Abstract
The large GTPase dynamin (Dyn2) has been demonstrated by us and others to interact with several different actin-binding proteins. To define how Dyn2 might participate in actin dynamics in livings cells we have expressed green fluorescent protein (GFP)-tagged Dyn2 in cultured cells and observed labeling of comet-like vesicles and macropinosomes. The comet structures progressed with a constant velocity and were reminiscent of actin comets associated with motile vesicles in cells expressing type I phosphatidylinositol phosphate 5-kinases. Based on these observations we sought to determine whether Dyn2 is an integral component of actin comets. Cells expressing type I phosphatidylinositol phosphate 5-kinase and Dyn2-GFP revealed a prominent colocalization of Dyn2 and actin in comet structures. Interestingly, comet formation and motility were normal in cells expressing wild-type Dyn2-GFP but altered markedly in Dyn2 mutant-expressing cells. Dyn2K44A-GFP mutant cells displayed a significant reduction in comet number, length, velocity, and efficiency of movement. In contrast, comets in cells expressing Dyn2ΔPRD-GFP appeared dark and did not incorporate the mutant Dyn2 protein, indicating that the proline-rich domain (PRD) is required for Dyn2 recruitment. Further, these comets were significantly longer and slower than those in control cells. These findings demonstrate a role for Dyn2 in actin-based vesicle motility.
Keywords: vesicle motility‖PIP5KI‖membrane trafficking
Dynamin is a 100-kDa large GTPase that functions to tubulate membranes and liberate nascent vesicles from the Golgi apparatus and plasma membrane (1, 2). Dynamin 2 (Dyn2) has been shown, using immunocytochemical and biochemical methods, to localize to clathrin-coated pits, the Golgi apparatus, and cortical actin ruffles (3–5). It has been reported that dynamin directly interacts with actin-associated proteins including the Wisckott-Aldrich Syndrome protein (N-WASP) binding partner syndapin and the actin-binding proteins profilin and actin-binding protein-1 (6–8). It is proposed that the actin cytoskeleton functions synergistically with other proteins, including dynamin, to coordinate vesicle formation and motility (8–10). Recently we showed that Dyn2 directly binds to and functions with the F-actin binding protein cortactin to regulate actin assembly in epithelial cells (5). This direct interaction is mediated by the proline-rich domain (PRD) and Src homology three domain of Dyn2 and cortactin, respectively. Interestingly, cortactin was shown to localize to the tails of actin-based motile vesicles (comets), induced by the activated form of the small GTPase Arf6, and to the tails of motile endosomal vesicles (11, 12). Actin comets also can be induced by expressing type I phosphatidylinositol phosphate 5-kinase α (PIP5KIα), resulting in the localized synthesis and accumulation of phosphatidylinositol 4,5 bisphosphate (13–15). The accumulation of phosphatidylinositol 4,5 bisphosphate, together with N-WASP and the Arp2/3 complex, induces de novo nucleation of actin-based comets that function to propel vesicles from donor compartments through the cytoplasm (16, 17). These vesicles form predominantly from the Golgi apparatus and plasma membrane (15), both locations at which Dyn2 mediates vesicle fission. To define how Dyn2 might regulate actin dynamics in living cells, we used green fluorescent protein (GFP)-tagged Dyn2 (Dyn2-GFP). Surprisingly, we observed labeling of comet-like vesicles in cultured rat hepatocytes (Clone 9). These structures contained a brightly stained dynamin head followed by a tail that resembled the actin comet tails of motile vesicles in PIP5KI-expressing cells and intracellular pathogens such as Listeria monocytogenes (15, 18). In addition, we also observed bright Dyn2-GFP puncta localized to the surface of macropinosomes formed from peripheral membrane ruffles of NIH 3T3 cells. To test whether Dyn2 plays a role in actin-based vesicular trafficking, we used immunocytochemistry, live cell imaging of Dyn2-GFP, expression of dominant negative Dyn2 proteins, and particle-tracking analysis. We find Dyn2 to be an integral component of actin comets associated with macropinosomes and show that the expression of mutant Dyn2 proteins results in decreased comet formation, defects in comet tail length, reduced comet velocity, and irregular comet movements. These observations implicate Dyn2 in mediating comet-based transport of macropinosomes in living cells.
Materials and Methods
Plasmid Constructs.
The Myc-tagged PIP5KIα expression construct was from L. M. Machesky. Mouse β-actin-GFP in CLONTECH pEGFP-C2 was from G. Marriot. Full-length Dyn2aa, Dyn2aaK44A, and Dyn2aaΔPRD were subcloned into pEGFP-N1 (CLONTECH; refs. 5 and 19). Dyn2aa was used for all experiments.
