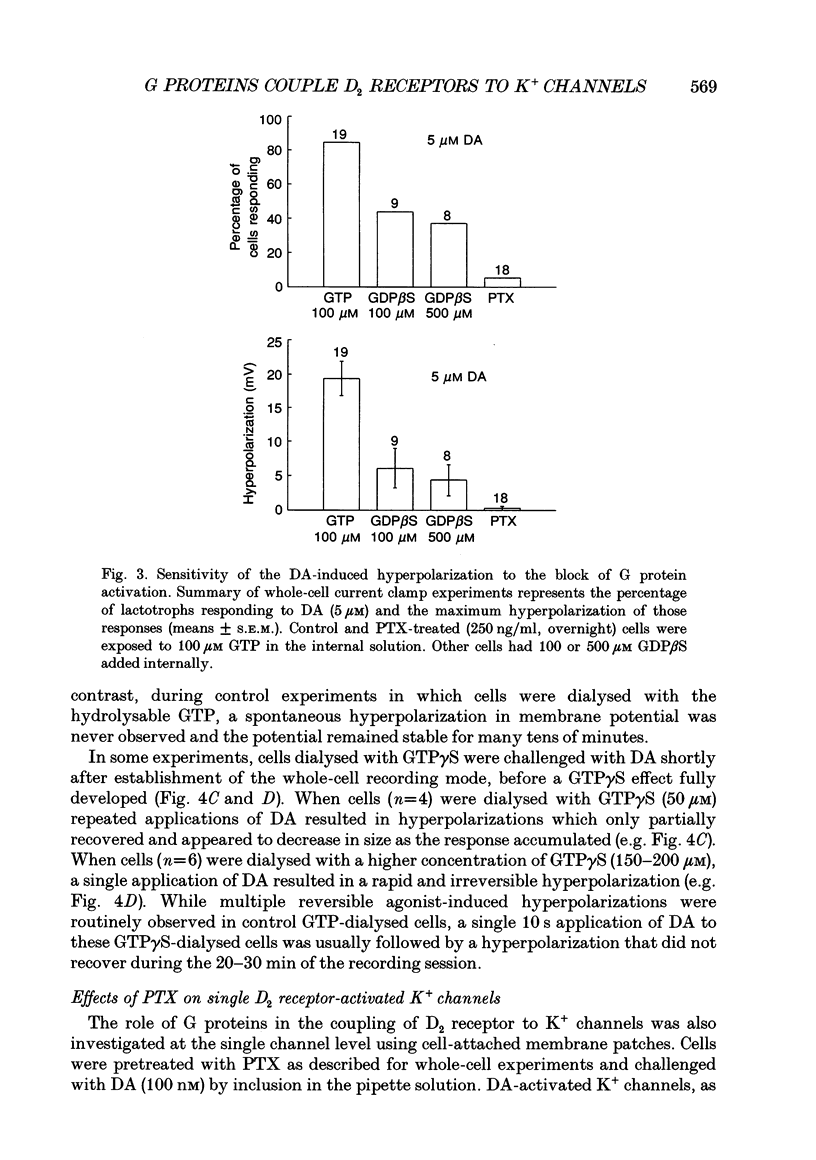
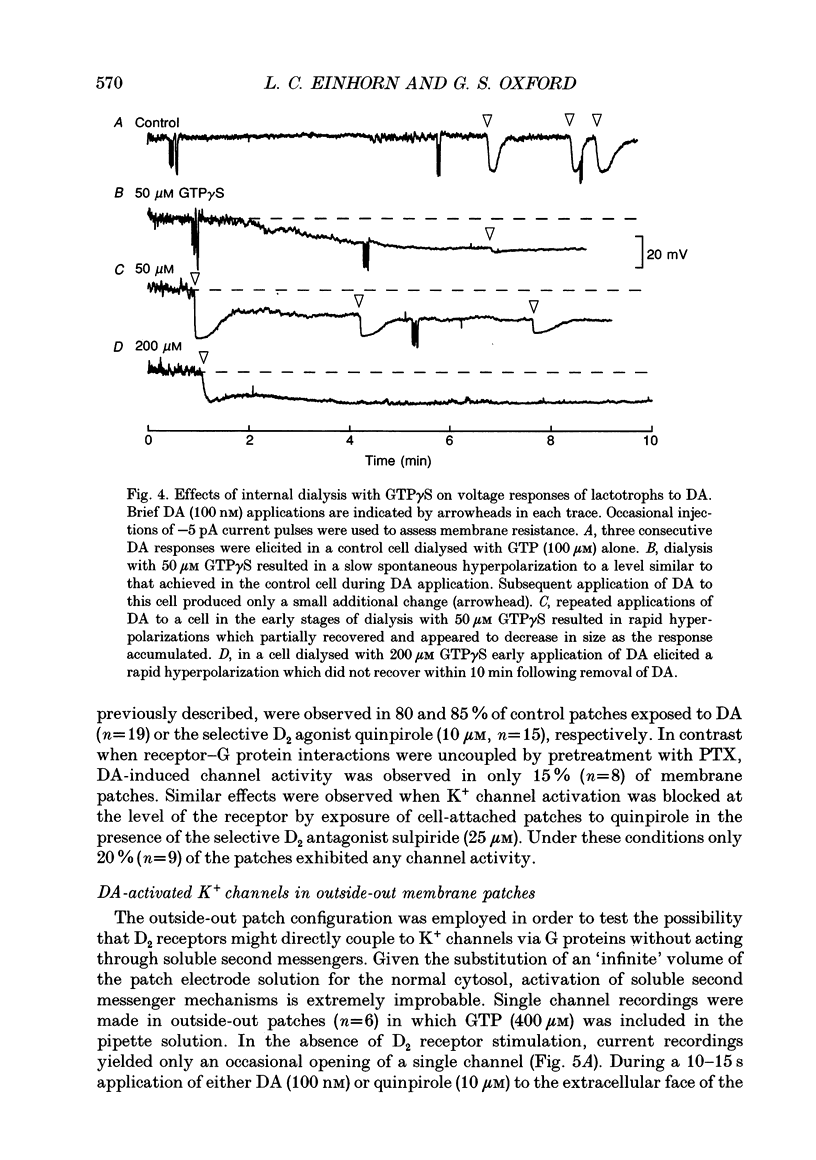
Abstract
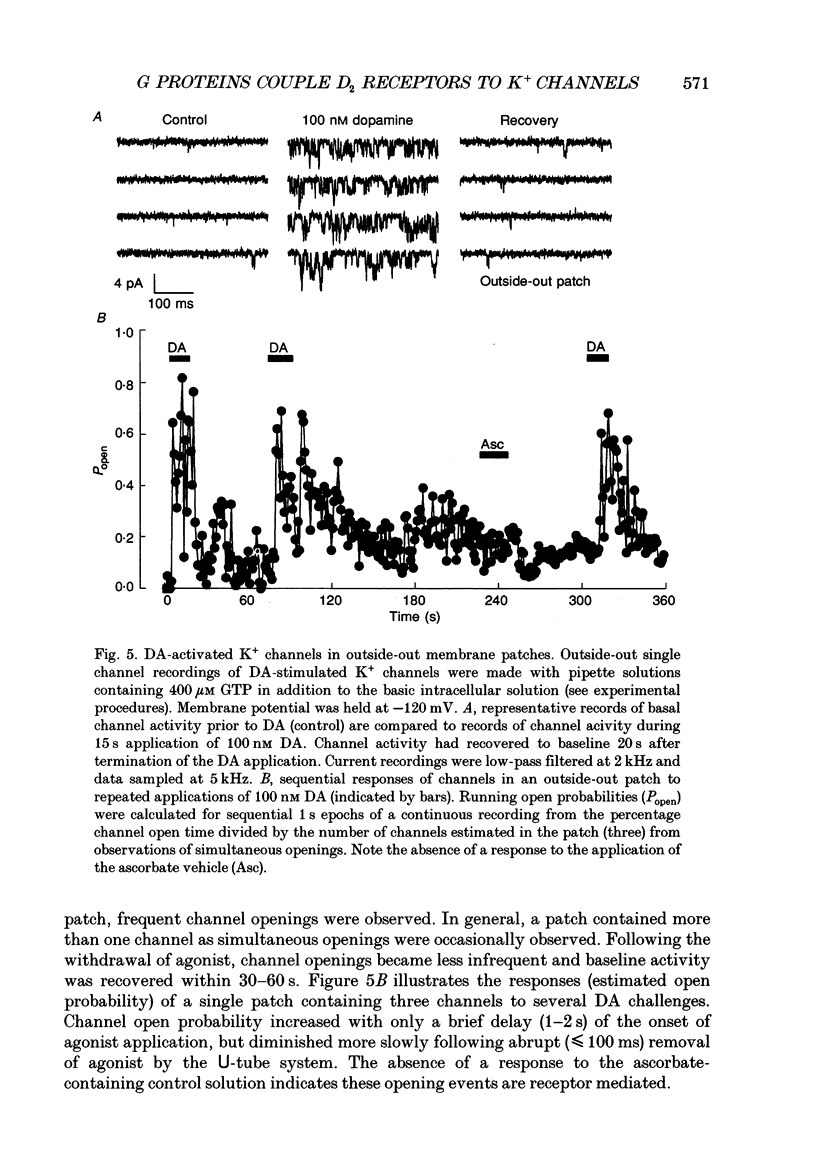
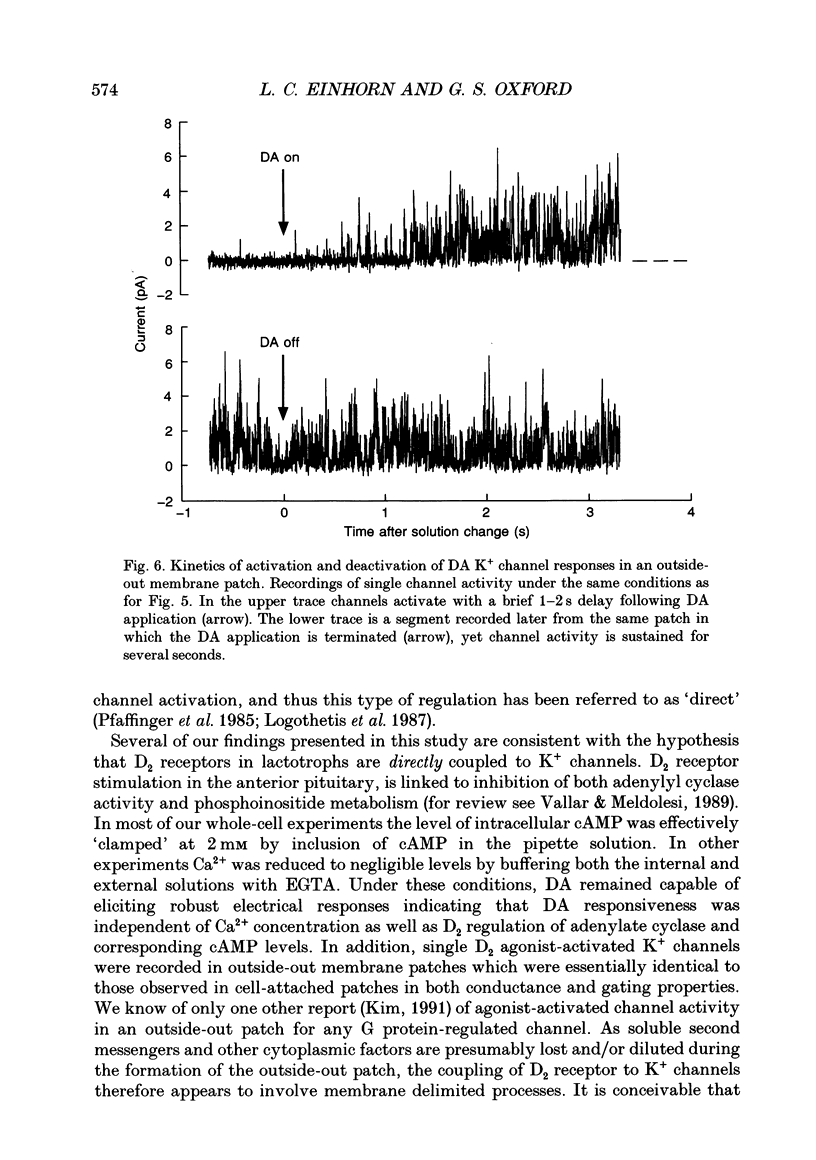
1. The involvement of guanine nucleotide binding proteins in the coupling of D2 dopamine (DA) receptors to single potassium channels was examined in rat pituitary lactotrophs. 2. Lactotrophs were unambiguously identified by the reverse haemolytic plaque assay (RHPA) and membrane potentials, whole-cell and single channel currents recorded using patch electrode methods. 3. DA or the D2 selective agonist, quinpirole, induced the opening of single K+ channels in cell-attached patches underlying robust hyperpolarizations of membrane potential in single cells. 4. Both whole-cell and single channel responses were independent of Ca2+ or cAMP concentrations. 5. Pertussis toxin (PTX) pretreatment (50-250 ng/ml, 6-12 h) blocked the action of DA on lactotroph membrane potential and uncoupled D2 receptors from single K+ channels in cell-attached patches. 6. Internal dialysis with GDP beta S (guanosine 5'-O-(2-thiodiphosphate) greatly reduced whole-cell responses to DA in a dose-dependent manner. 7. Internal dialysis of lactotrophs with GTP gamma S (guanosine 5'-O-(3-thiotriphosphate) potentiated DA responses in a dose-dependent manner while rendering the responses irreversible at higher doses. 8. DA (100 nM) or quinpirole (10 microM) activated K+ channels in excised outside-out membrane patches that were identical to those identified in cell-attached patches in terms of conductance and gating kinetics. 9. It is proposed that D2 receptors are coupled to non-voltage-dependent K+ channels by G proteins of the Gi/Go class and that this coupling is via a direct, membrane delimited pathway.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Jonathan N. Dopamine: a prolactin-inhibiting hormone. Endocr Rev. 1985 Fall;6(4):564–589. doi: 10.1210/edrv-6-4-564. