Abstract
Both astroglia and microglia show region-specific distribution in CNS and often maladapt to age-associated alterations within their niche. Studies on autopsied substantia nigra (SN) of Parkinson’s disease (PD) patients and experimental models propose gliosis as a trigger for neuronal loss. Epidemiological studies propose an ethnic bias in PD prevalence, since Caucasians are more susceptible than non-whites. Similarly, different mice strains are variably sensitive to MPTP. We had earlier likened divergent MPTP sensitivity of C57BL/6 J and CD-1 mice with differential susceptibility to PD, based on the numbers of SN neurons. We examined whether the variability was incumbent to inter-strain differences in glial features of male C57BL/6 J and CD-1 mice. Stereological counts showed relatively more microglia and fewer astrocytes in the SN of normal C57BL/6 J mice, suggesting persistence of an immune-vigilant state. MPTP-induced microgliosis and astrogliosis in both strains suggest their involvement in pathogenesis. ELISA of pro-inflammatory cytokines in the ventral-midbrain revealed augmentation of TNF-α and IL-6 at middle age in both strains that reduced at old age, suggesting middle age as a critical, inflamm-aging-associated time point. TNF-α levels were high in C57BL/6 J, through aging and post-MPTP, while IL-6 and IL-1β were upregulated at old age. CD-1 had higher levels of anti-inflammatory cytokine TGF-β. MPTP challenge caused upregulation of enzymes MAO-A, MAO-B, and iNOS in both strains. Post-MPTP enhancement in fractalkine and hemeoxygenase-1 may be neuron-associated compensatory signals. Ultrastructural observations of elongated astroglial/microglial mitochondria vis-à-vis the shrunken ones in neurons suggest a scale-up of their functions with neurotoxic consequences. Thus, astroglia and microglia may modulate aging and PD susceptibility.
Keywords: Parkinson’s disease; 1-Methyl-4-Phenyl-1, 2, 3, 6-Tetrahydropyridine (MPTP); Cytokine ELISA; Neuroinflammation; Monoamine oxidases a&b; Unbiased stereology, C57BL/6 J; CD-1 white mice; Substantia nigra pars compacta
Introduction
Inflamm-aging refers to the alterations in the neuron-glia communication during aging, that result from a persistent functional decline in the immune system, characterized by a generalized increase in the pro-inflammatory markers (Franceschi et al. 2006). The system copes by releasing anti-inflammatory cytokines; a process termed as “anti-inflamm-aging.” The imbalance between inflamm-aging and anti-inflamm-aging-associated processes, supported by the genetic make-up of the individual and environmental factors combine to trigger the onset or protect against age-related neurodegenerative diseases like Parkinson’s disease (PD). PD is characterized by a selective loss of dopaminergic (DA) neurons, primarily in the substantia nigra pars compacta (SNpc) (Bernheimer, 1973). The pattern of depletion in striatal dopamine and the dysfunction of the basal ganglia circuitry was considered as prognostic marker for cognitive dysfunction in drug-naïve PD cases (Chung et al., 2018). It affects the natural gait while altering the cortico-striatal and cerebellar processing (Gilat et al., 2017). Non-motor symptoms like constipation precede the motor symptoms by many years, thus suggesting a role for gut microbiota in the disease pathogenesis (Chaudhuri et al. 2006; Dutta et al 2019).
PD is characterized by T-cell infiltration (Galiano-Landeira et al., 2020; Subbarayan et al., 2020; Seo et al., 2020), microgliosis (Joers et al. 2017; review), and astrogliosis (Croisier and Graeber 2006), all of which affect the survival of substantia nigra neurons. Chronic neuroinflammation is considered to be a prototypical event preceding and accompanying neuronal dysfunction (Lee et al., 2014). It is marked by the presence of MHC class II-activated microglia (Imamura et al. 2003) and reactive astrocytes, direct participation of the adaptive immune system (Brochard et al. 2009; Harms et al., 2017) as well as increased synthesis of peripheral cytokines (Reale et al., 2009; Rydbirk et al., 2019), chemokines (Ahmadi Rastegar et al. 2019), inflammatory markers, and reactive oxygen and nitrogen species (reviewed by Williams et al., 2019). Midbrain DA neurons express the receptors for cytokines such as tumor necrosis factor (TNF-α) and interleukin-1β (IL-1β), hinting at their sensitivity to these cytokines (Boka, et al., 1994; Borrajo et al., 2014). Both neurons and glia express interferon-γ (IFN-γ) receptors and contribute to neuroinflammation (Hashioka et al. 2009, Kim et al., 2000).
Epidemiological studies on PD propose prevalence and incidence rates of approximately 108–257/100,000 and 11–19/100,000 person year, respectively (Van Den Eeden et al. 2003), in Europe. North America reported 329–107/100,000 in Nebraska-USA (Strickland and Bertoni 2004) and 224 per 100,000 person-years in persons above 65 years (Wirdefeldt et al. 2011). The incidence among Indians, Chinese, and Malays were less compared to the Westerners (Gourie-Devi M 2014; Abbas et al., 2017). Thus, the white populations have significantly higher prevalence than non-whites; yet the underlying mechanisms are unclear. We demonstrated preservation of substantia nigra neurons despite a non-logarithmic increase in α-synuclein with age, maintenance of GNDF receptors on substantia nigra neurons as well as other few neuroprotective factors in Asian-Indians (Alladi et al. 2009, 2010a, 2010b). We also found age-related morphological transformation of astrocytes and microglia (Jyothi et al. 2015). Direct comparisons on human tissues between populations were not possible due to unavailability of tissues. Besides, there are no studies in different human populations, based on ethnicities, which implored us to design an animal model to understand the mechanisms of differential prevalence and the role of glia therein.
Among the available small animal models for PD, a reliable recapitulation of PD is seen following administration of the neurotoxin 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) in mice (Jackson-Lewis and Przedborski 2007). In addition, different mice strains show varying responses to MPTP, for example, C57BL/6 J mice are more sensitive, while CD-1 white, BALB/c, and Swiss Webster are resistant. Thus, the neurobiological attributes of these strains can be compared to understand the mechanisms of differential susceptibility and may be extrapolated to the human phenomenon of differential susceptibility to the disease. C57BL/6 J dopaminergic neurons exposed to MPP+ (1-methyl-4-phenylpyridinium) demonstrated a 39% loss when cultured on C57BL/6 J glia compared to 17% neuron loss when cultured on SWR/J glia (Smeyne et al. 2001). Thus, glia may modulate the strain-dependent susceptibility of mice to MPTP and comparison of responses of different mice strains may provide a platform to understand the population-based differences (Kohutnicka et al. 1998).
We have earlier reported that the CD-1 had more substantia nigra DA neurons than C57BL/6 J and were better protected against MPTP (Vidyadhara et al. 2017, 2019). Soreq et al., (2017) reported that astroglia shows significant senescence-associated changes in gene expression patterns. Yet, the role of glia in aging, disease modulation, and differential vulnerability is not completely understood. In the present study, we systematically investigated the responses of astroglia and microglia in the substantia nigra of the two different mice strains, i.e., C57BL/6 J and CD-1, in terms of aging and in association with inflammatory responses to MPTP. The outcome may be useful to understand the role of glia and the underlying molecular mechanisms that contribute to the differences in the prevalence of PD between the Caucasians and Asian-Indians.
Materials and Methods
Experimental Animals and MPTP-HCl Administration
We used C57BL/6 J (MPTP-susceptible) and CD-1 (MPTP-resistant) mice at 15–17 week (young adults), 10–12 months (middle-aged), and 18–20 months (old/aged). All the experiments were conducted on male mice as there is a male preponderance in the prevalence and incidence of PD (reviewed by Georgiev et al., 2017).
They were housed under standard laboratory conditions of temperature 25° ± 2 °C, 12-h light:12-h dark cycle with ad libitum access to food and water. The mice received four intraperitoneal injections of MPTP hydrochloride (15 mg/kg/dose) in saline, at 2-h intervals. The controls received saline (Vidyadhara et al. 2017). The mice were sacrificed at days 1, 4, & 7 after MPTP injection to assess the temporal profile of the cytokines. The other parameters were studied at 7 days post-MPTP.
Tissue Processing for Immunohistochemistry (IHC)
Male mice anaesthetized using isoflurane were intracardially perfused with 0.9% heparinized saline followed by 4% ice-cold buffered paraformaldehyde (0.1 M). The brains were removed and post-fixed for 48 h at 4 °C; cryoprotected in buffered sucrose grades (10%, 20% & 30%). 40-µm-thick serial coronal sections of midbrains were collected on gelatin-coated slides.
Immunostaining
Two different series were used for Iba-1 and s100-β labeling (n = 3–4/strain/age group/experimental condition) with a section periodicity of 1 in 6. The antigen was unmasked using sodium citrate buffer (pH-6) at 80 °C for 20 min. The endogenous peroxidase was quenched using 0.1% hydrogen peroxide (H2O2) in 70% methanol (20 min in dark). The non-specific binding was blocked with 3% buffered bovine serum albumin (BSA) for 4 h at room temperature (RT). The primary antibody exposure (0.1 M PBS-TX; dilution 1:500; Table 1) lasted for 72 h at 4 °C in a closed chamber. Biotinylated secondary labeling (8 h; dilution 1:100, Vector labs, USA; Table 1) was followed by tertiary labeling with avidin–biotin complex (Vector labs, USA; 4 h at RT). The staining was visualized with 0.05% DAB (3'−3'-diaminobenzidine) and 0.01% nickel sulfate in 0.1 M acetate imidazole buffer (pH 7.4) containing 0.01% H2O2 resulting in a black-colored reaction. For the second antibody, similar procedure was followed (Table 1) except that the chromation was performed exclusively with DAB, resulting in brown-colored profiles [(TH (brown) and Iba-1/s100β black)]. Negative controls were processed without adding primary antibody. The sections were mounted with DPX following alcohol-based dehydration.
Table 1.
Details of Primary and Secondary antibodies used for immunohistochemistry
| Staining modality, primary antibody, and source | Dilution and incubation time | Secondary antibody and ABC kits | Dilution and incubation time | |
|---|---|---|---|---|
| Immuno-Peroxidase Staining | Anti-Goat Iba-1, Abcam, UK | 1:800 72 h at 4 °C | Goat Elite ABC kit, Vector laboratories, USA | 1:200, 4 h at RT |
| Anti-Mouse S100β, Sigma-Aldrich | 1:800 72 h at 4 °C | MOM* ABC kit, Vector laboratories,USA | 1:500, 3 h at RT | |
| Anti-Rabbit TH, Santa Cruz, USA | 1:1000 72 h at 4 °C | Rabbit Elite ABC kit, Vector laboratories,USA | 1:200, 4 h at RT | |
| Immuno-Fluorescence | Anti-Goat Iba-1, Abcam UK | 1:800 72 h at 4 °C | FITC, Sigma-Aldrich,USA | 1; 200 6–8 h at 4 °C |
| Anti-Rabbit GFAP, Abcam UK | 1:500 24 h at 4 °C | Cy3, Sigma-Aldrich,USA | 1; 200 6–8 h at 4 °C | |
| Anti-Rabbit Fractalkine, Abcam UK | 1:500 72 h at 4 °C | Cy3, Sigma-Aldrich, USA | 1; 200 6–8 h at 4 °C | |
| Anti-Mouse CNPase, Abcam UK | 1:1000 72 h at 4 °C | FITC, Sigma-Aldrich, USA | 1; 200 6–8 h at 4 °C | |
| Anti-Rabbit MFGE8, Cloude Clone crop | 1:800, 72 h at 4 °C | Cy3, Sigma-Aldrich, USA | 1; 200 6–8 h at 4 °C | |
*MOM Mouse-on-mouse kits
For immunofluorescence labeling, following the incubation with primary antibody, the sections were incubated in an appropriate fluorescently labeled secondary antibodies (Sigma-Aldrich, USA). The sections were then mounted using Vectashield hard set mounting medium (Vector Laboratories, USA) and images were captured using a laser scanning confocal microscope (DMIRE-TCS Leica Microsystems, Germany).
Stereological Quantification of Cells
The s100β and iba-1 immunoreactive (ir) cells in SNpc were quantified using optical fractionator method with an Olympus BX61 Microscope (Olympus Microscopes, Japan) equipped with Stereo Investigator software version 8.1 (Micro-brightfield Inc., Colchester, USA). The SNpc in TH-ir midbrain sections was delineated on both the sides under 10X (Paxinos, 2013). Pilot studies determined the grid interval and counting frame size. Cells were counted in every sixth Sect. (6–8 sections/animal) under 100X, with a grid interval of 10000μm2 (x = 100 μm, y = 100 μm) and counting frame of size 6400μm2 (x = 80 μm, y = 80 μm) as per our earlier report (Vidyadhara et al. 2017). The absolute numbers were derived and Gundersen’s coefficient of error was determined.