Plasmid Transfection and Microinjection.
All plasmids were transfected by using the GeneJammer transfection reagent (Stratagene). Transfection conditions were according to the manufacturer. Transfected cells were grown for 16–24 h before experimentation (6). Rat fibroblast cells were microinjected with PIP5KIα and β-actin-GFP plasmid DNA at 1 μg/ml each in microinjection buffer (10 mM KH2PO4, pH 7.2/75 mM KCl/400 μM Texas red-dextran) (20). The cells were allowed to recover 8–10 h before live-time confocal imaging.
Immunofluorescence Localization.
Rat fibroblasts or rat hepatocytes (Clone 9) were grown and prepared for indirect immunocytochemistry as described (4). Affinity-purified anti-Dyn2 polyclonal antibody, αDyn2, and anti-cortactin polyclonal antibody were used as described (5). The monoclonal antibody to myc (9E10) was used according to the manufacturer's recommendations (Zymed). Secondary antibodies were from Molecular Probes. To visualize F-actin, 80 nM rhodamine-phalloidin (Sigma) was included in the secondary antibody incubation. Digital images were acquired as described (5).
Live-Time Fluorescence Video Microscopy.
For conventional live-time microscopy, cells were transfected or microinjected into 35-mm imaging dishes. A Zeiss Axiovert 35 microscope equipped with a 37°C heated stage and Orca II charge-coupled device camera (Hamamatsu Photonics, Hamamatsu City, Japan) was used for imaging. Images were captured every 5 s. Live-time confocal imaging was performed by using a Zeiss LSM510 confocal microscope equipped with a heated stage.
Quantitation of Comet Formation, Tail Length, Velocity, and Movement.
Rat fibroblasts were used for quantitation. The cells were processed for immunocytochemistry and costained with anti-Myc (PIP5KIα), anti-dynamin, and rhodamine-phalloidin. The mean percentage of PIP5KIα-expressing cells that formed comets was determined by visual inspection in the actin channel. At least 100 cells were counted in each experiment. The mean number of comets per PIP5KIα-positive cell was determined by visual inspection from at least 70 cells in each case. To calculate comet velocity the distance traveled over 1.0 min was measured and divided by the elapsed-time (IPLab, Scanalytics, Fairfax, VA). The mean velocity was solved for at least 12 separate comets in each condition. To quantitate comet movement characteristics, 100 frames from GFP time-lapse videos were stacked, and a circle with a radius of 5.0 μm was centered at the comets' points of origin. The distance traveled to traverse the 5.0-μm radius was determined for at least 12 comets for each condition. To obtain the movement index (MI), 5.0 μm (the radius) was divided by the average distance traveled; this is a fraction of linearity with am MI = 1.0, equal to perfectly linear and smaller values, indicating less linear movement.
Results
Dynamin Is an Integral Component of Actin Comets.
Motile comet structures were observed initially while live-time imaging rat hepatocytes (Clone 9) expressing Dyn2-GFP (Fig. 1). These Dyn2 comets appeared as bright puncta with only localized movements (Movie 1, which is published as supporting information on the PNAS web site, www.pnas.org). Unexpectedly, the structures became rapidly motile and were comprised of a bright head followed by a tail structure (Fig. 1 b–e and Movie 1). As revealed by the projected time-lapse video, the comet structure progressed through the cytoplasm with a uniform velocity of 0.17 μm/s (Fig. 1f and Movie 1). Uniform velocity is characteristic of actin comets (15, 21). Additionally, video microscopy of Clone 9 and NIH 3T3 cells expressing Dyn2-GFP showed specific, punctate labeling of large, dark, vesicular structures that formed from peripheral membrane ruffles and progressed rapidly toward the cell center (Fig. 1 g–k and Movie 2, which is published as supporting information on the PNAS web site). These structures were consistent with the formation of macropinosomes, which form from zones of actin-dependent membrane ruffling at the cell periphery. As for the comet structures, the Dyn2 labeling was confined to the side of the macropinosome opposite from the direction of movement and formed short tails (Fig. 1 g–k and Movie 2). These structures appeared identical to the motile endosomes observed associated with the Dyn2 binding partner cortactin (12). Because these comet structures were rare, transient, and difficult to study, cells were transfected with Myc-PIP5KIα together with wild-type Dyn2-GFP and viewed by immunocytochemistry. Both Dyn2-GFP and anti-Dyn2 antibodies showed strong staining of all actin comets as confirmed by costaining with either cortactin or actin (Fig. 2). To observe colocalization of dynamin and actin comets in living cells we used dual-color live-time imaging of rat fibroblasts coexpressing PIP5KIα, Dyn2-GFP, and red fluorescent protein-tagged cortactin. It is clear that both Dyn2 and cortactin localize to actin comets in these cells (data not shown). These experiments together with live-time imaging of Dyn2-GFP (Movie 3) confirmed our original observations and showed clearly that Dyn2 is an integral component of comets and is actively incorporated into the comet head and tail as they are formed (Fig. 2 and Movie 3).