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Mechanism of muscarinic receptor-induced K+ channel activation as revealed by hydrolysis-resistant GTP analogues. J Gen Physiol. 1988 Apr;91(4):469–493. doi: 10.1085/jgp.91.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Caron M. G., Beaulieu M., Raymond V., Gagné B., Drouin J., Lefkowitz R. J., Labrie F. Dopaminergic receptors in the anterior pituitary gland. Correlation of [3H]dihydroergocryptine binding with the dopaminergic control of prolactin release. J Biol Chem. 1978 Apr 10;253(7):2244–2253. [PubMed] [Google Scholar]
- Castelletti L., Memo M., Missale C., Spano P. F., Valerio A. Potassium channels involved in the transduction mechanism of dopamine D2 receptors in rat lactotrophs. J Physiol. 1989 Mar;410:251–265. doi: 10.1113/jphysiol.1989.sp017531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina J., Olate J., Abramowitz J., Mattera R., Cook R. G., Birnbaumer L. Alpha i-3 cDNA encodes the alpha subunit of Gk, the stimulatory G protein of receptor-regulated K+ channels. J Biol Chem. 1988 May 15;263(14):6746–6750. [PubMed] [Google Scholar]
- Cronin M. J., Myers G. A., MacLeod R. M., Hewlett E. L. Pertussis toxin uncouples dopamine agonist inhibition of prolactin release. Am J Physiol. 1983 May;244(5):E499–E504. doi: 10.1152/ajpendo.1983.244.5.E499. [DOI] [PubMed] [Google Scholar]
- De Lean A., Kilpatrick B. F., Caron M. G. Guanine nucleotides regulate both dopaminergic agonist and antagonist binding in porcine anterior pituitary. Endocrinology. 1982 Mar;110(3):1064–1066. doi: 10.1210/endo-110-3-1064. [DOI] [PubMed] [Google Scholar]
- Delbeke D., Scammell J. G., Martinez-Campos A., Dannies P. S. Dopamine inhibits prolactin release when cyclic adenosine 3',5'-monophosphate levels are elevated. Endocrinology. 1986 Apr;118(4):1271–1277. doi: 10.1210/endo-118-4-1271. [DOI] [PubMed] [Google Scholar]