Immunoblotting
The mice (n = 5/strain/age group/experimental condition) were sacrificed by cervical dislocation and the midbrains were snap frozen in liquid nitrogen and stored at − 80 °C till further use. The brains were thawed to − 20 °C on a cryostat and 5-µm-thick sections of SN were solubilized in 100 ul of mammalian lysis buffer 10% protease inhibitor cocktail (Sigma-Aldrich, USA). Following sonication (Q sonica, India) and centrifugation at 12000 g (4 °C), the protein concentration in the supernatant was assayed by Bradford’s method and 60ug of protein/sample was electrophoresed (Bio-Rad, USA) on a 5%/10% (loading/separating) denaturing gel. The proteins were transferred onto a buffered polyvinylidene difluoride (PVDF) membrane (Millipore, Germany). The non-specific staining was blocked by 5% skimmed milk protein (1X TBST; 4 h) followed by overnight incubation with primary antibody solution (Table 2.). This was followed by incubation with appropriate HRP-conjugated secondary antibody for 2 h (Table 2.). The band was detected using chemi-luminescent substrate for HRP (Super Signal West Pico, USA) using a Gel documentation system (Syngene International Ltd., India) and quantified using Image J 1.48 v program (Vidyadhara et al. 2017). B-actin was used as a loading control and each protein concentration was normalized against it.
Table 2.
Details of primary and secondary antibodies used for immunoblotting
| Primary antibody | Dilution and incubation time | Secondary antibody | Dilution and incubation time |
|---|---|---|---|
| Anti-iNOS, Rabbit, Abcam USA | 1:1500, 8 h at 4 °C | Anti-Rabbit HRP-Conjugated secondary antibody, Merck | 1:2000, 2 h at RT |
| Anti-Fractalkine, Rabbit, Abcam USA | 1:800, 8 h at 4 °C | Anti-Rabbit HRP-Conjugated secondary antibody, Merck | 1:2000, 2 h at RT |
| Anti-β-actin, Mouse, Sigma-Aldrich | 1:2000, 8 h at 4 °C | Anti-Mouse HRP-Conjugated secondary antibody, Merck | 1:2000, 2 h at RT |
Enzyme-Linked Immunosorbent Assay (ELISA)
Protein lysates were obtained from ventral midbrains (n = 4–5/strain/age group/time point/experimental condition) of post-MPTP day (d)1, d4, and d7 mice. Both pro-inflammatory (TNF-α, IL-6, IL-1β) and anti-inflammatory cytokines (TGF-β, IL-4, 1L-10) were evaluated as per the manufacturer’s instructions (Ray Biotech, Inc, USA). MAO-A and MAO-B kits were obtained from Cloud-clone Corp, (Wuhan, China) and HO-1 ELISA kit was obtained from Abcam UK (mouse Simple Step, ab204524). Briefly 100 µl of standards/samples were added to each well except blanks/standards (2.5 h, 37 °C). Thereafter, 100 µl of biotinylated secondary antibody was added (1 h, RT) with gentle shaking followed by streptavidin (100 µl). The chromation was achieved with TMB (3,3’,5,5’-tetramethylbenzidine) one-step substrate reagent. The reaction was stopped with 50 µl stop solution/well and the absorbance was measured immediately at 450 nm (TECAN, Austria). All standards and samples were run in duplicates.
Electron Microscopy
The mice (n = 3/strain/age group/experimental condition) were anesthetized with isoflurane and transcardially perfused with a buffered mixture of 2.5% glutaraldehyde and 2% PFA (in 0.1 M phosphate buffer, pH 7.4). The dissected substantia nigra was cut into smaller pieces, post-fixed with 1% osmium tetroxide at RT, and dehydrated in grades of ethyl alcohol followed by clearing in propylene oxide. Infiltration (1:1 mixture of araldite and propylene oxide, overnight at RT, on a rotator) was followed by exposure to pure araldite for 4.5 h. The resin-embedded tissues (flat embedding molds) were allowed to polymerize (60 °C, 48 h). 1 µm semi-thin sections were stained with 1% toluidine blue to verify the region of interest and 60 nm ultrathin sections (Leica, Ultramicrotome, Austria) collected on copper grids were stained using saturated uranyl acetate for 1 h and 0.2% lead citrate (5–7 min). The washed and air-dried sections were examined under Transmission Electron Microscope (FEI, TECNAI G2 Spirit BioTWIN, Netherlands) and images were captured using Mega View-III CCD camera (Shruthi et al. 2017).
Statistics
The stereology-based data for quantification of astrocytes and microglia were analyzed by one-way ANOVA followed by Bonferroni post hoc analysis. The ELISA-related data were analyzed using Kruskal–Wallis test and Mann–Whitney U tests and represented as line graphs with median values with upper range for each strain and time point. The other observations were analyzed using two-way ANOVA followed by Tukey’s post hoc test using SSPS software and GraphPad Prism software (GraphPad Software version 6.01Inc, USA). A p value < 0.05 was considered as statistically significant. The data were expressed as mean ± standard error of mean (mean ± SEM).
Results
Differences in Number of Glia
Microglia
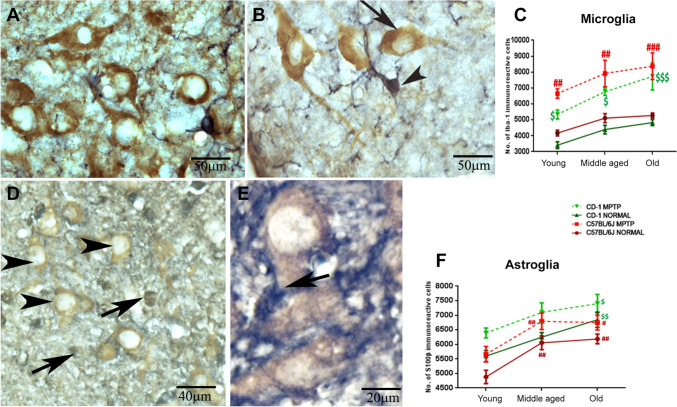
The strain-specific differences in the neuronal numbers of substantia nigra noted earlier (Vidyadhara et al. 2017) prompted us to verify if the differences extended to baseline numbers of glia. Iba-1 stained the microglia well (Fig. 1A&B; arrowheads) including their processes and were easily discriminated from neurons (arrows) in the vicinity. Based on unbiased stereology, CD-1 substantia nigra had relatively fewer Iba-1 ir cells across aging although not statistically significant (Fig. 1C; 18.2% at young age, 14% at middle age, and 8.5% at old age). In response to MPTP, an increase in microglia was noted in old animals of both strains [C57BL/6 J MPTP (young vs. middle-aged ##p < 0.01, young vs. old #p < 0.05), CD-1 MPTP (young vs. old $p < 0.05)]. Scale bar 50 µm for 1A&B.
Fig. 1.
Differences in numbers of microglia and astrocytes in the two strains following MPTP administration. A, B Representative DAB-stained photomicrographs of Iba-1 ir microglia (black) and TH-positive DA neurons (brown) within the SNpc of 18-month-old C57BL/6 J (A) and CD-1 mice (B). The line graph C shows no significant inter-strain differences in control mice. Note the gradual increase in the microglial numbers during aging in both strains. Note the significant increase in the number of microglia at all ages after MPTP injection [Young (C57BL/6 J normal vs. C57BL/6 J MPTP ## p < 0.01, CD-1 normal vs. CD-1 MPTP # p < 0.05); middle aged (C57BL/6 J normal vs. C57BL/6 J MPTP ## p < 0.01, CD-1 normal vs. CD-1 MPTP $$ p < 0.01); old (C57BL/6 J normal vs. C57BL/6 J MPTP ###p < 0.001, CD-1 normal vs. CD-1 MPTP $$$ p < 0.001), (*between strains, #within C57BL/6 J,$within CD-1)]. Scale bar: 50 μm. Astrocytes and susceptibility: Representative DAB-stained photomicrographs of s100β-ir astrocytes (black) and TH immunopositive DA neurons (brown) in SNpc of 18-month-old C57Bl/6 J (D) and CD-1 (E) mice. The line graph F a significant increase in the numbers of astrocytes during aging in both strains and in response to MPTP. C57BL/6 J normal (young vs. middle-aged ## p < 0.01, young vs. old ##p < 0.01), C57BL/6 J MPTP (young vs. middle-aged ##p < 0.01, young vs. old # p < 0.05), CD-1 normal (young vs. old $$p < 0.01) CD-1 MPTP (young vs. old $p < 0.05) (difference *between strains, #within C57BL/6 J, $within CD-1). Scale bar: 50 μm
Astrocytes
The astrocytes appeared much darkly stained (Fig. 1D&E; arrows) compared to the TH-ir dopaminergic neurons (arrowheads). The control CD-1 mice substantia nigra had moderately more s100β-positive astrocytes (Fig. 1F) than the C57BL/6 J at young (Fig. 1C&D, 14.68%) and old age (Fig. 1C&D, 10.67%). Under normal conditions, in C57BL/6 J, the numbers increased significantly from youth to middle age (C57BL/6 J young vs. middle-aged ##p < 0.01; 24.06%) to stabilize later (C57BL/6 J young vs. old ##p < 0.01; 26.75%). In contrast, the CD-1 substantia nigra showed a gradual age-related increase in the astrocytes (CD-1, young v/s middle age, 11.64%; young vs. old $$ p < 0.01; 22.32%). MPTP caused a significant increase in the numbers at all ages, in both the strains; notably higher in the young mice (C57BL/6 J control v/s MPTP 20%; CD-1 control vs. MPTP, 15.8%).
Cytokine Expression in the Ventral Midbrain
The increase in numbers of microglia and astrocytes in response to MPTP led us to hypothesize that gliosis may induce neuroinflammation. We therefore estimated the levels of pro- and anti-inflammatory cytokines.
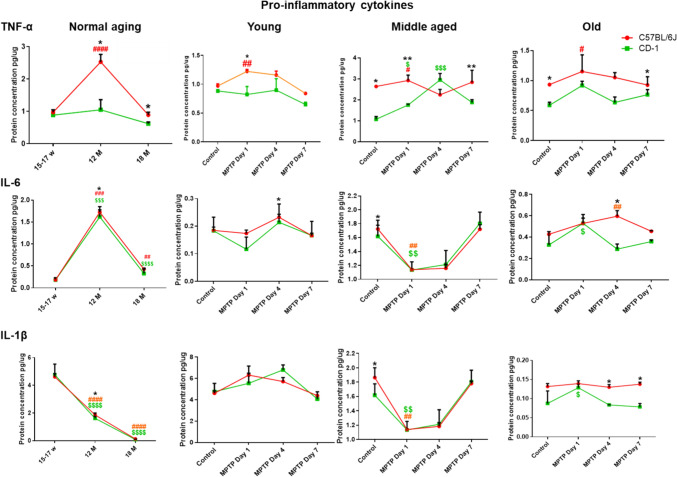
Pro-inflammatory Cytokine TNF-α was Relatively Higher in C57BL/6 J
The baseline TNF-α expression peaked at middle age in the C57BL/6 J (Fig. 2. top panel; normal aging; ####p < 0.0001; young vs. middle age), which was also significantly more than CD-1 (*p = 0.032; C57BL/6 J vs. CD-1). At old age too, the differences persisted, with higher levels in C57BL/6 J (*p = 0.016; C57BL/6 J vs. CD-1). MPTP caused an upregulation in the middle-aged C57BL/6 J at d1 (C57BL/6 J control vs. post-MPTP d1, #p = 0.032) and remained higher than CD-1 (C57BL/6 J vs. CD-1, **p = 0.008). In CD-1, the differences were noted at d4 (control vs. d4 post-MPTP; $$$p = 0.001). The levels were overall higher in C57BL/6 J, both at d1 and d7 (C57BL/6 J vs. CD-1; d1, **p = 0.008 and d7,*p = 0.016). At old age, C57BL/6 J showed a significant increase at d1 (control vs. post-MPTP d1, #p = 0.032). Further at d7, the differences were evident between the strains (C57BL/6 J vs.CD-1 *p = 0.032).
Fig. 2.