Figure 1.
Dyn2-GFP incorporates into endogenous comet-like vesicles and macropinosomes in living cells. (a) Confocal time-lapse imaging of Clone 9 hepatocytes expressing Dyn2-GFP. Dynamic comet-like structures were observed. N, nucleus. (b–e) Higher magnification revealed that these motile structures consisted of a brightly stained head followed by a tail structure (arrows) that extends 180° from the direction of movement. (f) The velocity of the movement was uniform as revealed by the consistent distances between projected frames of the time-lapse images (arrows). (g–k) Confocal time-lapse imaging of Dyn2-GFP in NIH 3T3 fibroblasts revealed large vesicular structures associated with small tails of Dyn2-GFP (arrows and boxes). (h′ and k′) High magnifications of individual macropinosomes from frames h and k, respectively. Consistent with the formation of macropinosomes, these structures formed from zones of active membrane ruffling at the cell periphery. (Bar, 10 μm.) See Movies 1 and 2, which are published as supporting information on the PNAS web site, www.pnas.org.
Figure 2.
Dyn2 is an integral component of actin comets. Cultured rat fibroblasts and Clone 9 hepatocytes showing dynamin incorporated into PIP5KIα-induced actin comets. (a and a′) Comets in a rat fibroblast expressing Dyn2aa-GFP and colabeled with cortactin. The Dyn2 labeling is brightest at the head of the comet (arrows) but is prominent also in the tail (Inset, arrows). In contrast, cortactin staining is localized predominantly to the comet tail. RF, rat fibroblast; αCortY, anti-cortactin. (b and b′) Comets in a Clone 9 hepatocyte immunolabeled for dynamin (αDyn2) and actin (rhodamine-phalloidin). Note the Dyn2 enrichment in the head and staining in the tails (arrows and Inset), whereas actin stains the tail only. (a: bar, 3 μm; b: bar, 2 μm.)
Dyn2 Mutants Induce Distinct Defects in Comet Formation and Tail Length.
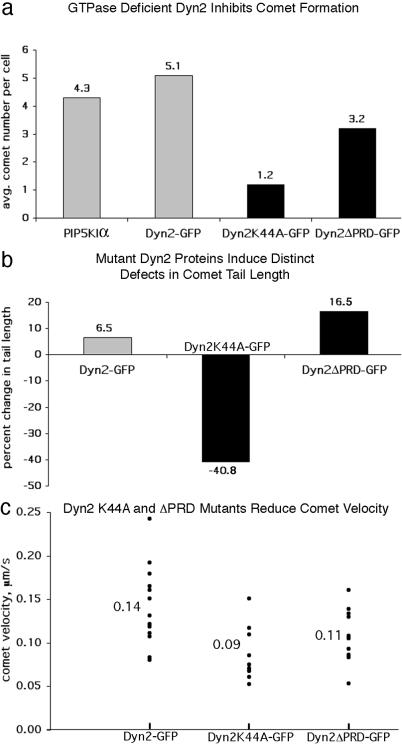
To test for a functional role of Dyn2 in actin comets, rat fibroblasts were transfected with PIP5KIα alone, PIP5KIα, and wild-type Dyn2-GFP or PIP5KIα and either of two distinct dominant negative Dyn2 mutants. The Dyn2K44A point mutant used in this study is GTPase-deficient and has been used to inhibit Dyn2 function in a variety of cells (20, 22). The second mutant used is a deletion of the last 124 amino acids comprising the PRD (Dyn2ΔPRD), which is known to interact directly with cortactin's Src homology three domain (5). This mutant has a diffuse, cytoplasmic distribution and results in an elongated cellular phenotype with increased actin stress fibers, suggesting an alteration in actin dynamics (5). Mutant Dyn2-expressing rat fibroblasts were fixed and stained for PIP5KIα (α-Myc) and actin, and the mean number of comets present per cell was scored. Cells expressing PIP5KIα alone and PIP5KIα with wild-type Dyn2 or Dyn2ΔPRD-GFP formed on average 4.2, 5.1, and 3.2 comets, respectively (Fig. 3a). In contrast, cells expressing the Dyn2K44A mutant displayed a 72.1% reduction in comet formation, with an average of only 1.2 comets per cell (Fig. 3a).
