Abstract
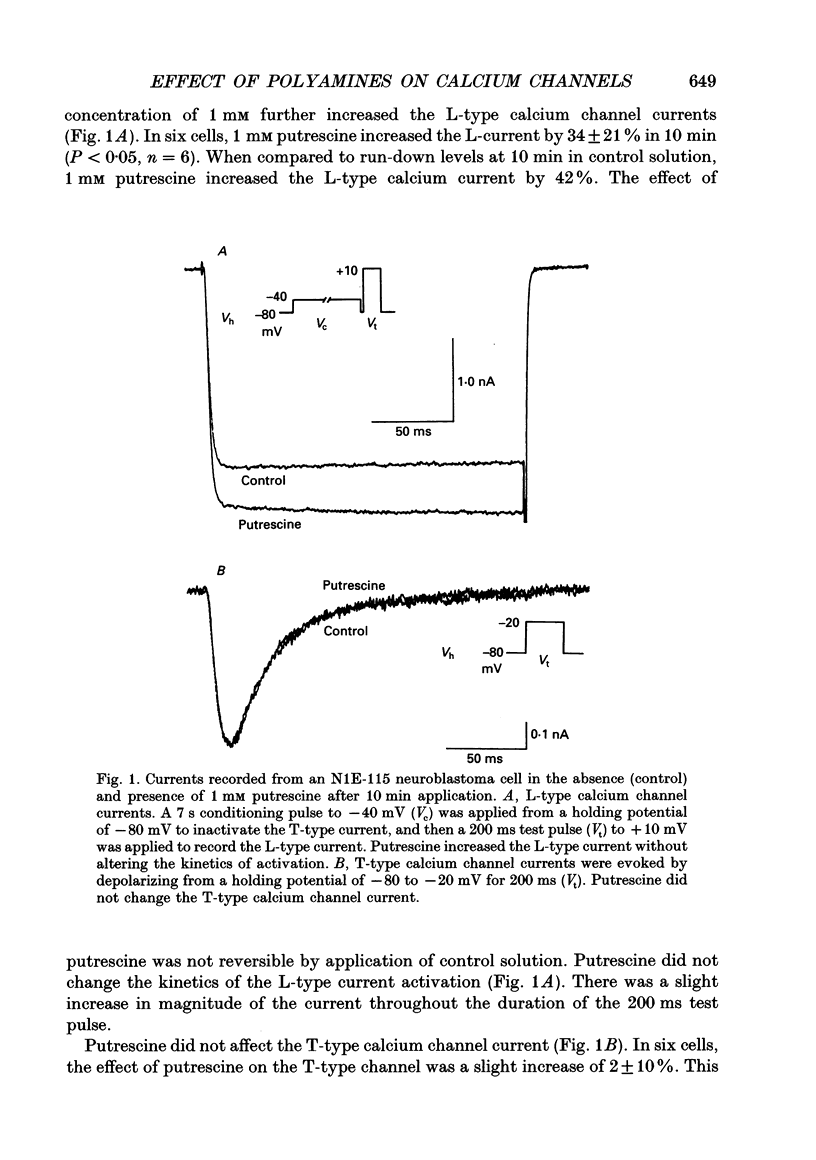
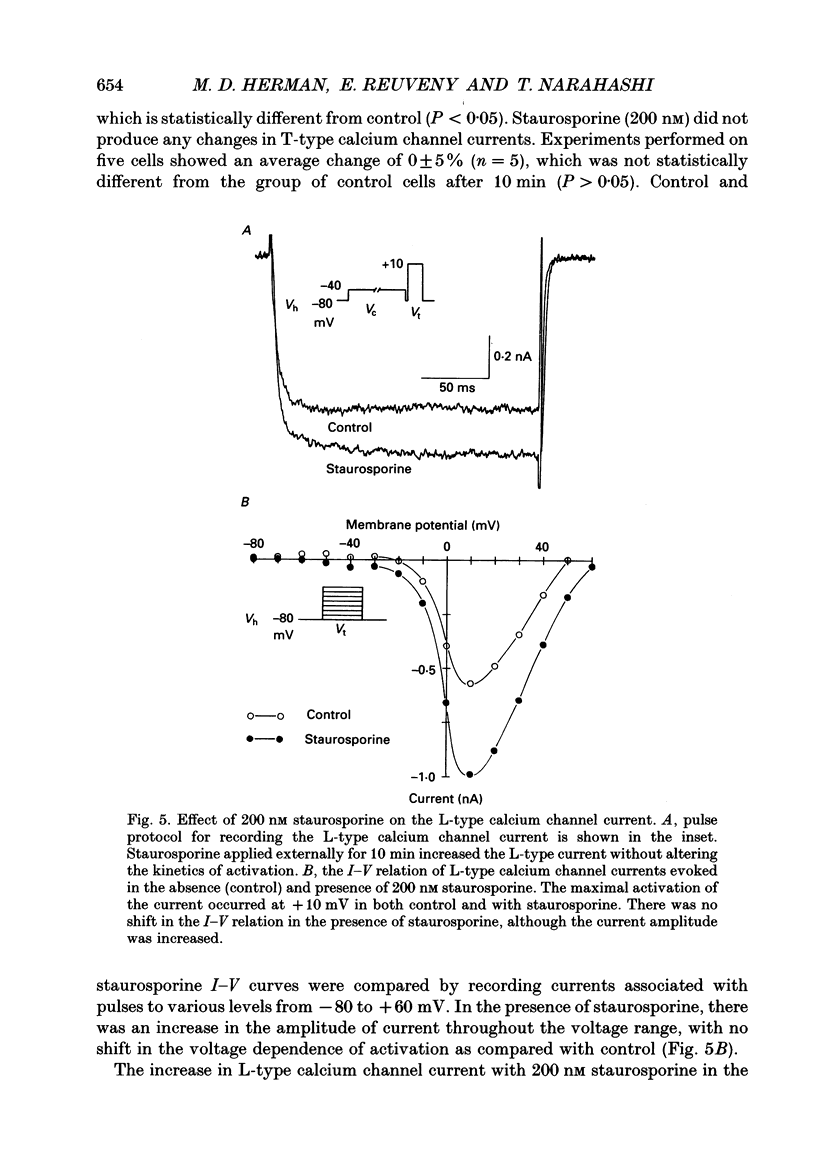
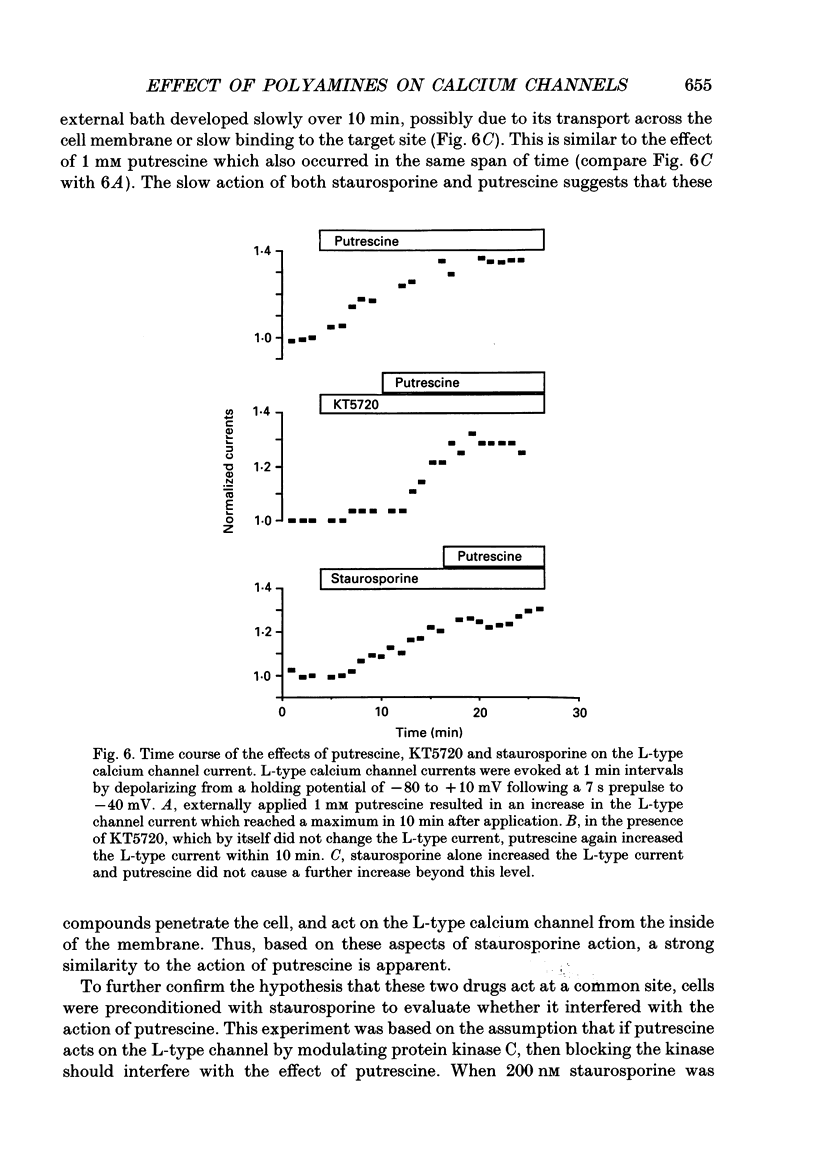
1. Putrescine has been implicated in modulating cytoplasmic calcium concentration and is correlated with selective neuronal vulnerability in cerebral ischaemia. In order to determine whether putrescine modulates voltage-activated calcium channels, whole-cell and single channel patch clamp experiments were performed with N1E-115 mouse neuroblastoma cells. 2. L-type calcium channel currents showed a 34 +/- 21% increase (n = 6 cells) during external application of 1 mM putrescine. There was no change in the kinetics of the current and no shift in the current-voltage relationship along the voltage axis. 3. T-type calcium channel currents were not affected by 1 mM putrescine. 