Levels of Pro-inflammatory cytokines: Line graphs representing different strains at different age periods and in response to MPTP. The top horizontal panel pertains to TNF-α, middle to IL-6, and bottom panel to IL-1β. Note, differences are depicted as * = between strains, # = within C57BL/6 J, $ = within CD-1. A Higher pro-inflammatory cytokine levels (TNF-α) in C57BL/6 J: Note higher levels in C57BL/6 J than CD-1 at middle (*p = 0.032) and old age (*p = 0.016). C57BL/6 J shows peak TNF-α expression at middle age (####p < 0.0001; young vs. middle age). At middle age MPTP induced increase in TNF-α in C57BL/6 J (control vs. post-MPTP d1, #p = 0.032) and CD-1 (control vs. d4 post-MPTP; $$$p = 0.001). The levels were higher in C57BL/6 J at d1 and d7 (C57BL/6 J vs. CD-1; d1, **p = 0.008 and d7,*p = 0.016). Old C57BL/6 J showed more expression at d1 (control vs. post-MPTP d1, #p = 0.032). Note the differences between strains at d7 (C57BL/6 J vs.CD-1 *p = 0.032). B Middle-aged animals showed significant increase in IL-6: Note the higher IL-6 expression at middle age (C57BL/6 J young vs. middle age ###p = 0.0007; CD-1 young vs. middle-aged $$$p < 0.0001). C57BL/6 J showed higher expression than CD-1 (*p = 0.032). Note the reduction at old age (C57BL/6 J middle age vs. old ##p = 0.0010; CD-1 middle age vs. old $$$$p < 0.0001). At 1d, a reduction is seen at middle age, in response to MPTP in C57BL/6 J (##p = 0.008) and CD-1 ($$p = 0.008). MPTP caused an upregulation in old CD-1 at d1 (control vs. d1, $p = 0.016), while at d4 in old C57BL/6 J (control vs. d4 ##p = 0.008), which is higher than CD-1 (C57BL/6 J vs. CD-1 post-MPTP d4*p = 0.032). C IL-1β is down-regulated during normal aging: Note the age-associated reduction in IL-1β in both strains (C57BL/6 J young vs. middle-aged ####p < 0.0001; CD-1 young vs. middle-aged $$$$p < 0.0001; C57BL/6 J middle-aged vs. old #### p < 0.001; CD-1 middle-aged vs. old $$$$ p < 0.001). Inter-strain differences are present at middle age, with higher levels in C57BL/6 J (C57BL/6 J vs. CD-1; *p = 0.032). Note the reduction upon MPTP in middle-aged C57BL/6 J (control vs. post-MPTP d1##p = 0.01) as well as CD-1 (control vs. post-MPTP d1; $$p = 0.01). Note the increase in old CD-1 at d1 (control vs. post-MPTP d1; CD-1.$p = 0.032). Post-MPTP at d4 and d7 the levels remain relatively higher in C57BL/6 J (C57BL/6 J vs. CD-1, *p = 0.032)
IL-6 and IL-1β show Largely Similar Patterns and MPTP Responses in Both Strains
Both the strains showed a significantly higher IL-6 expression at middle age (Fig. 2; C57BL/6 J young vs. middle age ###p = 0.0007; CD-1 young vs. middle-aged $$$p < 0.0001). Between the strains C57BL/6 J showed higher expression (C57BL/6 J vs. CD-1, *p = 0.032). Thereafter a significant reduction ensued at old age in both the strains (Fig. 2; C57BL/6 J middle age vs. old ##p = 0.0010; CD-1 middle age vs. old $$$$p < 0.0001). At middle age, in response to MPTP, both C57BL/6 J and CD-1 showed a notable reduction at d1 (##p = 0.008; $$p = 0.008) and were almost restored to the baseline at d7. At old age the MPTP-induced upregulation was appreciated at d1, in CD-1 ($p = 0.016, control vs. d1; CD-1). The old C57BL/6 J showed an increase at d4 (post-MPTP d4 ##p = 0.008), and was relatively more than CD-1 at that time point (d4,*p = 0.032).
IL-1β showed a significant reduction with aging in both strains (C57BL/6 J young vs. middle-aged ####p < 0.0001; CD-1 young vs. middle-aged $$$$p < 0.0001; C57BL/6 J middle-aged vs. old ####p < 0.001; CD-1 middle-aged vs. old $$$$p < 0.001). Baseline differences between strains were noted only at middle age, with higher expression in C57BL/6 J (C57BL/6 J vs. CD-1; *p = 0.032). The MPTP-injected middle-aged C57BL/6 J (control vs. post-MPTP d1##p = 0.01) as well as CD-1 (control vs. post-MPTP d1; $$p = 0.01) showed an acute decrease at d1.
The old CD-1 mice strains showed an augmented response at d1 (control vs. post-MPTP d1; CD-1 $p = 0.032). Post-MPTP at d4 and d7, the levels remained relatively higher in C57BL/6 J vis-à-vis the CD-1 (*p = 0.032; C57BL/6 J vs. CD-1).
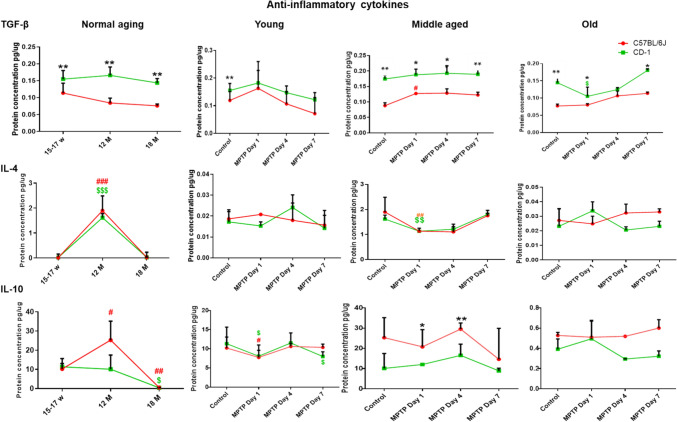
Levels of Anti-inflammatory Cytokine TGF-β are Relatively Lower in C57BL/6 J
The basal level of TGF-β was significantly lower in C57BL/6 J at all the age periods studied [(Fig. 3; top panel; C57BL/6 J vs. CD-1; young **p = 0.008), middle-aged (**p = 0.008), old (**p = 0.008)]. In response to MPTP, middle-aged C57BL/6 J showed an increase in expression (C57BL/6 J control vs. post-MPTP d1, #p < 0.032). Middle-aged CD-1 showed more expression than C57BL/6 J in normal conditions (C57BL/6 J vs. CD-1 **p = 0.08). Moreover, in response to MPTP, the levels remained higher at all the days studied (C57BL6/J v/s CD-1 d1 *p = 0.036; d4 *p = 0.029; d7 *p = 0.016). At old age too, the CD-1 showed more expression than C57BL/6 J, in normal conditions (C57BL/6 J vs. CD-1 **p = 0.008). In response to MPTP, at d1, despite the decrease in CD-1 (control vs. d1 $p = 0.016), the levels were comparatively higher than the C57BL/6 J (C57BL/6 J vs. CD-1 *p = 0.016), as also at d7 (C57BL/6 J vs. CD-1 *p = 0.05).
Fig. 3.
Levels of anti-inflammatory cytokines: Line graphs representing different strains at different age periods and in response to MPTP. The top horizontal panel pertains to TGF-β, middle to IL-4, and bottom panel to IL-10. Note, the differences are depicted as * = between strains, # = within C57BL/6 J, $ = within CD-1. A Lower levels of anti-inflammatory cytokine TGF-β in C57BL/6 J: Note the lower basal TGF-β levels in C57BL/6 J through aging (C57BL/6 J vs. CD-1; young **p = 0.008; middle-aged **p = 0.008; old **p = 0.008). MPTP caused upregulation in middle-aged C57BL/6 J at d1 (C57BL/6 J control vs. d1 #p < 0.032). However, overall expression was higher in CD-1 (C57BL6/J v/s CD-1 d1 *p = 0.036; d4 *p = 0.029; d7 *p = 0.016). Note the decrease in old CD-1 at d1, upon MPTP, (control vs. d1 $p = 0.016) and yet higher levels than the C57BL/6 J (d1 C57BL/6 J vs. CD-1 *p = 0.016; d7 C57BL/6 J vs. CD-1 *p = 0.05). B Increase in the basal level expression of IL-4 in middle-aged animals: Both the strains show an increase in IL-4 at middle age (C57BL/6 J young vs. middle age ###p = 0.0010; CD-1 young vs. middle age $$$p = 0.0009). Note the decrease at old age (C57BL/6 J middle age vs. old ###p = 0.0010; CD-1 middle age vs. old $$$p = 0.0002). Note the MPTP-induced reduction at d1 in both strains (C57BL/6 J##p = 0.008; CD-1 $$p = 0.008), at middle age. Old age observations are comparable between strains. C Compensatory response by IL-10 in C57BL/6 J: Note the increase in levels in C57BL/6 J from young to middle age (C57BL/6 J young vs. middle age #p = 0.0125) and a decrease at old age (C57BL/6 J middle age vs. old #p = 0.0033). The decrease in CD-1 is seen between middle and old age (CD-1 $p = 0.0317). Upon MPTP, young C57BL/6 J (#p = 0.032) and CD-1 ($p = 0.032) show a reduction at d1. Note, the slight increase at d4 and a reduction at d7 in CD-1 ($p < 0.033). At middle age, MPTP-induced inter-strain differences are appreciable at d1 and d4, with higher expression in C57BL/6 J (C57BL/6 J vs. CD-1; d1 *p = 0.016; d4 **p = 0.008). Note the non-significant differences at old age
Both the strains showed a significant increase in IL-4 expression at middle age (Fig. 3; C57BL/6 J young vs. middle age ###p = 0.0010; CD-1 young vs. middle age $$$p = 0.0009). Thereafter, a decrease was noted at old age (C57BL/6 J middle age vs. old ###p = 0.0010; CD-1 middle age vs. old $$$p = 0.0002). At middle age, MPTP elicited a reduction at d1 in both strains (C57BL/6 J##p = 0.008; CD-1 $$p = 0.008).
Anti-inflammatory Cytokine IL-10 levels are Relatively Higher in Middle-Aged C57BL/6 J
The control C57BL/6 J showed an increase in expression from young to middle age (C57BL/6 J young vs. middle age #p = 0.0125) and a much appreciable decrease by old age (C57BL/6 J middle age vs. old #p = 0.0033), whereas the CD-1 showed a decrease from middle to old age (CD-1 middle age vs. old, $p = 0.0317).
MPTP elicited strain-specific responses. At young age, both C57BL/6 J (#p = 0.032) and CD-1 ($p = 0.032) showed a reduction in IL-10 expression in response to MPTP at d1. The expression increased slightly at d4 only to reduce further in CD-1 at d7 ($p < 0.033). At middle age both strains showed differences in baseline expression, which were, however, not statistically significant. The MPTP-induced inter-strain differences were noted, with higher expression in C57BL/6 J at d1 and persisted till d4 (C57BL/6 J vs. CD-1; d1 *p = 0.016; d4 **p = 0.008). Further, the old C57BL/6 J showed mildly higher expression in response to MPTP at d4 and d7, however, the differences did not reach significance.
Differences in Enzyme Expression
MAO-A and MAO-B Levels
Monoamine oxidase (MAO) is a vital enzyme that metabolizes biogenic amines like noradrenaline, adrenaline, dopamine, etc. MAO-B present in glia is responsible for the breakdown of MPTP to MPP+. An interesting comparison between controls and PD patients showed that the PD striatum harbors significantly higher levels of MAO-B compared to controls, whereas the substantia nigra of both controls and PD showed no differences (Tong et al. 2017). We therefore conducted the MAO-B enzyme analyses in the striatal specimens. MAO-A-positive punctate were localized to the cytoplasm (Fig. 4A1, red) of the TH immunoreactive DA neurons (Fig. 4A2) as also in the neuropil (Fig. 4A3 pink coloration in neurons and red in the neuropil). The basal levels as measured by immunoblotting were higher in the substantia nigra of young CD-1 (C57BL/6 J vs. CD-1 *p < 0.05), however, middle age saw a peak in C57BL/6 J (young vs. middle-aged ###p < 0.001). In both strains, the levels reduced at old age (Fig. 4B, C57BL/6 J middle-aged vs. old ###p < 0.001; CD-1 middle-aged vs. old $$p > 0.01). MPTP induced a notable increase in the young C57BL/6 J, although both strains showed moderate enhancement at all ages (C57BL/6 J control vs. MPTP ###p < 0.001).
Fig. 4.
Essential Enzymes are differentially modulated in a strain-specific manner: A Representative photomicrographs of DA neurons (A1, TH, blue; cytoplasmic) co-labeled with MAO-A (A2, red; punctate staining), and merge showing the presence of MAO-A in dopaminergic neurons in SNpc (A3; pink). Note the punctate staining of MAO-A vis-a-vis the pan cytoplasmic staining of TH. B Representative immunofluorescence photomicrographs of astrocytes (GFAP, green, B1) co-labeled with MAO-B (red, B2) in striatum and the merge showing glial localization of MAO-B (B3, yellowish green) (B and D). The basal levels of MAO-A and MAO-B are higher in CD-1 (MAO-A C57BL/6 J vs. CD-1 * p < 0.05). Note the significant upregulation of MAO-A level in middle-aged C57BL/6 J (young vs. middle-aged ### p < 0.001). Aging increased the MAO-B levels in C57BL/6 J, whereas CD-1 showed a down-regulation. MPTP enhanced MAO-A and MAO-B levels in both strains. (Difference *between strains, #within C57BL/6 J, $within CD-1). E Representative immunofluorescence photomicrographs of microglia (E1, Iba-1, green) co-labeled with iNOS (E2, red) and their co-labeling (E3, merge, yellowish orange) in SNpc. Note the presence of some INOS ir cells that were not Iba-1 ir. (E3, red). F Histograms show that basal iNOS level was significantly higher in CD-1 mice in young adults (C57BL/6 J vs. CD-1 * p < 0.05); I Representative Western blots showing a single band of iNOS at 140 kDa. The lanes/blots that were run together are boxed together. Note the gradual increase at middle age in both strains. MPTP caused a strain independent augmentation in all age groups (middle-aged C57BL/6 J control vs. MPTP #p < 0.05, old C57BL/6 J control vs. MPTP #p < 0.05, old CD-1 control vs. MPTP$ p < 0.05). G Representative immunofluorescence photomicrographs of DA neurons (G1, TH, green) co-labeled with fractalkine (G2, red) and their co-labeling (G3, merge, yellow-green) in SNpc. H histograms show higher levels in CD-1 at all ages. Both strains show a gradual decrease with age (C57BL/6 J middle age vs. old #p < 0.05, CD-1 middle age vs. old $$p < 0.01). In C57BL/6 J, the response to MPTP at middle and old age was lesser (young vs. middle-aged ####p < 0.0001, middle age vs. old ####p < 0.0001). CD-1 shows a slow gradual reduction (Difference *between strains, #within C57BL/6 J, $within CD-1). J Representative Western blot bands of fractalkine showing single band at 76KDa in different study groups. The blots which were run together are boxed together. K Compensatory response by Hemeoxygenase-1: Histograms showing lower levels in young C57BL/6 J, whereas CD-1 shows stabilization at old age (C57BL/6 J young adult vs. middle-aged ####p < 0.0001, CD-1 mice young adult vs. middle-aged $$$p < 0.001). Both strains show increased expression after MPTP (C57BL/6 J vs. CD-1, young *p < 0.05; CD-1 old control vs. MPTP $$$p < 0.001), (Difference *between strains, #within C57BL/6 J, $ within CD-1)
The GFAP expressing glia (Fig. 4B1, green) showed MAO-B immunoreactivity (Fig. 4B2, red Fig. 4B3 merge, and yellowish green). MAO-B levels were also higher in young CD-1 than C57BL/6 J (Fig. 4fD). An age-associated gradual down-regulation in CD-1 was contrasted by a gradual increase in C57BL/6 J, although statistically significant. In summary, MPTP administration upregulated both MAO-A and MAO-B level in both strains.