Figure 3.
Dyn2 regulates comet formation, actin tail length, and comet velocity. For quantitation of comet formation and actin tail length, rat fibroblasts coexpressing Myc-PIP5KIα and wild-type or mutant Dyn2-GFP were fixed and stained for actin (rhodamine-phalloidin). (a) Cells expressing the K44A mutant show on average only 1.2 comets per cell, a 72.1% reduction compared with cells expressing PIP5KIα alone (4.3), and Dyn2ΔPRD expression shows a reduction in comet formation of 25.6% (3.2 comets) compared with PIP5Kα control. (b) The average comet tail length in cells expressing PIP5KIα and Dyn2 was only 6.5% longer than PIP5KIα control (5.7 μm), whereas those in cells expressing Dyn2K44A were 40.8% shorter (3.2 μm). In contrast, comet tails in Dyn2ΔPRD-expressing cells were 16.5% longer (6.2 μm) than control. (c) Particle-tracking analysis showed that Dyn2K44A-GFP and Dyn2ΔPRD-GFP comets progressed with reduced velocities of 0.09 and 0.11 μm/s, respectively, whereas wild-type Dyn2-GFP comets progressed at 0.14 μm/s. See Movies 3–5 (which are published as supporting information on the PNAS web site) for comparison of the Dyn2 comets.
Although both the Dyn2ΔPRD- and Dyn2K44A-expressing cells formed fewer comets, these organelles in the Dyn2K44A cells also appeared short and curled. Measurement of actin tails in K44A mutant cells revealed a 40.8% reduction in tail length (Fig. 3b), with the average comet being only 3.2 μm (data not shown) compared with 5.4 μm for control cells. In contrast, the actin tails in cells expressing Dyn2ΔPRD averaged 16.5% longer than control (Fig. 3b), with an average of 6.2 μm. Furthermore, only 9.9% of Dyn2K44A actin comets were greater than 5.0 μm, compared with 42.2% of wild type (data not shown). Expression of wild-type Dyn2 with PIP5KIα resulted in no significant change in comet formation or tail length (Fig. 3 a and b). In contrast to wild type (42.2%), 53.3% of actin tails in Dyn2ΔPRD cells were longer than 5.0 μm, supporting the observation that expression of Dyn2ΔPRD results in increased comet tail length (Fig. 3b). Appropriately, comets with the longest tails were in Dyn2ΔPRD-expressing cells, for example, 21.6, 18.3, and 15.7 μm, far exceeding the average of 5.4 μm for control. It should be noted that expression of wild-type Dyn2 showed no significant effect on comet tail length (Fig. 3b). The analysis presented here clearly demonstrates that expression of Dyn2K44A and Dyn2ΔPRD affected both comet formation and elongation of the actin tail, suggesting that dynamin is involved in actin filament turnover.
Dynamin Mutants Induce Defects in Comet Velocity and Movement.