- Einhorn L. C., Gregerson K. A., Oxford G. S. D2 dopamine receptor activation of potassium channels in identified rat lactotrophs: whole-cell and single-channel recording. J Neurosci. 1991 Dec;11(12):3727–3737. doi: 10.1523/JNEUROSCI.11-12-03727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert A., Bockaert J. Pharmacological characterization of the D2 dopamine receptor negatively coupled with adenylate cyclase in rat anterior pituitary. Mol Pharmacol. 1983 May;23(3):576–584. [PubMed] [Google Scholar]
- Enjalbert A., Musset F., Chénard C., Priam M., Kordon C., Heisler S. Dopamine inhibits prolactin secretion stimulated by the calcium channel agonist Bay-K-8644 through a pertussis toxin-sensitive G protein in anterior pituitary cells. Endocrinology. 1988 Jul;123(1):406–412. doi: 10.1210/endo-123-1-406. [DOI] [PubMed] [Google Scholar]
- Ewald D. A., Pang I. H., Sternweis P. C., Miller R. J. Differential G protein-mediated coupling of neurotransmitter receptors to Ca2+ channels in rat dorsal root ganglion neurons in vitro. Neuron. 1989 Feb;2(2):1185–1193. doi: 10.1016/0896-6273(89)90185-2. [DOI] [PubMed] [Google Scholar]
- Freedman J. E., Weight F. F. Single K+ channels activated by D2 dopamine receptors in acutely dissociated neurons from rat corpus striatum. Proc Natl Acad Sci U S A. 1988 May;85(10):3618–3622. doi: 10.1073/pnas.85.10.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S. R., Watanabe M., Di Paolo T., Falardeau P., Labrie F., Seeman P. The functional state of the dopamine receptor in the anterior pituitary is in the high affinity form. Endocrinology. 1985 Aug;117(2):690–697. doi: 10.1210/endo-117-2-690. [DOI] [PubMed] [Google Scholar]