4. The effect of putrescine on single L-type calcium channels was studied using the cell-attached configuration of the patch clamp technique. Putrescine (5 mM) applied to the bathing solution, but not present in the pipette, caused an increase in open time of the single channel current without changing the conductance of the channel. In 345 depolarizing steps compiled from three cells, the number of channel openings longer than 3 ms increased from six to seventy-six, and the number of channel openings longer than 9 ms increased from zero to twenty-seven. This single channel study supports the hypothesis that putrescine acts on the L-type channel from the inside of the cell. 5. External application of 1 mM spermine and 1 mM spermidine had no effect on T- and L-type calcium channels. Thus, the effect of putrescine is probably not mediated by the higher polyamines. 6. In order to test whether the effect of putrescine is mediated by a second messenger, specific protein kinase C and cyclic AMP-dependent protein kinase inhibitors, staurosporine and KT5720, respectively, were applied prior to putrescine. When cells were preconditioned with 200 nM staurosporine, the increase of the L-type calcium current by 1 mM putrescine was inhibited. By contrast, 200 nM KT5720 did not inhibit the putrescine effect. Therefore, the increase of L-type channel currents by putrescine may be mediated by protein kinase C but not the cyclic AMP-dependent protein kinase. 7. The putrescine-induced enhancement of the L-type calcium channel activity may play an important role in calcium-induced neurotoxicity.