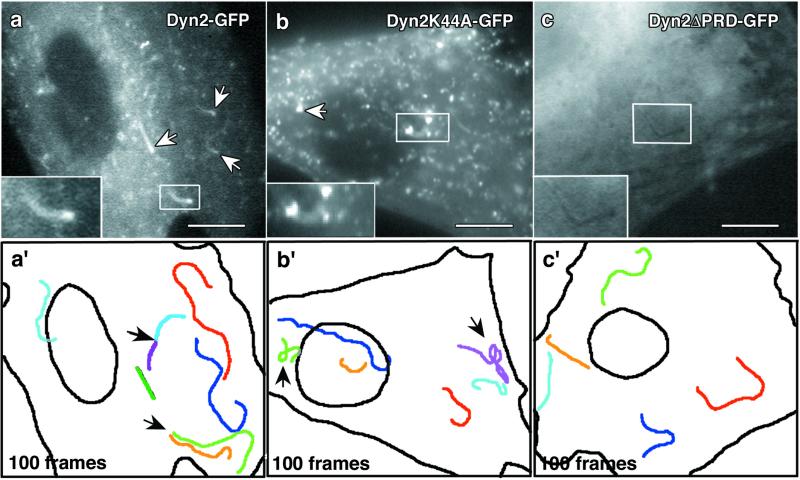
Because of the actin tail defects in cells expressing mutant Dyn2 and the fact that actin turnover in the tail is correlated directly to comet translocation (21, 23) we extended our study to include comet velocity and movement characteristics. Rat fibroblasts were transfected with PIP5KIα and wild-type Dyn2-GFP, Dyn2K44A-GFP, or Dyn2ΔPRD-GFP. Live-time video microscopy of expressing cells at 16–24 h posttransfection confirmed the analysis of fixed cells. Dyn2-GFP comets progressed with an average velocity of 0.14 μm/s (Fig. 3c), which is consistent with the velocity for actin comets reported by others (15, 24). Often times, multiple comets appeared to form from a single foci; this was observed by using Dyn2-GFP and actin-GFP (Fig. 4a′, Movie 3, and data not shown). Conversely, cells expressing Dyn2K44A-GFP rarely contained comets, which was consistent with the data from fixed cells. Those K44A mutant cells that produced comets usually had only one or two comets. Further, these comets were unusually small and dramatically slower than Dyn2-GFP comets, with an average velocity of only 0.09 μm/s (Fig. 3c and Movie 3 versus 4). Interestingly, cells expressing Dyn2ΔPRD-GFP possessed long dark comets that failed to incorporate the mutant GFP-tagged protein (Fig. 4 c and c′ and Movie 5), indicating that the PRD is required for Dyn2 assembly or recruitment into the comet, perhaps via an interaction with the Src homology three domain of cortactin (9). Comets in Dyn2ΔPRD-expressing cells were also 21.4% slower than wild-type Dyn2 comets, with an average velocity of 0.11 μm/s (Fig. 3c).
Figure 4.
Mutant dynamin proteins induce abnormal comet movements. Rat fibroblasts coexpressing Myc-PIP5KIα and wild-type or mutant forms of Dyn2-GFP were imaged for 100 frames, and the movement of comet structures was followed. (a) Cells expressing wild-type Dyn2-GFP formed numerous comets (arrows and Inset) that actively incorporated the tagged Dyn2 (see Movie 3). (a′) One hundred frames from the time-lapse images were stacked to show the movement characteristics of individual comets. Each color represents a distinct comet path. Note the linear quality of comet movement. Arrows, multiple comets appeared to form from a single domain. (b) A K44A-GFP-expressing cell with comets (arrows). The comets are fewer and much smaller than those of wild type (see Movie 4). (b′) One hundred stacked frames from the K44A-GFP time-lapse. These comets often had curved and wandering paths (arrows) and were less efficient in their translocation. See the Dyn2K44a-GFP video (Movie 4, arrow on right) for an example of defective movement. (c) Comets in cells expressing the truncated Dyn2ΔPRD-GFP are dark and do not incorporate the mutant protein (see Movie 5). (c′) One hundred frames from the ΔPRD-GFP video revealed that these comets moved in a similar manner to those of wild-type Dyn2, with smooth curvilinear paths. (Bars, 10 μm.)
The Dyn2K44A-GFP comets also displayed distinct movement defects. To chart the movement characteristics of comets, 100 frames from each Dyn2-GFP time-lapse video were projected into a single image. By using this type of analysis, the movement of individual comets in cells expressing different Dyn2 mutants were compared directly. Comets in cells expressing Dyn2K44A-GFP, unlike the smooth curvilinear movements of those in control cells, often moved in a wandering or spiraling manner (Fig. 4 a′ and b′ and Movie 3 versus 4, arrows). The comets in Dyn2ΔPRD-GFP-expressing cells moved similarly to those in wild-type cells (Fig. 4 a′ and c′ and Movie 3 versus 5). To quantitate the movement defect of Dyn2K44A comets, we defined a circular region of interest in the cytoplasm with a radius of 5.0 μm around the point of origin of comets and measured the path length for each individual comet to traverse the 5.0-μm radius (Fig. 5 a and b). The MI was obtained by dividing the radius (5.0 μm) by the path length. The MI represents the movement linearity, with 1 equal to perfectly linear and smaller values being progressively less linear. A radius of 5.0 μm was selected based on the velocity of the K44A comets and the rationale that a comet with normal movement characteristics (even at a slow velocity) would progress beyond the radius easily when given enough time. Thus, comets with highly disrupted movement would remain in the defined region of interest. The MI of K44A comets was only 0.54, whereas comets in cells expressing actin-GFP, wild-type Dyn2-GFP, and Dyn2ΔPRD-GFP were nearly 1.0 (Fig. 5c). It was particularly significant that although the average velocity of the K44A and ΔPRD comets was similar, only the K44A comets had a decreased MI (Fig. 5c).
