Abstract
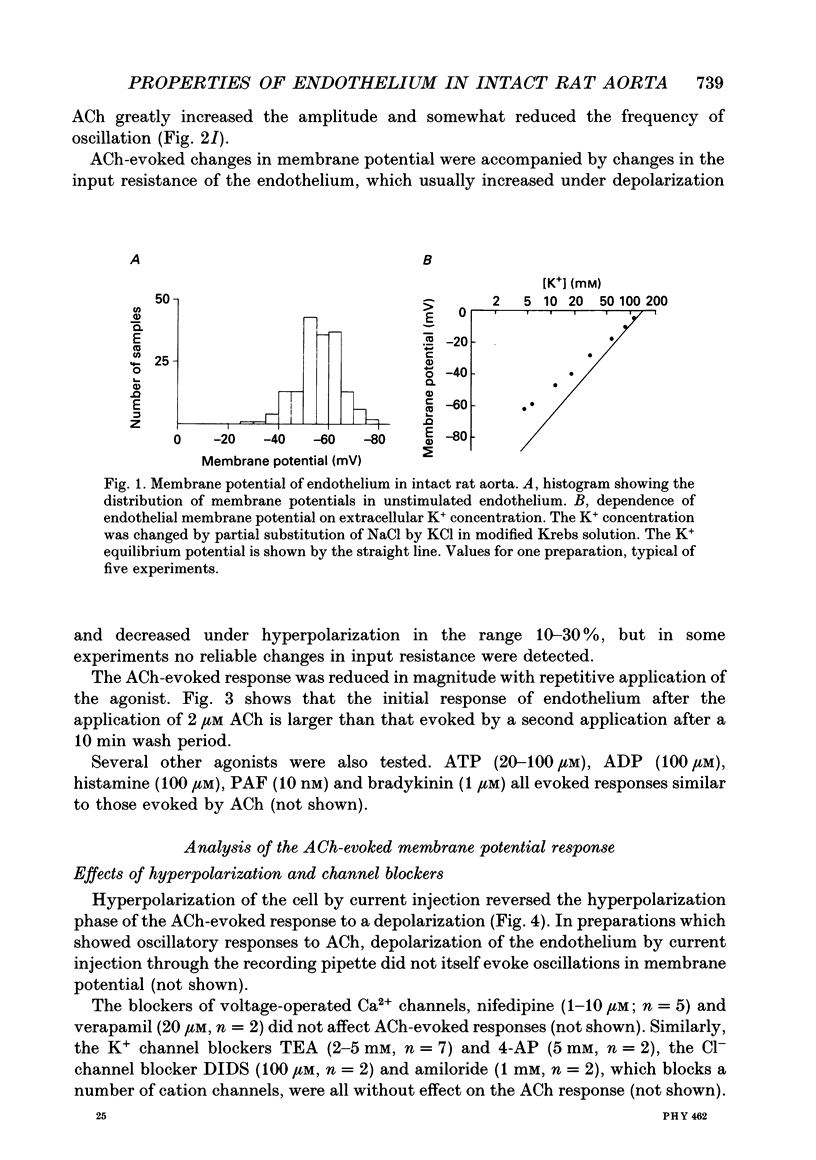
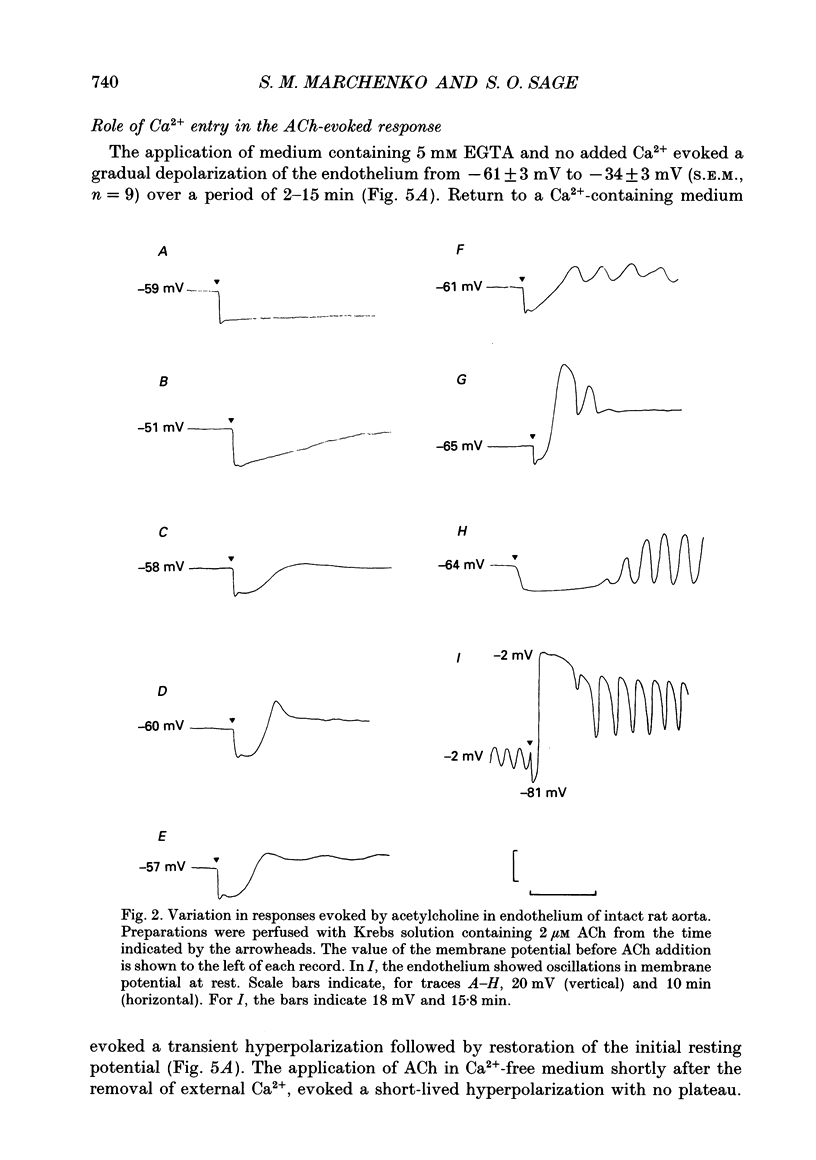
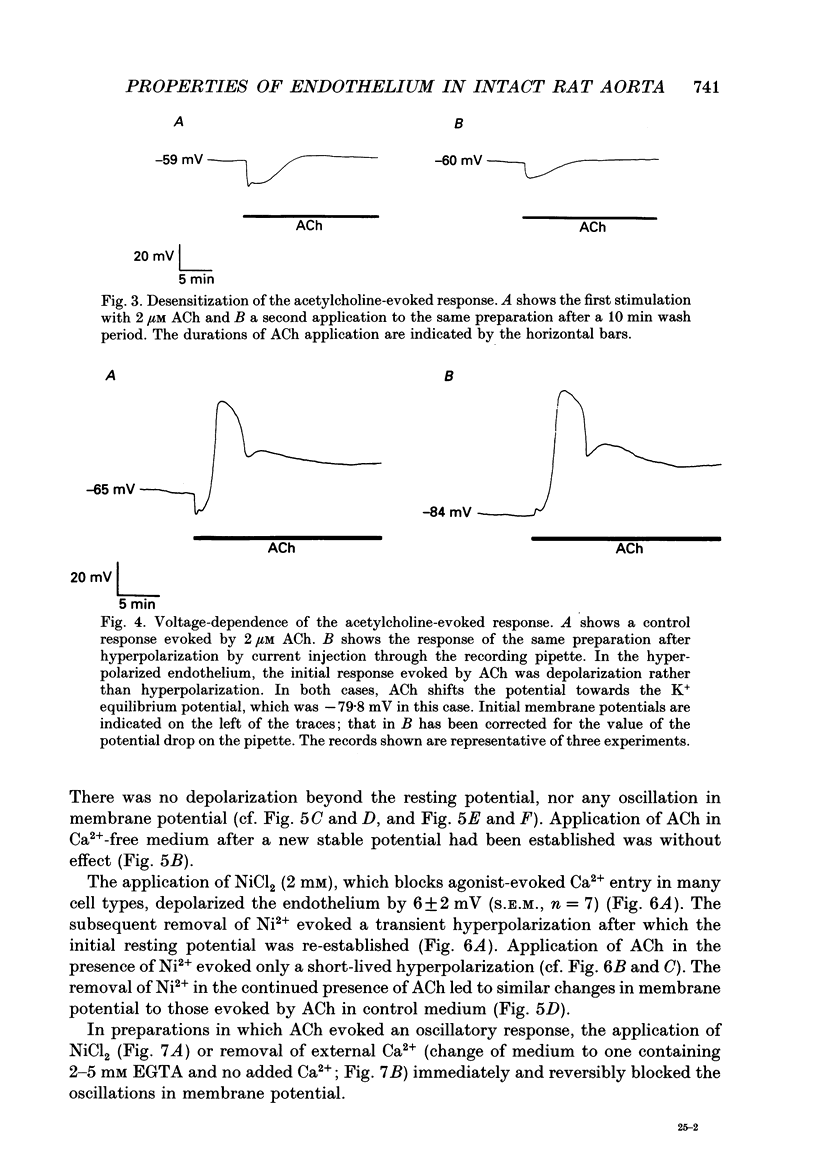
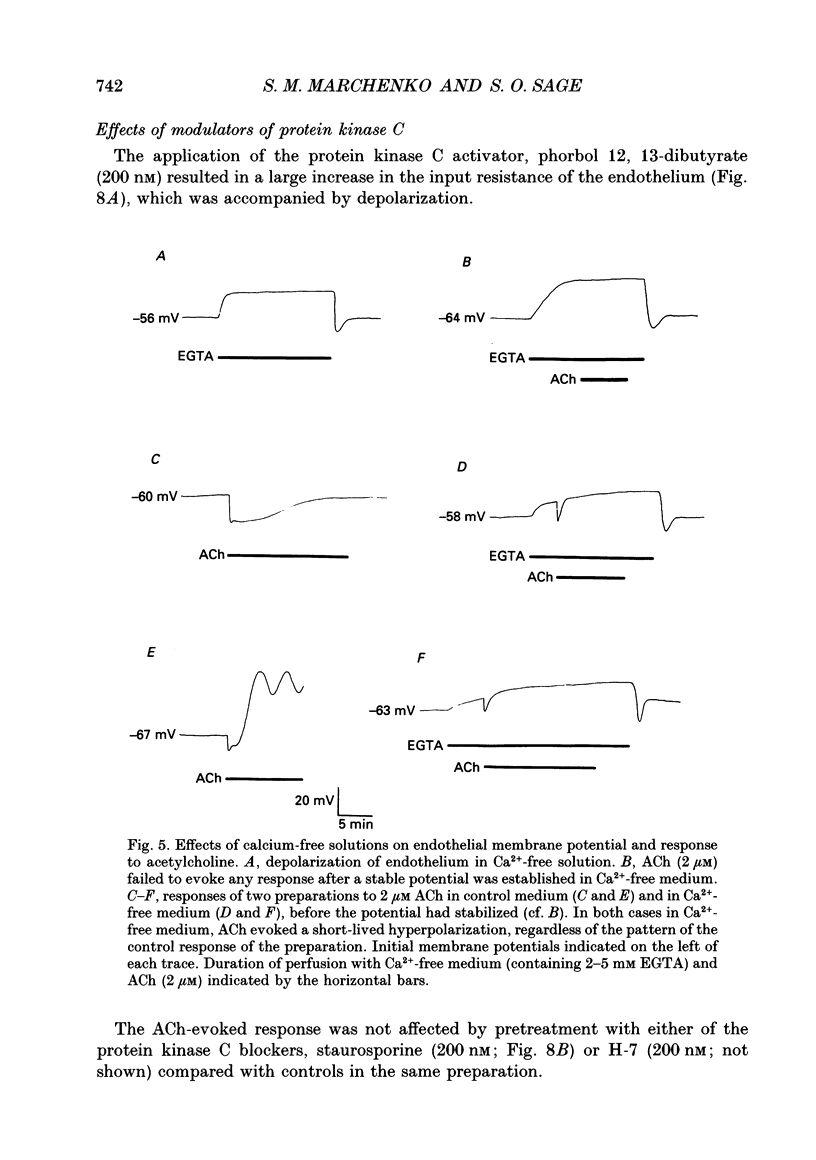
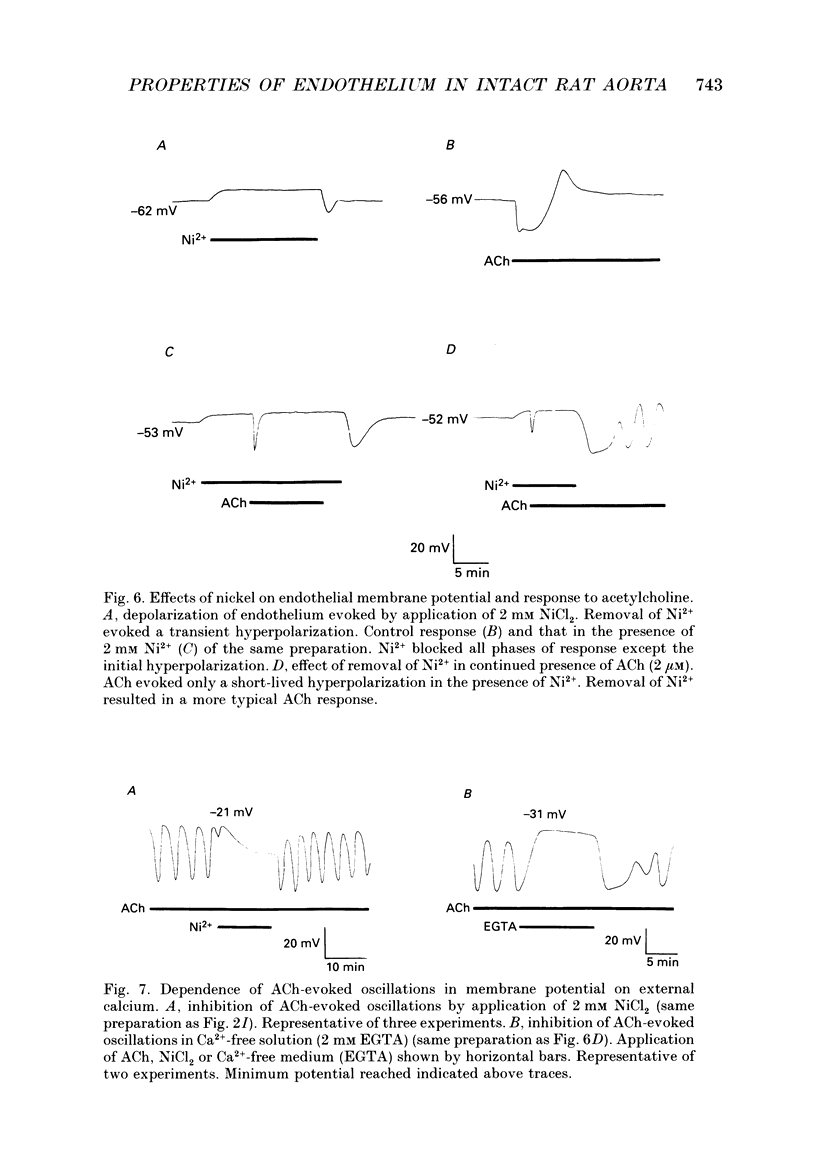
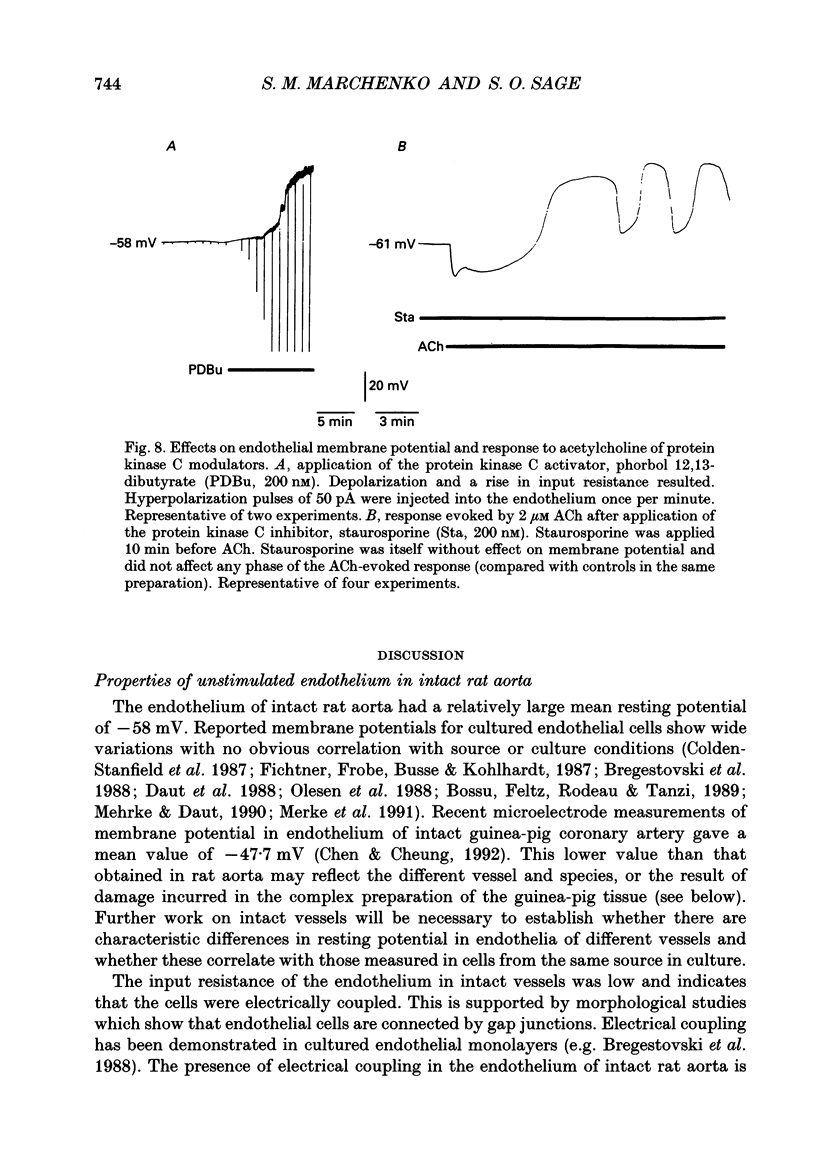
1. The passive electrical properties and the effects of acetylcholine on the membrane potential of the endothelium of intact rat aorta were investigated using the whole cell mode of the patch clamp technique. 2. Unstimulated endothelium had a membrane potential of -58 +/- 8 mV (S.E.M., n = 193; range -47 to -76 mV). The input resistance was 43 +/- 13 M omega (S.E.M., n = 8; range 26-64 M omega). KCl and BaCl2, but not tetraethylammonium (2 mM), 4-aminopyridine (5 mM) or 4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid (DIDS; 100 microM) depolarized the endothelium. 3. Acetylcholine (0.2-4 microM) evoked in most preparations a biphasic response with a transient hyperpolarization to a value close to the K+ reversal potential, followed by depolarization beyond the resting potential. In 46% of recordings, the depolarization was followed by oscillations in membrane potential. The duration of the hyperpolarization and magnitude of the depolarization was similar in all recordings from a given aorta, but varied greatly between different preparations. 