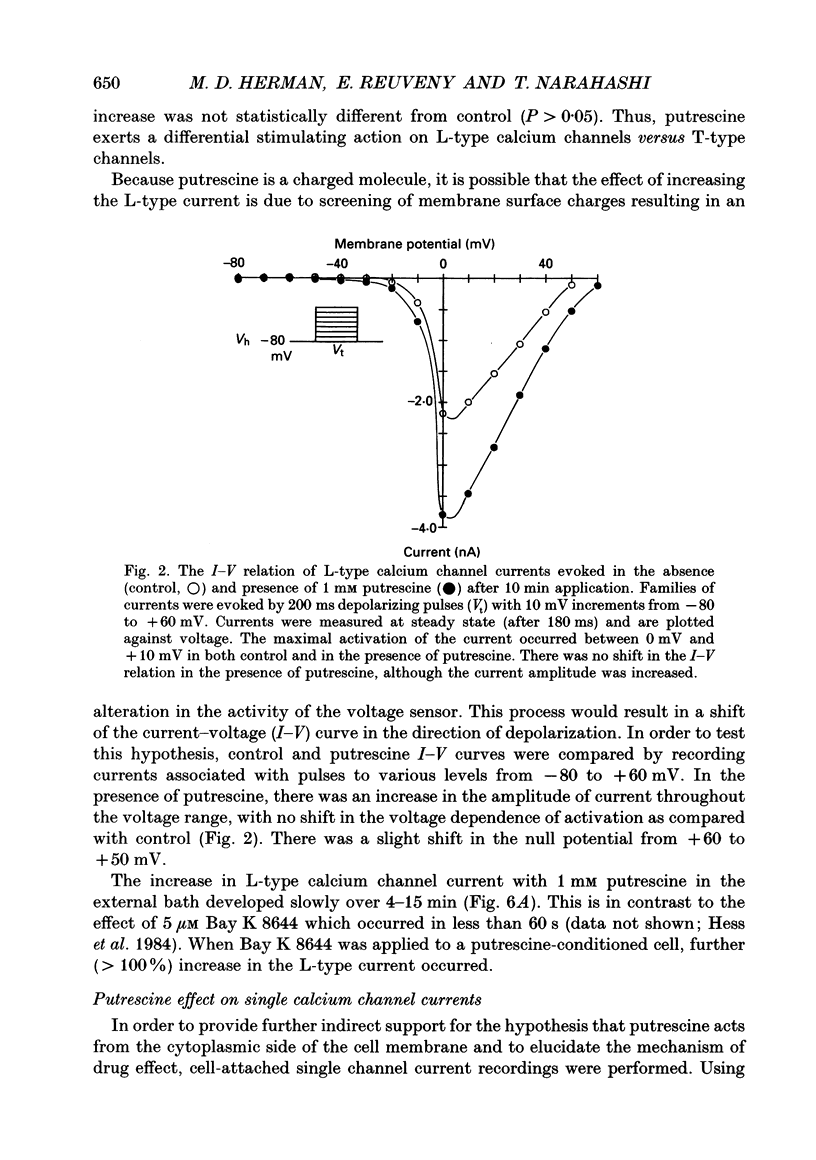
Full text
PDF



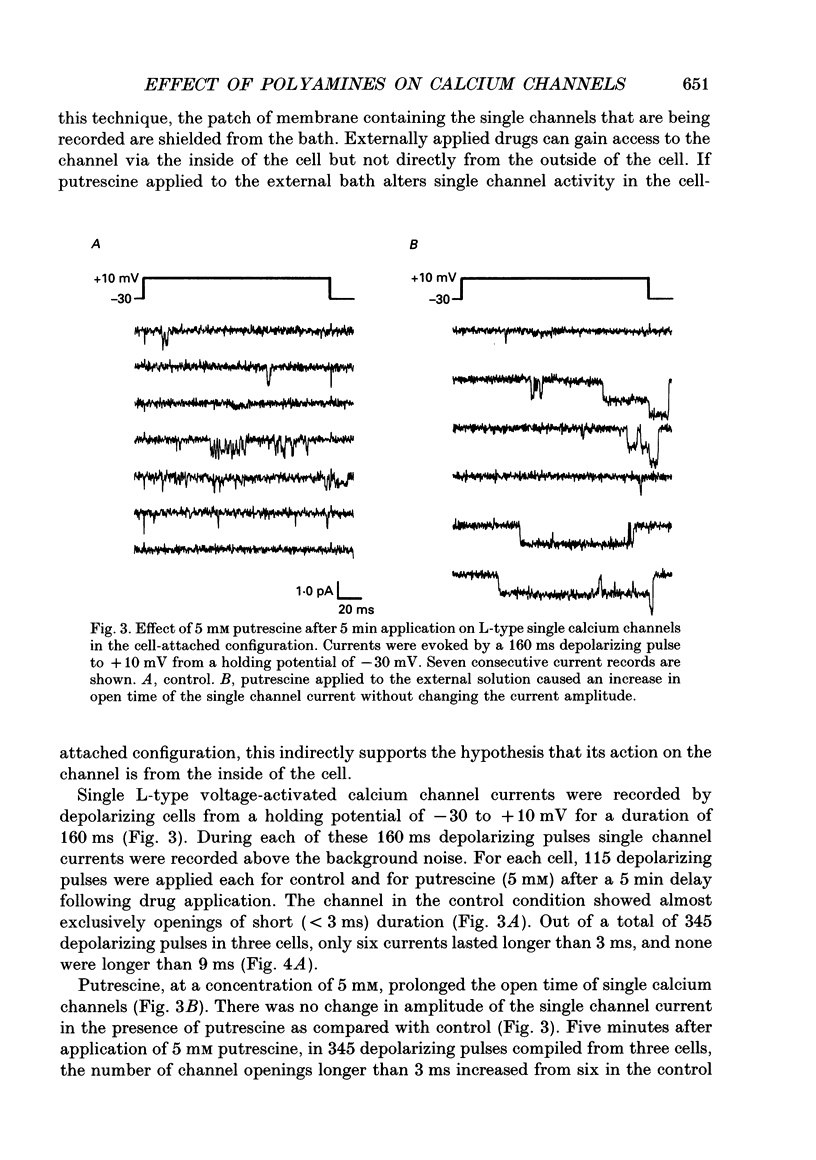
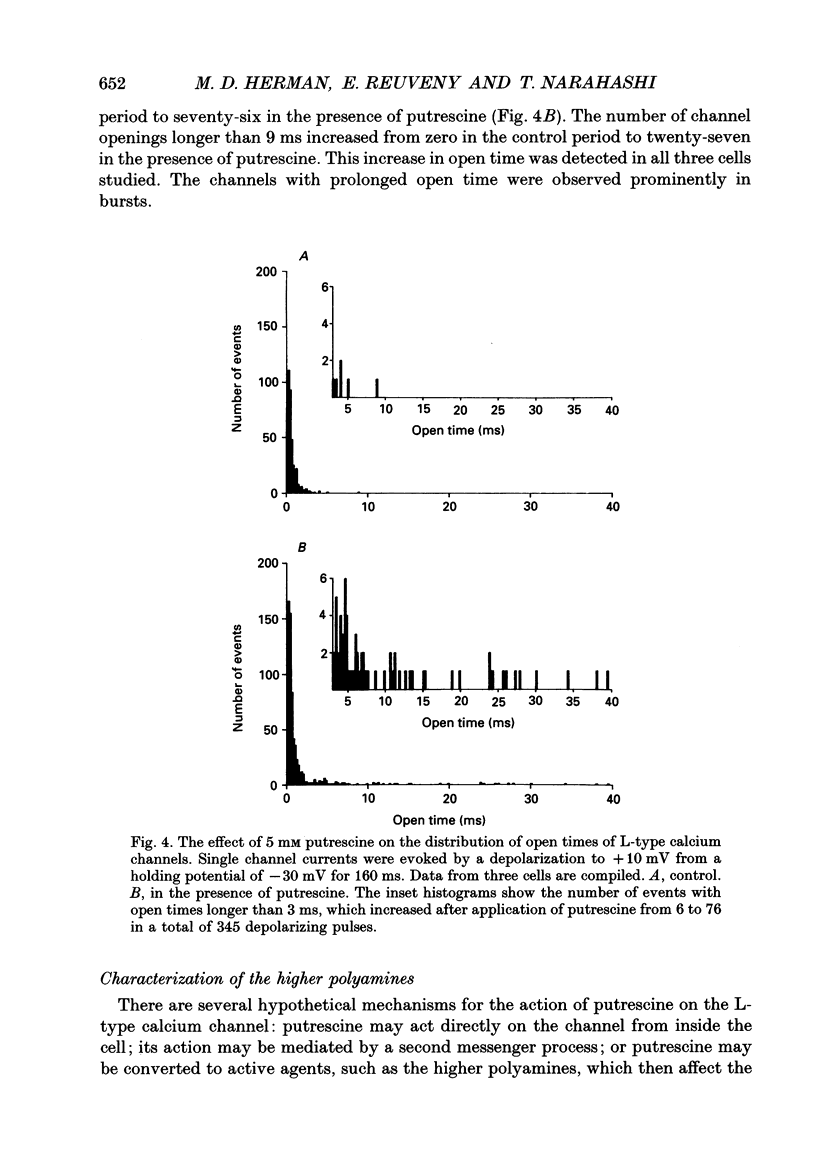






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy S. C., Walker C. H. Polyamines contribute to calcium-stimulated release of aspartate from brain particulate fractions. Brain Res. 1986 Apr 16;371(1):96–100. doi: 10.1016/0006-8993(86)90814-0. [DOI] [PubMed] [Google Scholar]
- Chan P. H., Fishman R. A. Transient formation of superoxide radicals in polyunsaturated fatty acid-induced brain swelling. J Neurochem. 1980 Oct;35(4):1004–1007. doi: 10.1111/j.1471-4159.1980.tb07100.x. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987 Feb;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande J. K., Siesjö B. K., Wieloch T. Calcium accumulation and neuronal damage in the rat hippocampus following cerebral ischemia. J Cereb Blood Flow Metab. 1987 Feb;7(1):89–95. doi: 10.1038/jcbfm.1987.13. [DOI] [PubMed] [Google Scholar]
- Frydman B., Frydman R. B., De los Santos C., Garrido D. A., Goldemberg S. H., Algranati I. D. Putrescine distribution in Escherichia coli studied in vivo by 13C nuclear magnetic resonance. Biochim Biophys Acta. 1984 Dec 11;805(4):337–344. doi: 10.1016/0167-4889(84)90016-8. [DOI] [PubMed] [Google Scholar]