- Giros B., Sokoloff P., Martres M. P., Riou J. F., Emorine L. J., Schwartz J. C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989 Dec 21;342(6252):923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- Grandy D. K., Marchionni M. A., Makam H., Stofko R. E., Alfano M., Frothingham L., Fischer J. B., Burke-Howie K. J., Bunzow J. R., Server A. C. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9762–9766. doi: 10.1073/pnas.86.24.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregerson K. A., Einhorn L., Smith M. M., Oxford G. S. Modulation of potassium channels by dopamine in rat pituitary lactotrophs: a role in the regulation of prolactin secretion? Soc Gen Physiol Ser. 1989;44:123–141. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick R. M., Hammond C., Paupardin-Tritsch D., Homburger V., Rouot B., Bockaert J., Gerschenfeld H. M. An alpha 40 subunit of a GTP-binding protein immunologically related to Go mediates a dopamine-induced decrease of Ca2+ current in snail neurons. Neuron. 1988 Mar;1(1):27–32. doi: 10.1016/0896-6273(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram C. D., Bicknell R. J., Mason W. T. Intracellular recordings from bovine anterior pituitary cells: modulation of spontaneous activity by regulators of prolactin secretion. Endocrinology. 1986 Dec;119(6):2508–2518. doi: 10.1210/endo-119-6-2508. [DOI] [PubMed] [Google Scholar]
- Israel J. M., Jaquet P., Vincent J. D. The electrical properties of isolated human prolactin-secreting adenoma cells and their modification by dopamine. Endocrinology. 1985 Oct;117(4):1448–1455. doi: 10.1210/endo-117-4-1448. [DOI] [PubMed] [Google Scholar]
- Katada T., Northup J. K., Bokoch G. M., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Subunit dissociation and guanine nucleotide-dependent hormonal inhibition. J Biol Chem. 1984 Mar 25;259(6):3578–3585. [PubMed] [Google Scholar]
- Kilpatrick B. F., Caron M. G. Agonist binding promotes a guanine nucleotide reversible increase in the apparent size of the bovine anterior pituitary dopamine receptors. J Biol Chem. 1983 Nov 25;258(22):13528–13534. [PubMed] [Google Scholar]