Inducible Nitric Oxide Synthase (iNOS)
Reactive microglia in the degenerating SN is involved in the secretion of iNOS (Boje and Arora 1992; Chhor et al. 2013) and HLA-DR (McGeer et al. 1988). Accordingly, in our specimens too, Iba-1 immunopositive microglia (Fig. 4E1; green) was primarily noted to express iNOS (Fig. 4E2; red) in addition to some non-microglial cells (Fig. 5E3 merge; red). The antibody showed a single band of 140 K Da (Fig. 4I). Note that the lanes/blots that were run together are boxed together (Bass et al. 2017). Young CD-1 had significantly higher basal iNOS (Fig. 4F; C57BL/6 J vs. CD-1 *p < 0.05). At middle age both strains showed a moderate increase in expression. With aging, CD-1 mice showed mild decrease in iNOS expression, whereas C57BL/6 J maintained the levels attained at middle age. MPTP caused iNOS augmentation across ages in both strains (middle-aged C57BL/6 J control vs. MPTP #p < 0.05, old C57BL/6 J control vs. MPTP #p < 0.05, old CD-1 control vs. MPTP $p < 0.05).
Fig. 5.
Age-related and MPTP-induced ultrastructural changes in substantia nigra neurons. Representative electron micrographs of substantia nigra neurons at different ages and in response to MPTP. Note that the mitochondria of the elderly C57BL/6 J were relatively shrunken (comparing A, E&I). MPTP caused mitochondrial shrinkage at all ages (comparing A&B and E&F, I&J; ‘M’ arrows). In CD-1, the mitochondrial size was fairly preserved with age (comparing C&D, D1 and G&H, K&L; ‘M’ arrows) or longer in response to MPTP (H, H1 and L, arrow). Note the shortening of ER strands with age (comparing A, E&I; ER) and in response to MPTP in C57BL/6 J (comparing A&B and E&F, I&J; ER). Note the dilated ER in CD-1 neurons upon MPTP in young (D2) and at middle age (ER, H & H1). Note the ER arrays in aged CD-1 (K1) and presence of many Golgi units in middle-aged and old mice of both strains (‘Go’ in I&K). MPTP-administered aged C57BL/6 J have circular Golgi with bloated saccules (J&L1; ‘Go’). Older C57BL/6 J have apoptotic bodies (I1; ‘Ab’) and CD-1 has crenelated neuronal nucleus. Note several lysosomes in young MPTP-injected CD-1 against few in C57BL/6 J (B&D ‘Ly’). The scale bar for A = B and others are specified for each photomicrograph
Fractalkine
Fractalkine (CX3CL1) is an enzyme synthesized by neurons, and it suppresses microglial activation (Thome et al. 2015). In our observation, it was localized to the DA neuronal cytoplasm (Fig. 4G1-G3). On Western blots, the antibody showed a band at 75 KDa (Fig. 4J). As depicted, the lanes/blots that were run together are boxed together (Bass et al. 2017). The CD-1 substantia nigra had moderately high levels at all the ages, compared to C57BL/6 J. A decrease was evident in both strains after middle age (Fig. 4H, C57BL/6 J middle age vs. old #p < 0.05, CD-1 middle age vs. old $$p < 0.01). MPTP elicited an increase in both the strains, but more appreciably in the young (C57BL/6 J, young adults vs. middle-aged ####p < 0.0001, middle-aged vs. old ####p < 0.0001).
HO-1
This family of enzymes, along with NADPH cytochrome P450 reductase, modulates the catabolism of cellular heme to biliverdin, carbon monoxide (CO), and free ferrous iron in brain (Ryter and Tyrrell 2000). The young CD-1 midbrains showed mildly higher levels of HO-1, which reduced following MPTP. Both strains showed a significant upregulation at middle age that persisted till old age (Fig. 4 k, C57BL/6 J young vs. middle-aged ####p < 0.001, CD-1 young vs. middle-aged $$$p < 0.001). Aged mice of both strains showed an increase in HO-1 expression in response to MPTP, moderate in C57BL/6 J, significant in CD-1 (CD-1 control vs. MPTP $$$p < 0.001), suggesting a compensatory induction.
Age-Related and MPTP-Induced Ultrastructural Changes
Effects on Substantia Nigra Neurons
The mitochondria of the young and middle-aged C57BL/6 J were relatively larger than those of the elderly (Fig. 5A, E&I). MPTP induced mitochondrial shrinkage at all ages (Fig. 5 comparing A&B and E&F, I&J; ‘M’ arrows). Shrunken albeit intact mitochondria were evident, in the MPTP-injected young CD-1 mice (Fig. 5D). However, at middle and old age they were preserved (Fig. 5 comparing C&D and G&H, K&L; ‘M’ arrows) or even elongated in response to MPTP (Fig. 5 H, H1 and L, arrow). The endoplasmic reticular (ER) strands shortened with age and in response to MPTP in C57BL/6 J (Fig. 5, comparing A&B and E&F, I&J; ER), while MPTP induced ER dilatation in the young (Fig. 5; D2) and middle-aged CD-1 mice neurons (Fig. 5H; ER). The normal-aged CD-1 showed the presence of ER arrays (Fig. 5K1). Interestingly, aged mice of both strains showed the presence of several Golgi apparatus units (Fig. 5I ‘Go’). In the MPTP-administered aged C57BL/6 J, they appeared circular and possessed bloated saccules with “pearl necklace like appearance” (Fig. 5, F&H; J&L1; ‘Go’). In the older C57BL/6 J, apoptotic bodies were noted (Fig. 5, I1; ‘Ab’), while in CD-1, the neuronal nucleus was at times crenelated. The neurons of MPTP-injected young CD-1 harbored several lysosomes, which were conspicuously absent in the neurons of young C57BL/6 J (Fig. 5 B&D ‘Ly’).
Astrocytes
The astrocytic nuclei were larger but not uniformly ovoid/round like those of oligodendrocytes. Their nuclear chromatin was fine and granular. The cytoplasm was sparse and granular within the perinuclear zone (Luse SA, 1956), but more electron-dense than the neuronal cytoplasm. The nucleus remained euchromatic through aging and in response to MPTP. The astrocytes of young and middle-aged C57BL/6 J had numerous long and tubular cytoplasmic mitochondria (Fig. 6, ‘M,’ comparing A&B and E&F), whereas those in the myelinated axons were spherical (‘Ma’). In CD-1, the cytoplasmic mitochondria were oval (Fig. 6, comparing C&D and G&H). The astrocytic mitochondria were relatively longer in the old CD-1 (Fig. 6, comparing E&G). The ER was relatively well maintained through aging and in response to MPTP in both strains. Golgi saccules were semicircular and dilated in the middle-aged MPTP-injected CD-1 (Fig. 6, Go, comparing G&H and K&L). The scale bar is 1 μm for all micrographs except ‘K.’
Fig. 6.
Astrocytic organelles mostly resist age and MPTP-induced alterations. Representative electron micrographs of astrocytes at different ages and in response to MPTP. Astrocytes had large and euchromatic nucleus. Note the numerous long and tubular cytoplasmic mitochondria in young and middle-aged C57BL/6 J (‘M,’ A vs. E) and the alterations following MPTP (B vs. F) and spherical ones (‘Ma’) in the myelinated axons; oval mitochondria in CD-1 cytoplasm (control C vs. G; post-MPTP D vs. H). In old C57BL/6 J, the mitochondria are smaller than old CD-1 (comparing I vs. K). MPTP caused qualitative increase in mitochondrial numbers and their elongation (comparing I vs. J). Golgi saccules were semicircular and dilated in the middle-aged CD-1 injected with MPTP (Go, comparing G vs. H and K vs. L). The scale bar is 1 μm for all micrographs except ‘K’
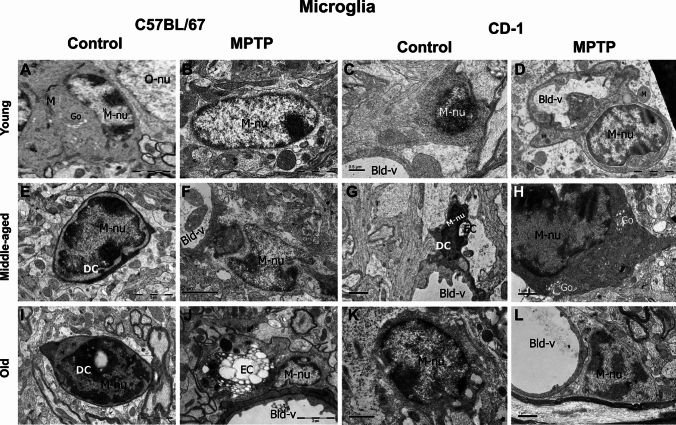
Microglia
Most of the microglia were present near the blood vessels and had electron-dense cytoplasm with a bean-shaped nucleus. Heterochromatin nets and electron-dense pockets were noted along the nuclear perimeter. In the young, the nuclei were euchromatic (Fig. 7A–D; ‘M-nu’), while those of middle aged and old were electron-dense (Fig. 7E–sL; ‘M-nu’). In the cytoplasm and neighboring tracks of MPTP-injected young C57BL/6 J, long tubular mitochondria were noted (Fig. 7B, ‘M’). The young CD-1 showed phagocytic microglia along with the engulfed cells (Fig. 7D; ‘EC’) as also the MPTP-injected old C57BL/6 J (Fig. 7 J).
Fig. 7.
Microglia and Dark cells. Representative electron micrographs of microglia at different ages and in response to MPTP. Most microglia are seen near the blood vessels, have bean-shaped nucleus, electron-dense cytoplasm, and electron-dense pockets along the nuclear perimeter. Note the euchromatic nuclei in young (A-D; ‘M-nu’) and electron-dense ones in middle-aged and old animals (E-L; ‘M-nu’). In the cytoplasm and neighboring tracks of MPTP-injected young C57BL/6 J, mitochondria are long and tubular (B, ‘M’). Phagocytic microglia along with the engulfed cells are seen in young CD-1 (D, ‘EC’) and MPTP-injected old C57BL/6 J (J). Amoeboid “dark cells” are seen around the blood vessels in both strains (E, G and I; DC), they have thin rim of cytoplasm containing fewer organelles. The scale bars are specified for each micrograph
Interestingly, amoeboid “dark cells” were seen around blood vessels in both strains after middle age (Fig. 7E, G, and I; ‘DC’) and had thin rim of cytoplasm containing few organelles.
Thickening of the Vascular Basement Membrane
Blood vessels showed comparable membrane architecture in the young mice of both strains in control conditions as well as upon MPTP challenge (Fig. 8 A-D). At middle age, MPTP-injected C57BL/6 J showed a membrane discontinuity suggesting a possible breach (E vs. F, arrowheads) and thickening of basement membrane in C57BL/6 J (arrows, E vs. F) but not in CD-1 (F vs. H). At old age, MPTP caused thickening of vascular basement membranes in both strains (arrows, I vs. J; K vs. L). Macrophage-like cells (** I & J) were seen in the lumen in old C57BL/6 J. Dark cells (DC) were noted too.
Fig. 8.