- Gilad G. M., Gilad V. H. Polyamine uptake, binding and release in rat brain. Eur J Pharmacol. 1991 Jan 25;193(1):41–46. doi: 10.1016/0014-2999(91)90198-y. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Iqbal Z., Koenig H. Polyamines appear to be second messengers in mediating Ca2+ fluxes and neurotransmitter release in potassium-depolarized synaptosomes. Biochem Biophys Res Commun. 1985 Dec 17;133(2):563–573. doi: 10.1016/0006-291x(85)90943-x. [DOI] [PubMed] [Google Scholar]
- Ishiura S. Calcium-dependent proteolysis in living cells. Life Sci. 1981 Sep 14;29(11):1079–1087. doi: 10.1016/0024-3205(81)90194-6. [DOI] [PubMed] [Google Scholar]
- Kase H., Iwahashi K., Nakanishi S., Matsuda Y., Yamada K., Takahashi M., Murakata C., Sato A., Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987 Jan 30;142(2):436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Kimhi Y., Palfrey C., Spector I., Barak Y., Littauer U. Z. Maturation of neuroblastoma cells in the presence of dimethylsulfoxide. Proc Natl Acad Sci U S A. 1976 Feb;73(2):462–466. doi: 10.1073/pnas.73.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D. J., Routtenberg A. cis-Fatty acids, which activate protein kinase C, attenuate Na+ and Ca2+ currents in mouse neuroblastoma cells. J Physiol. 1989 Dec;419:95–119. doi: 10.1113/jphysiol.1989.sp017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk J. F., Bennett M. V., Zukin R. S. Polyamines potentiate responses of N-methyl-D-aspartate receptors expressed in xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9971–9974. doi: 10.1073/pnas.87.24.9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. Ionic currents in cultured mouse neuroblastoma cells under voltage-clamp conditions. J Physiol. 