- Kim D. Calcitonin-gene-related peptide activates the muscarinic-gated K+ current in atrial cells. Pflugers Arch. 1991 May;418(4):338–345. doi: 10.1007/BF00550871. [DOI] [PubMed] [Google Scholar]
- Kim D., Lewis D. L., Graziadei L., Neer E. J., Bar-Sagi D., Clapham D. E. G-protein beta gamma-subunits activate the cardiac muscarinic K+-channel via phospholipase A2. Nature. 1989 Feb 9;337(6207):557–560. doi: 10.1038/337557a0. [DOI] [PubMed] [Google Scholar]
- Kleuss C., Hescheler J., Ewel C., Rosenthal W., Schultz G., Wittig B. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991 Sep 5;353(6339):43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987 Nov;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol. 1988 Jul;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. L., Weight F. F., Luini A. A guanine nucleotide-binding protein mediates the inhibition of voltage-dependent calcium current by somatostatin in a pituitary cell line. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9035–9039. doi: 10.1073/pnas.83.23.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo P. M., Legendre P., Israel J. M., Vincent J. D. Dopamine inhibits two characterized voltage-dependent calcium currents in identified rat lactotroph cells. Endocrinology. 1990 Sep;127(3):990–1001. doi: 10.1210/endo-127-3-990. [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Vallar L., Elahi F. R., Pozzan T., Spada A., Meldolesi J. Dopamine inhibits cytosolic Ca2+ increases in rat lactotroph cells. Evidence of a dual mechanism of action. J Biol Chem. 1987 Oct 15;262(29):13920–13927. [PubMed] [Google Scholar]
- Mason W. T., Sikdar S. K., Zorec R., Akerman S., Rawlings S. R., Cheek T., Moreton R., Berridge M. Ion channels, intracellular calcium, and exocytosis: control of hormone secretion in cultured bovine pituitary lactotrophs. Soc Gen Physiol Ser. 1989;44:225–238. [PubMed] [Google Scholar]