4. Hyperpolarization of the endothelium below the K+ reversal potential reversed the direction of the first phase of the acetylcholine-evoked response, which was unaffected by tetraethylammonium, 4-aminopyridine, or DIDS. 5. The removal of extracellular Ca2+ evoked a depolarization of the endothelium from -61 +/- 3 to -34 +/- 3 mV (S.E.M., n = 9) over 2-15 min. Restoration of external Ca2+ evoked a transient hyperpolarization. 6. ACh applied in nominally Ca(2+)-free medium shortly after Ca2+ removal evoked only a transient hyperpolarization. After the establishment of a stable membrane potential in Ca(2+)-free medium, acetylcholine was without effect. 7. NiCl2 (2 mM) evoked a small depolarization of the endothelium (6 +/- 2 mV; S.E.M., n = 7). The subsequent removal of Ni2+ evoked a transient hyperpolarization. 8. In the presence of Ni2+, acetylcholine evoked a short-lived hyperpolarization. Both the application of Ni2+ and the removal of extracellular Ca2+ immediately blocked oscillations in membrane potential evoked by acetylcholine. 9. The blockers of voltage-operated Ca2+ channels, nifedipine (1-10 microM) and verapamil (20 microM) were without effect on the biphasic acetylcholine-evoked responses. 10. In preparations in which acetylcholine evoked large (20-45 mV) oscillations in membrane potential, depolarization of the endothelium alone, by current injection or application of KCl, did not evoke oscillations. 