1978 May;278:265–286. doi: 10.1113/jphysiol.1978.sp012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruzzi M., Barbiroli B., Monti M. G., Tadolini B., Hakim G., Mezzetti G. Inhibitory action of polyamines on protein kinase C association to membranes. Biochem J. 1987 Oct 1;247(1):175–180. doi: 10.1042/bj2470175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W., Hallmayer J., Röhn G. Relationship between putrescine content and density of ischemic cell damage in the brain of mongolian gerbils: effect of nimodipine and barbiturate. Acta Neuropathol. 1988;76(4):388–394. doi: 10.1007/BF00686976. [DOI] [PubMed] [Google Scholar]
- Paschen W., Schmidt-Kastner R., Hallmayer J., Djuricic B. Polyamines in cerebral ischemia. Neurochem Pathol. 1988 Jul-Dec;9:1–20. doi: 10.1007/BF03160353. [DOI] [PubMed] [Google Scholar]
- Pullan L. M., Keith R. A., LaMonte D., Stumpo R. J., Salama A. I. The polyamine spermine affects omega-conotoxin binding and function at N-type voltage-sensitive calcium channels. J Auton Pharmacol. 1990 Aug;10(4):213–219. doi: 10.1111/j.1474-8673.1990.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Qi D. F., Schatzman R. C., Mazzei G. J., Turner R. S., Raynor R. L., Liao S., Kuo J. F. Polyamines inhibit phospholipid-sensitive and calmodulin-sensitive Ca2+-dependent protein kinases. Biochem J. 1983 Aug 1;213(2):281–288. doi: 10.1042/bj2130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehncrona S., Westerberg E., Akesson B., Siesjö B. K. Brain cortical fatty acids and phospholipids during and following complete and severe incomplete ischemia. J Neurochem. 1982 Jan;38(1):84–93. doi: 10.1111/j.1471-4159.1982.tb10857.x. [DOI] [PubMed] [Google Scholar]