- Monsma F. J., Jr, McVittie L. D., Gerfen C. R., Mahan L. C., Sibley D. R. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989 Dec 21;342(6252):926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983 Mar 10;258(5):3319–3326. [PubMed] [Google Scholar]
- Ohara K., Haga K., Berstein G., Haga T., Ichiyama A., Ohara K. The interaction between D-2 dopamine receptors and GTP-binding proteins. Mol Pharmacol. 1988 Mar;33(3):290–296. [PubMed] [Google Scholar]
- Oxford G. S., Wagoner P. K. The inactivating K+ current in GH3 pituitary cells and its modification by chemical reagents. J Physiol. 1989 Mar;410:587–612. doi: 10.1113/jphysiol.1989.sp017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Ray K. P., Gomm J. J., Law G. J., Sigournay C., Wallis M. Dopamine and somatostatin inhibit forskolin-stimulated prolactin and growth hormone secretion but not stimulated cyclic AMP levels in sheep anterior pituitary cell cultures. Mol Cell Endocrinol. 1986 May;45(2-3):175–182. doi: 10.1016/0303-7207(86)90145-0. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Sato M. A single GTP-binding protein regulates K+-channels coupled with dopamine, histamine and acetylcholine receptors. Nature. 1987 Jan 15;325(6101):259–262. doi: 10.1038/325259a0. [DOI] [PubMed] [Google Scholar]
- Senogles S. E., Amlaiky N., Falardeau P., Caron M. G. Purification and characterization of the D2-dopamine receptor from bovine anterior pituitary. J Biol Chem. 1988 Dec 15;263(35):18996–19002. [PubMed] [Google Scholar]
- Senogles S. E., Benovic J. L., Amlaiky N., Unson C., Milligan G., Vinitsky R., Spiegel A. M., Caron M. G. The D2-dopamine receptor of anterior pituitary is functionally associated with a pertussis toxin-sensitive guanine nucleotide binding protein. J Biol Chem. 1987 Apr 5;262(10):4860–4867. [PubMed] [Google Scholar]