Figure 5.
GTPase-defective Dyn2 comets move with decreased efficiency. Dyn2K44A-GFP comets show a dramatically reduced MI compared with actin-GFP, Dyn2-GFP, and Dyn2ΔPRD-GFP comets. One hundred frames from GFP time-lapse videos were stacked and superimposed over a circle with an outer radius of 5.0 μm (red) to provide a point of origin of individual comets. The distance traveled (yellow) to completely traverse the 5.0-μm radius was determined for at least 12 comets. The ratio of 5.0 μm and the distance traveled is the MI. (a and c) Actin-GFP, Dyn2-GFP, and Dyn2ΔPRD-GFP comets moved nearly linearly (1.0). (b and c) Dyn2K44A-GFP comets have an MI of only 0.54, indicating their efficiency of movement was impaired severely (decreased linearity). Note that Dyn2ΔPRD-GFP comets also had linear movement despite having a reduced velocity as shown in Fig. 3c. (Bar, 5.0 μm.)
Many Dyn2-GFP Comets Are Associated with Macropinosomes Formed at the Cell's Ruffled Edge.
The origin of comet-associated vesicles is currently unclear. Past studies have suggested that the origin of many actin-based vesicles is the Golgi apparatus and peripheral plasma membrane (12, 15). Interestingly, we have observed Dyn2-GFP labeling of macropinosomes formed from peripheral membrane ruffles in quiescent cells (Fig. 1). Confocal imaging of rat fibroblasts coexpressing PIP5KIα and actin-GFP revealed macropinosomes that formed directly from the peripheral membrane ruffles (Fig. 6 and Movie 6, which is published as supporting information on the PNAS web site). Three criteria define these structures as macropinosomes: (i) they can be seen forming directly from membrane ruffles; (ii) immunocytochemistry experiments together with internalization of labeled dextran confirmed that Dyn2 and actin are components of macropinosomes in cells (data not shown); and (iii) previous studies have identified a substantial population of comets as positive for fluid-phase markers (15, 25). The large vesicle head and short actin tail are resolved easily on these structures (Fig. 6, arrows). Similar to the endogenous Dyn2-GFP macropinosomes observed in non-PIP5Kα-expressing cells (Fig. 1), the tails remained 180° opposite from the direction of movement. We also observed that the tails on the macropinosomes were significantly shorter than other comet tails. Taken together, these observations showed that Dyn2 is a component of macropinosomes, consistent with our observation that Dyn2 functions to regulate actin-based vesicle motility.
Figure 6.
Comets assemble on macropinosomes forming from membrane ruffles in PIP5KIα-expressing cells. Time-lapse confocal imaging of microinjected rat fibroblasts expressing PIP5KIα and actin-GFP. These cells often formed large macropinocytic structures from peripheral membrane ruffles. (a) Actin-GFP fluorescence in a cell undergoing active membrane ruffling (box). (b–d) Enlarged image of the boxed area in a. Time-lapse frames at 10-s intervals revealed macropinosomes forming directly from the peripheral membrane ruffles (arrowhead). The large vesicle head and short, trailing, actin tail are resolved easily (arrows). Similar to the endogenous Dyn2-GFP macropinosomes (Fig. 1), the tail remained 180° opposed from the direction of movement. (Bar, 10 μm.) See Movie 6.
Discussion
Dynamin as an Integral Component of Actin Comets.
The present study demonstrates that the large GTPase dynamin is an important component of actin comets that functions to regulate the formation, velocity, and movement of these dynamic organelles. These findings report that: (i) using multiple affinity-purified antibodies and the expression of GFP-tagged proteins, Dyn2 associates both at the comet head and tail (Figs. 1 and 2) and (ii) Dyn2 is an essential component of the comet formation and translocation machineries. Not only do Dyn2 mutants inhibit tail formation (Fig. 3 a and b), but they significantly interfere with normal tail function, resulting in slower velocities and aberrant translocation properties (Figs. 3a and 5). The combination of immunocytochemistry using Dyn2 antibodies, combined with the incorporation of an expressed GFP-tagged Dyn2, provides strong evidence that this large GTPase is a bona fide component of the actin comets. Further, the observation that either Dyn2 point or truncation mutants alter comet length and motility indicates that it plays a functional role, as opposed to merely being recruited to the comet in a nonspecific manner. Although surprising, the localization of dynamin to actin-rich structures such as membrane ruffles of migrating cells or growth cones has been reported (5, 26). This localization is believed to be mediated by at least two different actin-binding proteins, cortactin (5) and profilin (7), both of which dynamin directly binds, and also are defined components of actin comets (11, 27). Thus, as discussed below, it is likely that dynamin could play a role in regulating actin assembly or mediating membrane interactions with actin filaments.