11. The activator of protein kinase C, phorbol 12, 13-dibutyrate (200 nM) depolarized and greatly increased the input resistance of the endothelium, presumably due to an effect on gap junctions.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bossu J. L., Feltz A., Rodeau J. L., Tanzi F. Voltage-dependent transient calcium currents in freshly dissociated capillary endothelial cells. FEBS Lett. 1989 Sep 25;255(2):377–380. doi: 10.1016/0014-5793(89)81126-3. [DOI] [PubMed] [Google Scholar]
- Bregestovski P., Bakhramov A., Danilov S., Moldobaeva A., Takeda K. Histamine-induced inward currents in cultured endothelial cells from human umbilical vein. Br J Pharmacol. 1988 Oct;95(2):429–436. doi: 10.1111/j.1476-5381.1988.tb11663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregestovski P., Redkozubov A., Alexeev A. Elevation of intracellular calcium reduces voltage-dependent potassium conductance in human T cells. 1986 Feb 27-Mar 5Nature. 319(6056):776–778. doi: 10.1038/319776a0. [DOI] [PubMed] [Google Scholar]
- Busse R., Fichtner H., Lückhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Sage S. O. Bradykinin-evoked changes in cytosolic calcium and membrane currents in cultured bovine pulmonary artery endothelial cells. J Physiol. 