- Senogles S. E., Spiegel A. M., Padrell E., Iyengar R., Caron M. G. Specificity of receptor-G protein interactions. Discrimination of Gi subtypes by the D2 dopamine receptor in a reconstituted system. J Biol Chem. 1990 Mar 15;265(8):4507–4514. [PubMed] [Google Scholar]
- Smith P. F., Luque E. H., Neill J. D. Detection and measurement of secretion from individual neuroendocrine cells using a reverse hemolytic plaque assay. Methods Enzymol. 1986;124:443–465. doi: 10.1016/0076-6879(86)24034-3. [DOI] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Stack J., Surprenant A. Dopamine actions on calcium currents, potassium currents and hormone release in rat melanotrophs. J Physiol. 1991 Aug;439:37–58. doi: 10.1113/jphysiol.1991.sp018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Structural basis of beta-adrenergic receptor function. FASEB J. 1989 May;3(7):1825–1832. doi: 10.1096/fasebj.3.7.2541037. [DOI] [PubMed] [Google Scholar]
- Tam S. W., Dannies P. S. The role of adenosine 3',5'-monophosphate in dopaminergic inhibition of prolactin release in anterior pituitary cells. Endocrinology. 1981 Aug;109(2):403–408. doi: 10.1210/endo-109-2-403. [DOI] [PubMed] [Google Scholar]
- Vallar L., Meldolesi J. Mechanisms of signal transduction at the dopamine D2 receptor. Trends Pharmacol Sci. 1989 Feb;10(2):74–77. doi: 10.1016/0165-6147(89)90082-5. [DOI] [PubMed] [Google Scholar]
- VanDongen A. M., Codina J., Olate J., Mattera R., Joho R., Birnbaumer L., Brown A. M. Newly identified brain potassium channels gated by the guanine nucleotide binding protein Go. Science. 1988 Dec 9;242(4884):1433–1437. doi: 10.1126/science.3144040. [DOI] [PubMed] [Google Scholar]
- Weiner R. I., Bethea C. L., Jaquet P., Ramsdell J. S., Gospodarowicz D. J. Culture of dispersed anterior pituitary cells on extracellular matrix. Methods Enzymol. 1983;103:287–293. doi: 10.1016/s0076-6879(83)03018-9. [DOI] [PubMed] [Google Scholar]