MPTP affects vasculature at middle age in C57BL/6 J. Representative electron micrographs of blood vessels from different age groups and in response to MPTP. Note the thin basement membrane of the blood vessels in the young (A–D). MPTP-injected middle-aged C57BL/6 J show a discontinuous membrane suggesting a possible breach, (‘F,’ arrowheads) and thickening of basement membrane in C57BL/6 J (arrows, E vs. F) but not in CD-1 (F vs. H). Note that at old age, in response to MPTP, the basement membranes of the vessels appear thicker in both strains (arrows, I vs. J; K vs. L)
Discussion
Gender and MPTP
Many studies have earlier used both genders for MPTP-associated experiments; albeit with mixed results. Antzoulatos et al., (2010) suggested that small and chronic doses of MPTP induce subtle, sexually dimorphic motor deficiencies. Female mice showed a larger loss of dopamine, DOPAC (3,4-dihydroxyphenylacetic acid) and HVA (homovanillic acid) following MPTP injections. Similarly, in another study, MPTP caused a pronounced loss of both striatal and midbrain TH as well as striatal DAT alongside prominent gliosis suggesting that MPTP affects the female mice more severely due to compromised DAT levels (Ookubo et al. 2009). Other studies reported that estrogen was neuroprotective against MPTP- and 6-OHDA (6-hydroxydopamine)-induced depletion of dopaminergic neurons, striatal dopamine, and DA its metabolites (Kenchappa et al., 2004; Murray et al. 2003, Ramirez et al., 2003, Quesada and Micevych, 2004, Tripanichkul et al., 2006). A gender effect on DAT heterogeneity was noted in humans, with females having a higher heterogeneity in the striatum (Kuikka et al., 1999). Moreover, DAT-binding site density is higher in females in both animals and human studies (Morissette and Di Paolo, 1993, Lavalaye et al., 2000). Hanamsagar et al., (2017) suggested that higher sensitivity to inflammatory events in male microglia lead them to faster aging and enhance the risk of disorders.
Microglial density varies significantly in region-specific and spatiotemporal fashion. In early development, female hippocampus is microglial-dense, while it is amygdala in males. In the cortex, striatum, and cerebellum, both sexes map similar densities. At early adulthood, males show higher density in the cortex, hippocampus, and amygdala, although the striatum and cerebellum show no differences (Bordt et al., 2020). We used only male mice for our experiments in view of the male preponderance in the prevalence of PD (review by Georgiev et al., 2017).
The Baseline Number of Microglia and Astrocytes Show Minor Inter-Strain Differences
Microglia are selectively more populous in the hippocampus and SNpc (Lawson et al. 1990), the target regions of the common age-associated diseases like Alzheimer’s and Parkinson’s disease, respectively, endorsing their role in neurodegeneration and susceptibility. In aging mice, microglial priming is equated to an immune-vigilant state that results in their de-ramification, hypertrophy, increase in numbers, and exaggerated response to sub-threshold challenges. They synthesize ROS and pro-inflammatory cytokines like TNF-α, IL-6, IL-1β, while curtailing the synthesis of anti-inflammatory cytokines (Damani et al. 2011; Grabert et al. 2016). The presence of more microglia at middle age corroborates with the possibility of a pre-inflammed status and the presence of damage-associated molecular patterns (DAMPs) in-situ, thereby inflammasomes too may be vital in determining susceptibility. We found that normal C57BL/6 J mice have fewer substantia nigra neurons (Vidyadhara et al. 2017) and more baseline apoptosis (Yarreiphang et al., 2023) vis-à-vis the CD-1 mice. Neuronal apoptosis has been shown to flag the entry of microglial precursor cells into the zebra fish brain (Casano et al. 2016). Thus, just moderately more microglia (8–18%) in the control C57BL/6 J substantia nigra suggests a partial influence of numbers on susceptibility.
Aging selectively affects the already-sparse astrocytes in the substantia nigra, as against the other mesencephalic niche (Damier et al. 1996, 1999). Under physiological conditions, they secrete trophic factor GDNF (Grondin et al. 2003), therefore, the presence of moderately fewer astrocytes in C57BL/6 J (10–15% less than CD-1) and an acute increase in their numbers at middle age that persisted till old age suggest age-related reduction in neuroprotection and gliosis-assisted inflamm-aging. The latter could be linked to cytokines. However, the sedate increase in astrocytic numbers in CD-1 implicated graded changes in the milieu.
Higher TNF-α Expression in the Susceptible Strain may be a Steering Factor
DA neurons are sensitive to the pro-inflammatory cytokine TNF-α (Sriram et al. 2006), which is primarily secreted by microglia and in synchrony with IFN-γ/IL-1β induces neurodegeneration (Chao et al. 1995; Jeohn et al. 1998). Thereby, the dying neurons hold microglia in their cytotoxic state, to escalate synthesis of TNF-α leading to a self-perpetuating neuroinflammation. The higher levels of TNF-α released by the glia recruit their neighbors, to set off a vicious cycle, wherein the pro-inflammatory environment becomes self-propagating (Noh et al. 2014). Although only mildly higher in numbers, the persistently higher baseline TNF-α level through aging in C57BL/6 J suggest that in their substantia nigra, the microglia may be consistently primed, contributing to neuronal susceptibility. Elevated TNF-α levels are reported in mouse models as well as in autopsied brain tissue/CSF of PD patients (Mogi et al. 1994). Low levels of TNF-α are protective (Chertoff et al. 2011). Thus, the lower levels in CD-1 in addition to being a mark of low susceptibility may be potentially neuroprotective. Our auxiliary finding of TNF-α upregulation in middle-aged C57BL/6 J indicates that an imbalance in cytokine milieu precedes senescence.
IL-6, a pleiotropic cytokine, is upregulated in SN, CSF, and serum of PD patients (Blum-Degena et al. 1995; Hofmann et al. 2009). Conversely, Bolin et al., (2002) reported amplified MPTP susceptibility in IL-6−/− mice. In our study, since IL-6 followed a similar age-related expression pattern in both strains, it may not dictate baseline susceptibility. Yet, the differences following MPTP validate its role as a toxicity signal. The middle age demarcates a period of enhanced susceptibility. MPTP-induced IL-6 expression in old C57BL/6 J at later stages of the challenge suggests a link with astroglial responses or excessive neuronal death.
Reactive microglia in the degenerating SN overproduce IL-1β to activate astrocytes and promote iNOS secretion (Chhor et al. 2013). Chronic IL-1β expression in Wistar-rat substantia nigra induced progressive neurodegeneration, microgliosis, and motor disabilities (Ferrari et al. 2006). The acute increase in IL-1β at d1 post-MPTP that persists till d4 in the young C57BL/6 J vis-à-vis CD-1 endorses immediate microglial priming and activation in the former. Thus, mice differ in the immediacy or respondence indices. Moreover, noticeably higher basal TNF-α in C57BL/6 J at middle and old age underlines its role in basal susceptibility, aging, and neurodegeneration, whereas IL-6 and IL-1β appear to be weak responders to MPTP.
The Resistant Strain has Higher TGF-β Through Aging, but Lower IL-10 Levels at Middle Age
TGF-β1 inhibits microglial activation and protects DA neurons against MPTP-toxicity (Arimoto et al. 2007; Pintado et al. 2011). The low-baseline TGF-β expression in C57BL/6 J through aging and following MPTP suggest that this sensitive strain is ill equipped against neuroinflammation. Since CD-1 have more substantia nigra DA neurons and many of them resist MPTP (Vidyadhara et al. 2017, 2019), higher TGFβ1 levels allude to neuroprotection and DA neuronal survival through aging.
IL-4 protects DA neurons against MPP+ toxicity by up-regulating CD200, a microglial resting signal (Lyons et al. 2009). The moderate upsurge at d4 in MPTP-injected young CD-1, suggests an auxiliary course by the microglia (Hirsch and Hunot 2009). In view of its pronounced increase in basal levels of the MPTP-sensitive C57BL/6 J at middle age, it is likely that IL-4 may also have a pro-inflammatory role. Bok et al., (2018) while showing the microglia-specific expression of IL-4 demonstrated an LPS-induced upregulation in expression and rescue of substantia nigra DA neuronal loss by antibodies against IL-4.
IL-10 stimulates CD200 expression in neurons and induces astrocytes to synthesize anti-inflammatory TGF-β. It inhibits microglial synthesis of TNF-α, NO, and ROS, in-vitro, to neutralize oxidative stress (Balasingam and Yong 1996; Ledeboer et al. 2002). The upregulated baseline IL-10 expression in C57BL/6 J and upon MPTP at middle/old age may be a compensatory increase. However, these increases do not parallel a raise in TGF-β levels, hinting at a failed rescue attempt.
Thus, the noticeable increase in basal levels of both TNF-α and IL-6 as well as the anti-inflammatory IL-1β and IL-10 at middle age expound a hovering imbalance of pro- and anti-inflammatory cytokines, therefore tempting one to speculate that middle age imitates the prodromal period in the susceptible strain.
Strain-Specific Variability in Enzyme Responses
MAO-B inhibitors are promising candidates in the treatment of early-PD (Rabey et al. 2000). MAO-B level increases with age (Irwin et al. 1997) and is doubled in SN of PD patients (Damier et al. 1996). The age-associated upregulation in MAO-A and MAO-B levels in C57BL/6 J suggests a functional decline. The gradual age-related reduction in CD-1 may be a natural phenomenon that assists in combating MPTP-related stress. Although the reasons for higher basal level of MAO-A and MAO-B in young CD-1 are unclear, it may be due to higher number of neurons or DA terminals or it may be a bystander susceptibility marker. MPTP may cause a feed-forward effect on glia, triggering off a toxicity cycle.
HO-1 a cytoprotective, anti-apoptotic, and anti-inflammatory enzyme; down-regulates pro-inflammatory cytokines like TNF-α1 and IL-1β and up-regulates anti-inflammatory cytokine IL-10 in-vitro (Doré et al. 1999; Petrache et al. 2000). Predominantly expressed by astrocytes (Dwyer et al. 1995), it is positively correlated with aging and PD (Schipper et al. 1998). It was projected as a potential biomarker due to high levels in the patient saliva (Song et al. 2018). HO-1 overexpression tendered neuroprotection in MPP+ treated Parkinsonian rats via BDNF and GDNF (Hung et al. 2008). Thus, the gradual age-related increase in its levels in C57BL/6 J and in response to MPTP may indicate a cellular offset response to oxidative stress. Induction of iNOS causes NO release, which when protracted triggers oxidative damage in DA neurons (Nathan and Xie, 1994). The MPTP-induced up-scaling of iNOS in both strains supports this hypothesis.
The higher baseline iNOS and MAO-B levels alongside lower HO-1 levels in CD-1 are presently un-explained. Alternatively, these may be markers of sub-threshold susceptibility in them.
Neurons secrete the chemokine fractalkine (CX3CL1), to maintain microglia in resting state (Cardona et al. 2006). The inherently higher fractalkine levels in CD-1 indicate their healthy status. Age-related decrease in C57BL/6 J relays enhanced phagocytic signals, an indirect indicator of neuronal loss. Overexpression in response to MPTP in young mice implies the activation of compensatory responses during youth which decline with age.
Surviving Neurons Harbor Strain-Specific Ultrastructural Signatures
This is the first study on the alterations in the ultrastructure of mice substantia nigra with aging and in response to MPTP. The age-related and MPTP-induced shrinkage of mitochondria in C57BL/6 J validates the ensuing mitochondrial dysfunction and aging as a risk factor for PD. We earlier found higher DRP-1 levels in the lateral/ventral substantia nigra of C57BL/6 J, earmarking the inherent susceptibility of the mitochondria. On similar grounds, upregulation of mitochondrial fission protein dynamin-like protein 1 (DLP1/DRP-1) as well as down-regulation of fusion proteins Mfn1 and Mfn2 were noted in the substantia nigra of PD patients (Zhao et al. 2017). The relatively better-preserved mitochondrial structure with age and upon MPTP in CD-1 complements the higher HSD-10 (mitochondrial fusion-associated protein) expression (Seshadri and Alladi 2019). Elongated mitochondria are deft in energy generation (reviewed by Galloway et al., 2010) and calcium uptake (Lewis et al. 2018). In Caenorhabditis elegans, modulation of mitochondrial proteases SPG-7 and PPGN-1 enhanced mitofusion (Chaudhari and Kipreos 2017) to extend their overall survival. Thus enhanced HSD-10 expression (Seshadri and Alladi 2019) and elongated mitochondria in old CD-1 neurons may be the survival modalities, which may be lacking in C57BL/6 J.
Both fragmented and dilated ER are major pathological notations in A53TαS Tg mice substantia nigra (Colla et al. 2012) and rotenone model of sub-cutaneous administration (Zhang et al. 2017), suggesting ER dysfunction in PD. The MPTP-induced ER shortening in C57BL/6 J and its dilation in CD-1 suggests existence of strain-specific differences as well as different aspects of ER dysfunction in PD, which needs to be studied in detail. The presence of ER arrays in old CD-1 could be rejoinders of enhanced protein synthesis to compensate for the concurrent protein loss or mis-folding.
The presence of many intact Golgi units in the aged substantia nigra of both strains suggest a compensatory increase to circumvent age effects on protein packaging and post-translational processing. The “pearl necklace like globose” bloated saccules of Golgi units upon MPTP injection in old C57BL/6 J, hint at disease-induced functional impairment. Knockdown of adhesion proteins like GRASP 55/65 cause Golgi cisternae swelling (Lee et al. 2014). Simulation studies suggest that aberrations in biophysical properties like osmotic pressure, adsorption and adhesion energies, and precise vesicle addition frequency in addition to biological properties like rim stabilizer proteins cause abnormal self-organization into circular/fused Golgi complexes (Tachikawa and Mochizuki 2017). The presence of lysosomes in neurons of MPTP-injected young CD-1 that were visibly fewer in C57BL/6 J may suggest either the activation of lysosomal/UPR pathway or lysosomal accumulation due to impaired late endocytic pathway (Guerra et al. 2019). The apoptotic bodies in older C57BL/6 J, signal the occurrence of age-associated apoptosis. The crenelated nuclei in MPTP-injected old CD-1, suggest necroptosis as seen in striatal cells of a Huntington’s mouse (Turmaine et al. 2000). Thus, a majority of organelles were affected in C57BL/6 J both with aging and upon MPTP indicating affliction of associated cellular processes. Contrarily, elongation of mitochondria, preservation of Golgi apparatus and presence of lysosomes may be pointers of resilience in CD-1. Among the organellar defects, ER dilation is a sure sign of susceptibility in CD-1.