1989 Dec;419:555–568. doi: 10.1113/jphysiol.1989.sp017886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. F., Cheung D. W. Characterization of acetylcholine-induced membrane hyperpolarization in endothelial cells. Circ Res. 1992 Feb;70(2):257–263. doi: 10.1161/01.res.70.2.257. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Ritchie A. K., Eskin S. G., Navarro L. T., Kunze D. L. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res. 1987 Nov;61(5):632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- Daut J., Mehrke G., Nees S., Newman W. H. Passive electrical properties and electrogenic sodium transport of cultured guinea-pig coronary endothelial cells. J Physiol. 1988 Aug;402:237–254. doi: 10.1113/jphysiol.1988.sp017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner H., Fröbe U., Busse R., Kohlhardt M. Single nonselective cation channels and Ca2+-activated K+ channels in aortic endothelial cells. J Membr Biol. 1987;98(2):125–133. doi: 10.1007/BF01872125. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gillespie J. I., Greenwell J. R., Johnson C. Agonist-induced fluctuations in cytoplasmic calcium in primary cultures of bovine endothelial cells. Exp Physiol. 1991 Sep;76(5):667–676. doi: 10.1113/expphysiol.1991.sp003534. [DOI] [PubMed] [Google Scholar]
- Gillespie J. I., Johnson C., Nicholls J., Lynch M., Greenwell J. R. Is there a calcium paradox in isolated bovine aortic endothelial cells? Exp Physiol. 1992 Mar;77(2):393–396. doi: 10.1113/expphysiol.1992.sp003601. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Influx of bivalent cations can be independent of receptor stimulation in human endothelial cells. Biochem J. 1989 Apr 1;259(1):125–129. doi: 10.1042/bj2590125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Buckley N. J. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Johns A., Lategan T. W., Lodge N. J., Ryan U. S., Van Breemen C., Adams D. J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., Cannell M., van Breemen C. Calcium entry-dependent oscillations of cytoplasmic calcium concentration in cultured endothelial cell monolayers. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1690–1694. doi: 10.1073/pnas.89.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Loomis J. L., Segal S. S. Preservation of endothelial cells in excised rat carotid arteries. Effects of transmural pressure and segment length. Circ Res. 1991 Oct;69(4):997–1002. doi: 10.1161/01.res.69.4.997. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Mehrke G., Daut J. The electrical response of cultured guinea-pig coronary endothelial cells to endothelium-dependent vasodilators. J Physiol. 