Dynamin as a Regulator of Actin Dynamics in Motile Comets.
It is known that actin comets translocate through the cytoplasm using a dendritic flow model of actin polymerization in which G-actin monomers assemble into actin filaments at the head domain of the comet and collectively form a branched network that functions to push the organelle forward. This regulated treadmilling, assembly/disassembly reaction is mediated by the joint efforts of accessory proteins such as profilin, N-WASP, the Arp2/3 complex, phosphatidylinositol 4,5 bisphosphate, and possibly cortactin (25, 28). Previous studies have demonstrated that actin treadmilling is the major, if not the only, source of motility, because the rate of polymer exchange is equal to comet velocity (21, 23). The findings reported here provide strong evidence that Dyn2 is a member of the comet regulatory machinery. This prediction is based on the fact that the Dyn2K44A mutant protein reduces the amount of comet-forming vesicles and greatly reduces comet length as well as velocity and efficiency of movement. How Dyn2, when arrested in the GTP-bound state, could reduce the actin assembly and treadmilling rate is unclear. Previous studies have shown that Dyn2K44A inhibits actin dynamics, but the mechanism of how this occurs was not defined (22). Therefore, we have initiated in vitro actin assembly experiments to study the kinetics of filament nucleation, elongation, and branching/debranching.
In contrast to the Dyn2K44A mutant, the Dyn2ΔPRD truncation mutant behaved quite differently. As for Dyn2K44A-expressing cells, fewer comets formed in Dyn2ΔPRD expressers, but they were significantly longer than both the Dyn2K44A-GFP mutant (93.8% longer) and wild-type Dyn2 (8.7% longer) comets. Most striking is the fact that this truncated Dyn2ΔPRD did not assemble into the comets, making them appear as dark profiles in the GFP mutant-expressing cells (Fig. 4 and Movie 6). These findings support our previous studies that tested the effects of the Dyn2ΔPRD mutant on actin dynamics in cultured Clone 9 cells (5). In this study we observed that cells expressing this mutant protein exhibited numerous large actin stress fibers, indicating that the equilibrium of actin dynamics was pushed toward the polymerized state. Further, the truncated Dyn2ΔPRD protein failed to localize to the actin-rich cortex along with its binding partner cortactin, whereas some endogenous Dyn2 did. We concluded that Dyn2 lacking the PRD was unable to bind to cortactin, resulting in significant changes in the polymerized state of actin in the cell. Longer, slower comets in Dyn2ΔPRD-expressing cells are consistent with the premise that, without Dyn2 incorporated into the comet, normal actin treadmilling is altered to favor the polymerized state.
Dynamin as a Component of Macropinosomes.
The fact that Dyn2-GFP is observed as bright, well defined puncta in intimate association with motile vesicles and forming macropinosomes (Fig. 1) in non-PIP5KIα-expressing cells is informative on three fronts. First, Dyn2 may participate in the nucleation of actin from the membrane surface. Second, this study demonstrates that Dyn2 associates with motile membranes, and subsequently with comet formation, occurring in normal cells without increased levels of phosphatidylinositol 4,5 bisphosphate or ARF6. Although the length of the comet tails associated with the macropinosomes are smaller than in the PIP5KIα- or ARF6-expressing cells, the fact that comets actually do form, perhaps at a more physiological length, is significant. Third, the combined observations of Figs. 1 and 6 strongly suggest that Dyn2 is a component of the macropinocytotic process, one of a small number of proteins to be implicated in this enigmatic endocytotic pathway. Because we and others have been unable to inhibit macropinocytosis in past studies using inhibitory antibodies or dominant negative proteins, we assumed that Dyn2 was not involved in this pathway (29). These current observations indicate that Dyn2, although not participatory in the initial internalization steps of macropinosomes, is active in the important subsequent step, that being actin-based motility.
Supplementary Material
Acknowledgments
We thank L. M. Machesky and G. Marriot for the Myc-PIP5KIα and actin-GFP expression constructs, respectively. We also thank Matthew J. Ferber for technical contributions and H. M. Thompson and N. W. Gray for critical reading of this manuscript. This research was supported by grants from the National Institutes of Health and the Mayo Foundation (to M.A.M.).