Elongation of Glial Mitochondria, a Distinctive Feature of Pathogenesis
In an interesting spin off, unlike the neurons, ultrastructure of glial organelles was preserved during aging and following MPTP. The glial mitochondria that were smaller and fewer in controls C57BL/6 J, appeared enlarged/elongated in response to MPTP. Hoekstra et al., (2015) showed a reduction in fission protein DLP1/DRP-1 in both neurons and astrocytes in PD cortex along with fused elongated mitochondria in primary cortical astrocytes transfected with DLP1-siRNA. Co-culturing them with cortical neurons caused neuronal atrophy and excessive calcium release; suggesting neurotoxic effect of fused astrocytic mitochondria. In a stroke model, the penumbral astrocytes displayed hypertrophic and polarized processes; elongated mitochondria and lost their neuroprotective ability (Fiebig et al. 2019). Overexpression of mutant ubiquitin (UBB + 1) protected astrocytes from oxidative stress and H2O2-induced cell death by destabilizing mitochondrial fission-specific proteins, leading to mitochondrial fusion (Yim et al. 2014). Although the exact corollaries are not clear, the combination of astrogliosis and increased pro-inflammatory cytokines; tempts one to speculate that elongated mitochondria assist astroglial propagation into neurodegenerative sequels.
Mitochondrial elongation in microglia of MPTP-injected young C57BL/6 J mice, may too have neurodegenerative consequences. LPS-activated mouse cerebral microglia, stimulate DRP-1 and ROS synthesis in-vitro, to elongate tract-borne mitochondria (Katoh et al. 2017). The dilated Golgi apparatus in microglia indicate that while aggravating neuroinflammation, the microglial protein packaging process is also affected. Under chronic stress, in aging and in AD; microglia had condensed, electron-dense cytoplasm and nucleoplasm which imparted a striking “dark” appearance (Bisht et al. 2016). Dark cells noted in both strains from middle age, may have similar pathological objective as described earlier in aging and Alzheimer’s disease (St-Pierre et al., 2022). In most cells, the cytoplasm appeared as a thin rim, reducing the scope to visualize other organelles.
Our findings of mitochondrial elongation may have clinical implications. For instance, the antioxidant coenzyme Q10 (CoQ10) supports mitochondrial function while reducing the DA neuron loss in an animal model of PD (Spindler et al. 2009), yet it failed in the phase III clinical trials (The Parkinson Study Group QE3 Investigators 2014). Similarly, CoQ10 derivative MitoQ also failed (Snow et al. 2010). It is likely that these molecules stabilized the glial mitochondria too, thereby nullifying the neuronal outcome. It is vital to study the glial mitochondrial responses in isolation and on a temporal scale, to better understand the phenomenon.
Earlier Age at Onset of MPTP-Induced Basement Membrane Features in C57BL/6 J
MPTP caused basement membrane thickening similar to that reported in PD (Farkas et al. 2000). The presence of gaps in the middle-aged C57BL/6 J and old CD-1 implies that the blood–brain barrier (BBB) is vulnerable earlier in life in C57BL/6 J, an additional signal of negative impact of aging or on the gut microbiota (Montagne et al. 2015) or of inherent susceptibility.
Broader Views
The role of glia in basal ganglia disorders has been reviewed earlier (Bhaduri and Alladi 2022). Most of the studies confirm the involvement of single pathways in PD pathogenesis; only a few have compared mechanisms of resilience or susceptibility through aging. Classical studies demonstrated that ibotenate, another neurotoxin causing PD-like symptoms, induces gliogenic effects through a fourfold increase in GFAP mRNA (Rataboul et al. 1989). The reactive astrocytes release an otherwise neuron-specific protein clusterin, involved in neuronal apoptosis (Pasinetti and Finch 1991), which can cause ballooning of DA axons, synapse loss, vacuolation of cytoplasm, and dendrites (Danik et al. 1993; Smith et al. 1990). Over time, the astrocytes within the vicinity develop inclusions. Ibotenate-induced cortical ablation upregulated the striatal GFAP, depleted DA by 50% and reduced the catalytic action of TH (Pasinetti et al. 1991).
GFAP knockout mice have higher GDNF levels and are resilient to quinolinic acid or 3-nitroproprionic acid-induced damage, as seen by lower metabolic insult, and an increase in striatal projection neurons (Hanbury et al. 2003). Astrocytes provide limited neuroprotection via GDNF synthesis following a degenerative insult (Yarreiphang et al., 2023). Via a novel glial pathway, MPP+ and 6-OHDA disturb the activation of TRPC3 to exacerbate neurotoxicity (Streifel et al. 2014). The pro-inflammatory cytokines TNF-α, IL-6, etc. released by the microglia are promulgated to influence the activity of 6-OHDA in rat models of PD by depleting BDNF, leading to neuronal apoptosis (Nagatsu and Sawada. 2005). Unilateral 6-OHDA lesions, initiated astrogliosis in the aged rat nigrostriatal system, along with excessive release of gliotrophic factors and heightened glial hypersensitivity (Gordon et al. 1997). Contrarily, Pasinetti et al. (1999) showed that 6-OHDA-injected aged F344 rats show no changes in GFAP. These differences may be species associated. Thus, while earlier studies address glia-associated singular mechanisms at a particular age period, however, our current study surveyed additional pertinent mechanisms in two distinct mice strains through aging, and provided an encompassing understanding of PD pathogenesis.
Conclusion
In summary, neuro-glial interactions, senescence-related changes in glia, cytokine levels, etc. are vital determinants of neuronal survival and differ greatly in the MPTP-resistant C57BL/6 J and MPTP-susceptible CD-1 white mice strain. The intended use of male animals for the study may be a limiting factor, however, in view of the male preponderance of the disease, our observations provide vital clues of disease pathogenesis. Besides, female mice are also known to show higher fatality in response to MPTP, due to differences in peripheral metabolism of MPTP, independent of its effects on dopaminergic neurons. It is also pertinent to compare all these factors between male and female animals to better understand the premise for neuroprotection in females.
Our findings in general may be extrapolated to different human populations that are either vulnerable or resistant to PD. For instance, in our study, the CD-1 may represent Asian-Indians who have inherently lower prevalence rate than the Caucasians. The prominent differences in pro- and anti-inflammatory cytokine levels at middle age suggest that by design, the middle-age milieu is acquiescent to neurodegeneration and may well be the critical soft period for the onset of neurodegenerative diseases. It is likely that senescence may result from differences in the in-situ inflammasomes, which merit detailed investigations. The pathogenesis is effectively assisted by the diabolic differences between the neuronal and glial mitochondria. Thus, glia are major players in aging and disease and may explain the ethnic bias in prevalence of PD.
Acknowledgements
We thank Dr. M.M. Srinivas Bharath, Head, Department of Clinical Psychopharmacology and Neurotoxicology; Dr. Gayathri N, Department of Neuropathology for laboratory facilities.
Abbreviations
- MPTP
1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine
- BBB
Blood–brain barrier
- BSA
Bovine serum albumin
- CoQ10
Coenzyme Q10
- D1
Day 1 post-MPTP
- D4
Day 4 post-MPTP
- D7
Day 7 post-MPTP
- DAMP
Damage-associated molecular patterns
- DLP1/DRP-1
Dynamin-like protein 1
- ELISA
Enzyme-linked immunosorbent assay
- ER
Endoplasmic reticulum
- GFAP
Glial Fibrillary Acidic Protein
- H2O2
Hydrogen peroxide
- HO-1
Hemeoxygenase-1
- Iba-1
Ionized calcium-binding adaptor protein 1
- IFN-γ
Interferon-γ
- IL-1β
Interleukin-1β
- iNOS
Inducible nitric oxide synthase
- Ir
Immunoreactive
- MAO-A
Monoamine oxidase A
- MAO-B
Monoamine oxidase B
- MPP+
1-Methyl-4-phenylpyridinium
- PD
Parkinson’s disease
- PVDF
Polyvinylidene difluoride
- RT
Room temperature
- SNpc
Substantia nigra pars compacta
- TMB
3,3’,5,5’-Tetramethylbenzidine
- TNF-α
Tumor necrosis factor- α
Author Contributions
APL contributed to study design, performed experiments, and collected data and provided 1st draft; UB performed experiments and collected data; MP performed statistical analysis of the data; RSK & BKCS performed electron microscopy and data analysis; TRR and BMK contributed to data analysis and revision of MS; PAA conceptualized the study, performed data analysis, edited the manuscript, and obtained funds.
Funding
The study was funded by DBT (No.BT/PR12518/MED/30/1462/2014) to PAA. APL was a UGC SRF.
Data Availability
The raw datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
None of the authors have any conflict of interest.
Ethics Approval
All the experimental protocols on mice were approved by the Institutional Biosafety committee and Institutional (NIMHANS) Animal Ethics Committee, in accordance with the guidelines of the CPCSEA, India, and NIH, USA.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas MM, Xu Z, Tan LCS (2017) Epidemiology of parkinson’s disease-east versus west. Mov Disord Clin Pract 5(1):14–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi Rastegar D, Ho N, Halliday GM, Dzamko N (2019) Parkinson’s progression prediction using machine learning and serum cytokines. NPJ Parkinsons Dis 25(5):14. 10.1038/s41531-019-0086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alladi PA, Mahadevan A, Yasha TC, Raju TR, Shankar SK, Muthane U (2009) Absence of age-related changes in nigral dopaminergic neurons of Asian Indians: relevance to lower incidence of Parkinson’s. Neuroscience 159(1):236–245. 10.1016/j.neuroscience.2008.11.051 [DOI] [PubMed] [Google Scholar]
- Alladi PA, Mahadevan A, Shankar SK, Raju TR, Muthane U (2010a) Expression of GDNF receptors GFRalpha1 and RET is preserved in substantia nigra pars compacta of aging Asian Indians. J Chem Neuroanat 40(1):43–52. 10.1016/j.jchemneu.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Alladi PA, Mahadevan A, Vijayalakshmi K, Muthane U, Shankar SK, Raju TR (2010b) Ageing enhances alpha-synuclein, ubiquitin and endoplasmic reticular stress protein expression in the nigral neurons of Asian Indians. Neurochem Int 5:530–539. 10.1016/j.neuint.2010.06.018 [DOI] [PubMed] [Google Scholar]
- Antzoulatos E, Jakowec MW, Petzinger GM, Wood RI (2010) Sex differences in motor behavior in the MPTP mouse model of Parkinson’s disease. Pharmacol Biochem Behav 95(4):466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimoto T, Choi DY, Lu X, Liu M, Nguyen XV, Zheng N, Bing G (2007) Interleukin-10 protects against inflammation-mediated degeneration of dopaminergic neurons in substantia nigra. Neurobiol Aging 28(6):894–906. 10.1016/j.neurobiolaging.2006.04.011 [DOI] [PubMed] [Google Scholar]
- Balasingam V, Yong VW (1996) Attenuation of astroglial reactivity by interleukin-10. J Neurosci 16(9):2945–2955. 10.1523/jneurosci.16-09-02945.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass JJ, Wilkinson DJ, Rankin D, Phillips BE, Szewczyk NJ, Smith K, Atherton PJ (2017) An overview of technical considerations for Western blotting applications to physiological research. Scand J Med Sci Sports 27(1):4–25. 10.1111/sms.12702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington Clinical, morphological and neurochemical correlations. J Neurol Sci 20(4):415–455. 10.1016/0022-510X(73)90175-5 [DOI] [PubMed] [Google Scholar]
- Bhaduri B, Alladi PA (2022) Glial cells are key orchestrators of neural degeneration in basal ganglia disorders in glial biology: role of Glia in health and disease. In: Tandon PN, Seth P, Patro N, Patro I (eds) The biology of Glial cells: recent advances. Springer-Nature. 10.1007/978-981-16-8313-8_15
- Bisht K, Sharma KP, Lecours C, Gabriela Sánchez M, El Hajj H, Milior G, Tremblay MÈ (2016) Dark microglia: a new phenotype predominantly associated with pathological states. Glia 64(5):826–839. 10.1002/glia.22966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum-Degena D, Müller T, Kuhn W, Gerlach M, Przuntek H, Riederer P (1995) Interleukin-1β and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 202(1–2):17–20. 10.1016/0304-3940(95)12192-7 [DOI] [PubMed] [Google Scholar]
- Boje KM, Arora PK (1992) Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res 587(2):250–256. 10.1016/0006-8993(92)91004-X [DOI] [PubMed] [Google Scholar]