1990 Nov;430:251–272. doi: 10.1113/jphysiol.1990.sp018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrke G., Pohl U., Daut J. Effects of vasoactive agonists on the membrane potential of cultured bovine aortic and guinea-pig coronary endothelium. J Physiol. 1991 Aug;439:277–299. doi: 10.1113/jphysiol.1991.sp018667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylon C. B., Irvine R. F. Synchronized repetitive spikes in cytoplasmic calcium in confluent monolayers of human umbilical vein endothelial cells. FEBS Lett. 1990 Nov 26;275(1-2):173–176. doi: 10.1016/0014-5793(90)81465-z. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Davies P. F., Clapham D. E. Muscarinic-activated K+ current in bovine aortic endothelial cells. Circ Res. 1988 Jun;62(6):1059–1064. doi: 10.1161/01.res.62.6.1059. [DOI] [PubMed] [Google Scholar]
- Peach M. J., Singer H. A., Loeb A. L. Mechanisms of endothelium-dependent vascular smooth muscle relaxation. Biochem Pharmacol. 1985 Jun 1;34(11):1867–1874. doi: 10.1016/0006-2952(85)90300-4. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Rhodin J. A. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967 Apr;18(1):181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Adams D. J., van Breemen C. Synchronized oscillations in cytoplasmic free calcium concentration in confluent bradykinin-stimulated bovine pulmonary artery endothelial cell monolayers. J Biol Chem. 1989 Jan 5;264(1):6–9. [PubMed] [Google Scholar]