Abbreviations
- Dyn2
dynamin 2
- PRD
proline-rich domain
- PIP5KIα
type I phosphatidylinositol phosphate 5-kinase α
- GFP
green fluorescent protein
- MI
movement index
References
- 1.McNiven M A, Cao H, Pitts K R, Yoon Y. Trends Biochem Sci. 2000;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- 2.Hinshaw J E. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takei K, Mundigl O, Daniell L, De Camilli P. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henley J R, McNiven M A. J Cell Biol. 1996;133:761–775. doi: 10.1083/jcb.133.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNiven M A, Kim L, Krueger E W, Orth J D, Cao H, Wong T W. J Cell Biol. 2000;151:187–198. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qualmann B, Roos J, DiGregorio P J, Kelly R B. Mol Biol Cell. 1999;10:501–513. doi: 10.1091/mbc.10.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witke W, Podtelejnikov A V, Di Nardo A, Sutherland J D, Gurniak C B, Dott C, Mann M. EMBO J. 1998;17:967–976. doi: 10.1093/emboj/17.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kessels M M, Engqvist-Goldstein A E Y, Drubin D G, Qualmann B. J Cell Biol. 2001;153:351–366. doi: 10.1083/jcb.153.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qualmann B, Kessels M M, Kelly R B. J Cell Biol. 2000;150:F111–F116. doi: 10.1083/jcb.150.5.f111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeng R L, Welch M D. Curr Biol. 2001;11:R691–R694. doi: 10.1016/s0960-9822(01)00410-9. [DOI] [PubMed] [Google Scholar]
- 11.Schafer D A, D'Souza-Schorey C, Cooper J A. Traffic. 2000;1:892–903. doi: 10.1034/j.1600-0854.2000.011108.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaksonen M, Peng H B, Rauvala H. J Cell Sci. 2000;113:4421–4426. doi: 10.1242/jcs.113.24.4421. [DOI] [PubMed] [Google Scholar]
- 13.De Camilli P, Emr S D, McPherson P S, Novick P. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- 14.Ma L, Cantley L C, Janmey P A, Kirschner M W. J Cell Biol. 1998;140:1125–1136. doi: 10.1083/jcb.140.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozelle A L, Machesky L M, Yamamoto M, Driessens M H E, Insall R H, Roth M G, Luby-Phelps K, Marriott G, Hall A, Yin H L. Curr Biol. 2000;10:311–320. doi: 10.1016/s0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- 16.Martin T F J. Curr Opin Cell Biol. 2001;13:493–499. doi: 10.1016/s0955-0674(00)00241-6. [DOI] [PubMed] [Google Scholar]
- 17.Higgs H N, Pollard T D. Annu Rev Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- 18.Welch M D, Iwamatsu A, Mitchison T J. Nature (London) 1997;385:265–269. doi: 10.1038/385265a0. [DOI] [PubMed] [Google Scholar]
- 19.Cao H, Garcia F, McNiven M. Mol Biol Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao H, Thompson H M, Krueger E W, McNiven M A. J Cell Sci. 2000;113:1993–2002. doi: 10.1242/jcs.113.11.1993. [DOI] [PubMed] [Google Scholar]
- 21.Theriot J A, Mitchison T J, Tilney L G, Portnoy D A. Nature (London) 1992;357:257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- 22.Ochoa G-C, Slepnev V I, Neff L, Ringstad N, Takei K, Daniell L, Cao H, McNiven M, Baron R, De Camilli P. J Cell Biol. 2000;150:377–389. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameron L A, Footer M J, van Oudenaarden A, Theriot J A. Proc Natl Acad Sci USA. 1999;96:4908–4913. doi: 10.1073/pnas.96.9.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taunton J, Rowning B A, Coughlin M L, Wu M, Moon R T, Mitchison T J, Larabell C A. J Cell Biol. 2000;148:519–530. doi: 10.1083/jcb.148.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taunton J. Curr Opin Cell Biol. 2001;13:85–91. doi: 10.1016/s0955-0674(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 26.Scaife R, Margolis R L. J Cell Biol. 1990;111:3023–3033. doi: 10.1083/jcb.111.6.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loisel T P, Boujemaa R, Pantaloni D, Carlier M F. Nature (London) 1999;401:613–616. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- 28.Higgs H. Trends Biochem Sci. 2001;26:219. doi: 10.1016/s0968-0004(01)01829-1. [DOI] [PubMed] [Google Scholar]
- 29.Gold E S, Underhill D M, Morrissette N S, Guo J B, McNiven M A, Aderem A. J Exp Med. 1999;190:1849–1856. doi: 10.1084/jem.190.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.