- Bok E, Cho EJ, Chung ES, Shin WH, Jin BK (2018) Interleukin-4 contributes to degeneration of dopamine neurons in the lipopolysaccharidetreated substantia nigra in vivo. Exp Neurobiol 27(4):309–319. 10.5607/en.2018.27.4.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC (1994) Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci Lett 172(1–2):151–154. 10.1016/0304-3940(94)90684-X [DOI] [PubMed] [Google Scholar]
- Bolin LM, Strycharska-Orczyk I, Murray R, Langston JW, Di Monte D (2002) Increased vulnerability of dopaminergic neurons in MPTP-lesioned interleukin-6 deficient mice. J Neurochem 83(1):167–175. 10.1046/j.1471-4159.2002.01131.x [DOI] [PubMed] [Google Scholar]
- Bordt EA, Ceasrine AM, Bilbo SD (2020) Microglia and sexual differentiation of the developing brain: a focus on ontogeny and intrinsic factors. Glia 68(6):1085–1099. 10.1002/glia.23753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrajo A, Rodriguez-Perez AI, Diaz-Ruiz C, Guerra MJ, Labandeira-Garcia JL (2014) Microglial TNF-α mediates enhancement of dopaminergic degeneration by brain angiotensin. Glia 62(1):145–157. 10.1002/glia.22595. (PMID: 24272709) [DOI] [PubMed] [Google Scholar]
- Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S (2009) Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 119(1):182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Ransohoff RM (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9(7):917–924. 10.1038/nn1715 [DOI] [PubMed] [Google Scholar]
- Casano AM, Albert M, Peri F (2016) Developmental apoptosis mediates entry and positioning of microglia in the zebrafish brain. Cell Rep 16(4):897–906. 10.1016/j.celrep.2016.06.033 [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu SX, Ehrlich L, Peterson PK (1995) Interleukin-1 and tumor necrosis factor-α synergistically mediate neurotoxicity: Involvement of nitric oxide and of n-methyl-d-aspartate receptors. Brain Behav Immun 9(4):355–365. 10.1006/brbi.1995.1033 [DOI] [PubMed] [Google Scholar]
- Chaudhari SN, Kipreos ET (2017) Increased mitochondrial fusion allows the survival of older animals in diverse C Elegans longevity pathways. Nat Commun 8(1):182. 10.1038/s41467-017-00274-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, Healy DG, Schapira AHV (2006) Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5(3):235–245. 10.1016/S1474-4422(06)70373-8 [DOI] [PubMed] [Google Scholar]
- Chertoff M, Di Paolo N, Schoeneberg A, Depino A, Ferrari C, Wurst W, Pitossi F (2011) Neuroprotective and neurodegenerative effects of the chronic expression of tumor necrosis factor α in the nigrostriatal dopaminergic circuit of adult mice. Exper Neurol 227(2):237–251. 10.1016/j.expneurol.2010.11.010 [DOI] [PubMed] [Google Scholar]
- Chhor V, Le Charpentier T, Lebon S, Oré MV, Celador IL, Josserand J, Fleiss B (2013) Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia In vitro. Brain, Behav, Immun 32:75–80. 10.1016/j.bbi.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SJ, Yoo HS, Oh JS, Kim JS, Ye BS, Sohn YH, Lee PH (2018) Effect of striatal dopamine depletion on cognition in de novo Parkinson’s disease. Parkinsonism Relat Disord 51:43–48. 10.1016/j.parkreldis.2018.02.048. (Epub 2018 Mar 2 PMID: 29526657) [DOI] [PubMed] [Google Scholar]
- Colla E, Coune P, Liu Y, Pletnikova O, Troncoso JC, Iwatsubo T, Lee MK (2012) Endoplasmic reticulum stress is important for the manifestations of α-synucleinopathy in vivo. J Neurosci 32(10):3306–3320. 10.1523/JNEUROSCI.5367-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisier E, Graeber MB (2006) Glial degeneration and reactive gliosis in alpha-synucleinopathies: the emerging concept of primary gliodegeneration. Acta Neuropathol 112(5):517–530. 10.1007/s00401-006-0119-z [DOI] [PubMed] [Google Scholar]
- Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT (2011) Age-related alterations in the dynamic behavior of microglia. Aging Cell 10(2):263–276. 10.1111/j.1474-9726.2010.00660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P, Kastner A, Agid Y, Hirsch EC (1996) Does monoamine oxidase type B play a role in dopaminergic nerve cell death in Parkinson’s disease? Neurology 46(5):1262–1269. 10.1212/wnl.46.5.1262 [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122(8):1437–1448. 10.1093/brain/122.8.1437 [DOI] [PubMed] [Google Scholar]
- Danik M, Chabot J-G, Hassan-gonzalez D, Suh M, Quirion R (1993) Localization of sulfated glycoprotein-2/clusterin mRNA in the rat brain by in situ hybridization. J Comp Neurol 334:209–227. 10.1002/cne.903340205 [DOI] [PubMed] [Google Scholar]
- Doré S, Takahashi M, Ferris CD, Hester LD, Guastella D, Snyder SH (1999) Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Nat Acad Sci USA 96(5):2445–2450. 10.1073/pnas.96.5.2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta SK, Verma S, Jain V, Surapaneni BK, Vinayek R, Phillips L, Nair PP (2019) Parkinson’s disease: the emerging role of gut dysbiosis, antibiotics, probiotics, and fecal microbiota transplantation. J Neurogastro Motil 25(3):363–376. 10.5056/jnm19044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer BE, Nishimura RN, Lu SY (1995) Differential expression of heme oxygenase-1 in cultured cortical neurons and astrocytes determined by the aid of a new heme oxygenase antibody. Response to oxidative stress. Mol Brain Res 30(1):37–47. 10.1016/0169-328X(94)00273-H [DOI] [PubMed] [Google Scholar]
- Farkas E, De Jong GI, de Vos RA, Jansen Steur EN, Luiten PG (2000) Pathological features of cerebral cortical capillaries are doubled in Alzheimer’s disease and Parkinson’s disease. Acta Neuropathol 100(4):395–402 [DOI] [PubMed] [Google Scholar]
- Ferrari CC, Pott Godoy MC, Tarelli R, Chertoff M, Depino AM, Pitossi FJ (2006) Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1β in the substantia nigra. Neurobiol Dis 24(1):183–193. 10.1016/j.nbd.2006.06.013 [DOI] [PubMed] [Google Scholar]
- Fiebig C, Keiner S, Ebert B, Schäffner I, Jagasia R, Lie DC, Beckervordersandforth R (2019) Mitochondrial dysfunction in astrocytes impairs the generation of reactive astrocytes and enhances neuronal cell death in the cortex upon photothrombotic lesion. Front Mol Neurosci. 10.3389/fnmol.2019.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2006) Inflamm-aging: an evolutionary perspective on immunosenescence. Ann New York Acad Sci. 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- Galiano-Landeira J, Torra A, Vila M, Bové J (2020) CD8 T cell nigral infiltration precedes synucleinopathy in early stages of Parkinson’s disease. Brain 143(12):3717–3733. 10.1093/brain/awaa269. (PMID: 33118032) [DOI] [PubMed] [Google Scholar]
- Georgiev D, Hamberg K, Hariz M, Forsgren L, Hariz GM (2017) Gender differences in Parkinson’s disease: a clinical perspective. Acta Neurol Scand 136(6):570–584. 10.1111/ane.12796. (Epub 2017 Jul 2 PMID: 28670681) [DOI] [PubMed] [Google Scholar]
- Gilat M, Bell PT, Ehgoetz Martens KA, Georgiades MJ, Hall JM, Walton CC, Lewis SJG, Shine JM (2017) Dopamine depletion impairs gait automaticity by altering cortico-striatal and cerebellar processing in Parkinson’s disease. Neuroimage 15(152):207–220. 10.1016/j.neuroimage.2017.02.073. (Epub 2017 Mar 3 PMID: 28263926) [DOI] [PubMed] [Google Scholar]
- Gordon MN, Schreier WA, Ou X, Holcomb LA, Morgan DG (1997) Exaggerated astrocyte reactivity after nigrostriatal deafferentation in the aged rat. J Comp Neurol 388(1):106–119 (PMID: 9364241) [PubMed] [Google Scholar]
- Gourie-Devi M (2014) Epidemiology of neurological disorders in India: review of background, prevalence and incidence of epilepsy, stroke Parkinson’s disease and tremors. Neurol India 62(6):588–598. 10.4103/0028-3886.149365 [DOI] [PubMed] [Google Scholar]
- Grabert K, Michoel T, Karavolos MH, Clohisey S, Kenneth Baillie J, Stevens MP, McColl BW (2016) Microglial brain regionâ ’dependent diversity and selective regional sensitivities to aging. Nat Neurosci 19(3):504–516. 10.1038/nn.4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin R, Cass WA, Zhang Z, Stanford JA, Gash DM, Gerhardt GA (2003) Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J Neurosci 23(5):1974–1980. 10.1523/jneurosci.23-05-01974.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra F, Girolimetti G, Beli R, Mitruccio M, Pacelli C, Ferretta A, Gasparre G, Cocco T, Bucci C (2019) Synergistic effect of mitochondrial and lysosomal dysfunction in Parkinson’s disease. Cells. 10.3390/cells8050452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, Alter MD, Block CS, Sullivan H, Bolton JL, Bilbo SD (2017) Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 65(9):1504–1520. 10.1002/glia.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanbury R, Ling ZD, Wuu J, Kordower JH (2003) GFAP knockout mice have increased levels of GDNF that protect striatal neurons from metabolic and excitotoxic insults. J Comp Neurol 461:307–316. 10.1002/cne.10667 [DOI] [PubMed] [Google Scholar]
- Harms AS, Delic V, Thome AD, Bryant N, Liu Z, Chandra S, Jurkuvenaite A, West AB (2017) α-Synuclein fibrils recruit peripheral immune cells in the rat brain prior to neurodegeneration. Acta Neuropathol Commun 5(1):85. 10.1186/s40478-017-0494-9.PMID:29162163;PMCID:PMC5698965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashioka S, Klegeris A, Schwab C, McGeer PL (2009) Interferon-gamma-dependent cytotoxic activation of human astrocytes and astrocytoma cells. Neurobiol Aging 30(12):1924–1935. 10.1016/j.neurobiolaging.2008.02.019. (Epub 2008 Mar 28 PMID: 18375019) [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8(4):382–397. 10.1016/S1474-4422(09)70062-6 [DOI] [PubMed] [Google Scholar]
- Hoekstra JG, Cook TJ, Stewart T, Mattison H, Dreisbach MT, Hoffer ZS, Zhang J (2015) Astrocytic dynamin-like protein 1 regulates neuronal protection against excitotoxicity in Parkinson disease. Am J Pathol 185(2):536–549. 10.1016/j.ajpath.2014.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann KW, Schuh AFS, Saute J, Townsend R, Fricke D, Leke R, Rieder CRM (2009) Interleukin-6 serum levels in patients with parkinson’s disease. Neurochem Res 34(8):1401–1404. 10.1007/s11064-009-9921-z [DOI] [PubMed] [Google Scholar]
- Hung SY, Liou HC, Kang KH, Wu RM, Wen CC, Fu WM (2008) Overexpression of heme oxygenase-1 protects dopaminergic neurons against 1-methyl-4-phenylpyridinium-induced neurotoxicity. Mol Pharmacol 74(6):1564–1575. 10.1124/mol.108.048611 [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y (2003) Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol 106(6):518–526. 10.1007/s00401-003-0766-2. (Epub 2003 Sep 25 PMID: 14513261) [DOI] [PubMed] [Google Scholar]
- Irwin I, Delanney L, Chan P, Sandy MS, Monte DAD, Langston JW (1997) Nigrostriatal monoamine oxidase A and B in aging squirrel monkeys and C57BL/6 mice. Neurobiology of Aging Mar-Apr 18(2):235–241. 10.1016/S0197-4580(97)00003-1 [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S (2007) Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc 2(1):141–151. 10.1038/nprot.2006.342 [DOI] [PubMed] [Google Scholar]
- Jeohn GH, Kong LY, Wilson B, Hudson P, Hong JS (1998) Synergistic neurotoxic effects of combined treatments with cytokines in murine primary mixed neuron/glia cultures. J Neuroimmunol 85(1):1–10. 10.1016/S0165-5728(97)00204-X [DOI] [PubMed] [Google Scholar]
- Joers V, Tansey MG, Mulas G, Carta AR (2017) Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog Neurobiol 155:57–75. 10.1016/j.pneurobio.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyothi HJ, Vidyadhara DJ, Mahadevan A, Philip M, Parmar SK, Manohari SG, Alladi PA (2015) Aging causes morphological alterations in astrocytes and microglia in human substantia nigra pars compacta. Neurobiol Aging 36(12):3321–3333. 10.1016/j.neurobiolaging.2015.08.024 [DOI] [PubMed] [Google Scholar]
- Katoh M, Wu B, Nguyen HB, Thai TQ, Yamasaki R, Lu H, Ohno N (2017) Polymorphic regulation of mitochondrial fission and fusion modifies phenotypes of microglia in neuroinflammation. Sci Rep 7(1):4942. 10.1038/s41598-017-05232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchappa RS, Diwakar L, Annepu J, Ravindranath V (2004) Estrogen and neuroprotection: higher constitutive expression of glutaredoxin in female mice offers protection against MPTP-mediated neurodegeneration. FASEB J 18(10):1102–1104. 10.1096/fj.03-1075fje. (Epub 2004 May 7 PMID: 15132975) [DOI] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS (2000) Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci 20(16):6309–6316. 10.1523/jneurosci.20-16-06309.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohutnicka M, Lewandowska E, Kurkowska-Jastrzȩbska I, Członkowski A, Członkowska A (1998) Microglial and astrocytic involvement in a murine model of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Immunopharmacol 39(3):167–180. 10.1016/S0162-3109(98)00022-8 [DOI] [PubMed] [Google Scholar]