- Sage S. O., van Breemen C., Cannell M. B. Sodium-calcium exchange in cultured bovine pulmonary artery endothelial cells. J Physiol. 1991;440:569–580. doi: 10.1113/jphysiol.1991.sp018725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve R., Parent L., Simoneau C., Roy G. External ATP triggers a biphasic activation process of a calcium-dependent K+ channel in cultured bovine aortic endothelial cells. Pflugers Arch. 1988 Oct;412(5):469–481. doi: 10.1007/BF00582535. [DOI] [PubMed] [Google Scholar]
- Spagnoli L. G., Villaschi S., Neri L., Palmieri G. Gap junctions in myo-endothelial bridges of rabbit carotid arteries. Experientia. 1982 Jan 15;38(1):124–125. doi: 10.1007/BF01944566. [DOI] [PubMed] [Google Scholar]
- Tracey W. R., Peach M. J. Differential muscarinic receptor mRNA expression by freshly isolated and cultured bovine aortic endothelial cells. Circ Res. 1992 Feb;70(2):234–240. doi: 10.1161/01.res.70.2.234. [DOI] [PubMed] [Google Scholar]
- Yotti L. P., Chang C. C., Trosko J. E. Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter. Science. 1979 Nov 30;206(4422):1089–1091. doi: 10.1126/science.493994. [DOI] [PubMed] [Google Scholar]