- Röhn G., Kocher M., Oschlies U., Hossmann K. A., Paschen W. Putrescine content and structural defects in isolated fractions of rat brain after reversible cerebral ischemia. Exp Neurol. 1990 Mar;107(3):249–255. doi: 10.1016/0014-4886(90)90142-f. [DOI] [PubMed] [Google Scholar]
- Rüegg U. T., Burgess G. M. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989 Jun;10(6):218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- Schoemaker H. Polyamines allosterically modulate [3H]nitrendipine binding to the voltage-sensitive calcium channel in rat brain. Eur J Pharmacol. 1992 Feb 13;225(2):167–169. doi: 10.1016/0922-4106(92)90097-f. [DOI] [PubMed] [Google Scholar]
- Siman R., Noszek J. C. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988 Jun;1(4):279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Thams P., Capito K., Hedeskov C. J. An inhibitory role for polyamines in protein kinase C activation and insulin secretion in mouse pancreatic islets. Biochem J. 1986 Jul 1;237(1):131–138. doi: 10.1042/bj2370131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twombly D. A., Herman M. D., Kye C. H., Narahashi T. Ethanol effects on two types of voltage-activated calcium channels. J Pharmacol Exp Ther. 1990 Sep;254(3):1029–1037. [PubMed] [Google Scholar]