- Kuikka JT, Tupala E, Bergström KA, Hiltunen J, Tiihonen J (1999) Iodine-123 labelled PE2I for dopamine transporter imaging: influence of age in healthy subjects. Eur J Nucl Med 26(11):1486–1488. 10.1007/s002590050483. (PMID: 10552092) [DOI] [PubMed] [Google Scholar]
- Lavalaye J, Booij J, Reneman L, Habraken JB, van Royen EA (2000) Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med 27(7):867–869. 10.1007/s002590000279. (PMID: 10952500) [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39(1):151–170. 10.1016/0306-4522(90)90229-W [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Brevé JJP, Wierinckx A, Van Der Jagt S, Bristow AF, Leysen JE, Van Dam AM (2002) Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur J Neurosci 16(7):1175–1185. 10.1046/j.1460-9568.2002.02200.x [DOI] [PubMed] [Google Scholar]
- Lee I, Tiwari N, Dunlop MH, Graham M, Liu X, Rothman JE (2014) Membrane adhesion dictates Golgi stacking and cisternal morphology. Proc Nat Acad Sci USA 111(5):1849–1854. 10.1073/pnas.1323895111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Kwon SK, Lee A, Shaw R, Polleux F (2018) MFF-dependent mitochondrial fission regulates presynaptic release and axon branching by limiting axonal mitochondria size. Nat Commun. 10.1038/s41467-018-07416-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, McQuillan K, Deighan BF, O’Reilly JA, Downer EJ, Murphy AC, Lynch MA (2009) Decreased neuronal CD200 expression in IL-4-deficient mice results in increased neuroinflammation in response to lipopolysaccharide. Brain, Behav, Immun 23(7):1020–1027. 10.1016/j.bbi.2009.05.060 [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the: Substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38(8):1285–1291. 10.1212/wnl.38.8.1285 [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T (1994) Tumor necrosis factor-α (TNF-α) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett 165(1–2):208–210. 10.1016/0304-3940(94)90746-3 [DOI] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Zlokovic BV (2015) Blood-Brain barrier breakdown in the aging human hippocampus. Neuron 85(2):296–302. 10.1016/j.neuron.2014.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette M, Di Paolo T (1993) Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology 58(1):16–22. 10.1159/000126507. (PMID: 8264850) [DOI] [PubMed] [Google Scholar]
- Murray HE, Pillai AV, McArthur SR, Razvi N, Datla KP, Dexter DT, Gillies GE (2003) Dose- and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: differential actions of estrogen in males and females. Neuroscience 116(1):213–222. 10.1016/s0306-4522(02)00578-x. (PMID: 12535954) [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M (2005) Inflammatory process in Parkinson’s disease: role for cytokines. Curr Pharm des 11:999–1016. 10.2174/1381612053381620 [DOI] [PubMed] [Google Scholar]
- Nathan C, Xie Q-W (1994) Nitric oxide synthases: Roles, tolls, and controls. Cell 78(6):915–918. 10.1016/0092-8674(94)90266-6 [DOI] [PubMed] [Google Scholar]
- Noh H, Jeon J, Seo H (2014) Systemic injection of LPS induces region-specific neuroinflammation and mitochondrial dysfunction in normal mouse brain. Neurochem Int 69:35–40. 10.1016/j.neuint.2014.02.008 [DOI] [PubMed] [Google Scholar]
- Ookubo M, Yokoyama H, Kato H, Araki T (2009) Gender differences on MPTP (1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine) neurotoxicity in C57BL/6 mice. Mol Cell Endocrinol 311(1–2):62–68 [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Finch CE (1991) Sulfated glycoprotein-2 (SGP-2) mRNA is expressed in rat striatal astrocytes following ibotenic acid lesions. Neurosci Lett 130:1–4. 10.1016/0304-3940(91)90213-D [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Morgan DG, Finch CE (1991) Disappearance of GAD-mRNA and tyrosine hydroxylase in substantia nigra following striatal ibotenic acid lesions: evidence for transneuronal regression. Exp Neurol 112:131–139. 10.1016/0014-4886(91)90063-I [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Hassler M, Stone D, Finch CE (1999) Glial gene expression during aging in rat striatum and in long-term responses to 6-OHDA lesions. Synapse 31:278–284. 10.1002/(SICI)1098-2396(19990315)31:4%3c278::AID-SYN5%3e3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- Paxinos and Franklin's the mouse brain in stereotaxic coordinates 2013. George Paxinos, Keith B.J. Franklin Eds. 4th ed. Amsterdam : Boston : Elsevier/Academic Press
- Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AMK (2000) Heme oxygenase-1 inhibits TNF-α-induced apoptosis in cultured fibroblasts. Am J Physiol - Lung Cell Mol Physiol 278(2):L312–L319. 10.1152/ajplung.2000.278.2.l312 [DOI] [PubMed] [Google Scholar]
- Pintado C, Revilla E, Vizuete ML, Jiménez S, García-Cuervo L, Vitorica J, Castaño A (2011) Regional difference in inflammatory response to LPS-injection in the brain: Role of microglia cell density. J Neuroimmunol 238(1–2):44–51. 10.1016/j.jneuroim.2011.06.017 [DOI] [PubMed] [Google Scholar]
- Quesada A, Micevych PE (2004) Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res 75(1):107–116 [DOI] [PubMed] [Google Scholar]
- Rabey JM, Sagi I, Huberman M, Melamed E, Korczyn A, Giladi N, Inzelberg R, Djaldetti R, Klein C, Berecz G (2000) Rasagiline mesylate, a new MAO-B inhibitor for the treatment of Parkinson’s disease: a double-blind study as adjunctive therapy to levodopa. Clin Neuropharmacol 23(6):324–330. 10.1097/00002826-200011000-00005 [DOI] [PubMed] [Google Scholar]
- Ramirez AD, Liu X, Menniti FS (2003) Repeated estradiol treatment prevents MPTP-induced dopamine depletion in male mice. Neuroendocrinology 77(4):223–231. 10.1159/000070277. (PMID: 12766322) [DOI] [PubMed] [Google Scholar]
- Rataboul P, Vernier P, Biguet NF, Mallet J, Poulat P, Privat A (1989) Modulation of GFAP mRNA levels following toxic lesions in the basal ganglia of the rat. Brain Res. Bull Spinal Cord-Recent Work Trauma Rec 22:155–161. 10.1016/0361-9230(89)90140-8 [DOI] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Onofrj M (2009) Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun 23(1):55–63. 10.1016/j.bbi.2008.07.003. (Epub 2008 Jul 17 PMID: 18678243) [DOI] [PubMed] [Google Scholar]
- Rydbirk R, Elfving B, Folke J, Pakkenberg B, Winge K, Brudek T, Aznar S (2019) Increased prefrontal cortex interleukin-2 protein levels and shift in the peripheral T cell population in progressive supranuclear palsy patients. Sci Rep 9(1):7781. 10.1038/s41598-019-44234-y.PMID:31123295;PMCID:PMC6533275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Tyrrell RM (2000) The heme synthesis and degradation pathways: role in oxidant sensitivity Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 28(2):289–309 [DOI] [PubMed] [Google Scholar]
- Schipper HM, Liberman A, Stopa EG (1998) Neural heme oxygenase-1 expression in idiopathic Parkinson’s disease. Exper Neurol 150(1):60–68. 10.1006/exnr.1997.6752 [DOI] [PubMed] [Google Scholar]
- Seo J, Park J, Kim K, Won J, Yeo HG, Jin YB, Koo BS, Lim KS, Jeong KJ, Kang P, Lee HY, Choi WS, Baek SH, Jeon CY, Hong JJ, Huh JW, Kim YH, Park SJ, Kim SU, Lee DS, Lee SR, Lee Y (2020) Chronic Infiltration of T Lymphocytes into the Brain in a Non-human Primate Model of Parkinson’s Disease. Neuroscience 1(431):73–85. 10.1016/j.neuroscience.2020.01.043. (Epub 2020 Feb 7 PMID: 32036014) [DOI] [PubMed] [Google Scholar]
- Seshadri A, Alladi PA (2019) Divergent expression patterns of Drp1 and HSD10 in the nigro-striatum of two mice strains based on their MPTP susceptibility. Neurotoxicity Res 36(1):27–38. 10.1007/s12640-019-00036-8 [DOI] [PubMed] [Google Scholar]
- Shruthi S, Sumitha R, Varghese AM, Ashok S, Chandrasekhar Sagar BK, Sathyaprabha TN, Nalini A, Kramer BW, Raju TR, Vijayalakshmi K, Alladi PA (2017) Brain-derived neurotrophic factor facilitates functional recovery from ALS-cerebral spinal fluid-induced neurodegenerative changes in the NSC-34 motor neuron cell line. Neurodegener Dis 17(1):44–58. 10.1159/000447559. (Epub 2016 Sep 13 PMID: 27617773) [DOI] [PubMed] [Google Scholar]
- Smeyne M, Goloubeva O, Smeyne RJ (2001) Strain-dependent susceptibility to MPTP and MPP+-induced parkinsonism is determined by glia. Glia 34(2):73–80. 10.1002/glia.1042 [PubMed] [Google Scholar]
- Smith GM, Rutishauser U, Silver J, Miller RH (1990) Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev Biol 138:377–390. 10.1016/0012-1606(90)90204-V [DOI] [PubMed] [Google Scholar]
- Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Taylor KM (2010) A double-blind, placebo-controlled study to assess the mitochondria- targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord 25(11):1670–1674. 10.1002/mds.23148 [DOI] [PubMed] [Google Scholar]
- Song W, Kothari V, Velly AM, Cressatti M, Liberman A, Gornitsky M, Schipper HM (2018) Evaluation of salivary heme oxygenase-1 as a potential biomarker of early Parkinson’s disease. Mov Disord 33(4):583–591. 10.1002/mds.27328 [DOI] [PubMed] [Google Scholar]
- Soreq L, Rose J, Soreq E, Hardy J, Trabzuni D, Cookson MR, Ule J (2017) Major shifts in glial regional identity are a transcriptional hallmark of human brain aging. Cell Rep 18(2):557–570. 10.1016/j.celrep.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler M, Flint Beal M, Henchcliffe C (2009) Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatric Dis Treat 5:597–610. 10.2147/ndt.s5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Miller DB, O’Callaghan JP (2006) Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: Role of tumor necrosis factor-α. J Neurochemis 96(3):706–718. 10.1111/j.1471-4159.2005.03566.x [DOI] [PubMed] [Google Scholar]
- St-Pierre MK, Carrier M, González Ibáñez F, Šimončičová E, Wallman MJ, Vallières L, Parent M, Tremblay MÈ (2022) Ultrastructural characterization of dark microglia during aging in a mouse model of Alzheimer’s disease pathology and in human post-mortem brain samples. J Neuroinflammat 19(1):235. 10.1186/s12974-022-02595-8.PMID:36167544;PMCID:PMC9513936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streifel KM, Gonzales AL, Miranda BD, Mouneimne R, Earley S, Tjalkens R (2014) Dopaminergic neurotoxicants cause biphasic inhibition of purinergic calcium signaling in astrocytes. PLoS ONE 9:e110996. 10.1371/journal.pone.0110996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland D, Bertoni JM (2004) Parkinson’s prevalence estimated by a state registry. Mov Disord 19(3):318–323. 10.1002/mds.10619 [DOI] [PubMed] [Google Scholar]
- Subbarayan MS, Hudson C, Moss LD, Nash KR, Bickford PC (2020) T cell infiltration and upregulation of MHCII in microglia leads to accelerated neuronal loss in an α-synuclein rat model of Parkinson’s disease. J Neuroinflammation 17(1):242. 10.1186/s12974-020-01911-4.PMID:32799878;PMCID:PMC7429710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachikawa M, Mochizuki A (2017) Golgi apparatus self-organizes into the characteristic shape via postmitotic reassembly dynamics. Proc Nat Acad Sci USA 114(20):5177–5182. 10.1073/pnas.1619264114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome AD, Standaert DG, Harms AS (2010) Fractalkine signaling regulates the inflammatory response in an alpha-synuclein model of Parkinson disease. PLoS ONE 10(10):e0140566. 10.1371/journal.pone.0140566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Rathitharan G, Meyer J, Furukawa Y, Ang LC, Boileau I, Guttman M, Hornykiewicz O, Kish SJ (2017) Brain monoamine oxidase B and A in human parkinsonian dopamine deficiency disorders. Brain 140(9):2460–2474. 10.1093/brain/awx172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripanichkul W, Sripanichkulchai K, Finkelstein DI (2006) Estrogen down-regulates glial activation in male mice following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxication. Brain Res 1084(1):28–37. 10.1016/j.brainres.2006.02.029. (Epub 2006 Mar 27 PMID: 16564034) [DOI] [PubMed] [Google Scholar]
- Turmaine M, Raza A, Mahal A, Mangiarini L, Bates GP, Davies SW (2000) Nonapoptotic neurodegeneration in a transgenic mouse model of Huntington’s disease. Proc Nat Acad Sci USA 97(14):8093–8097. 10.1073/pnas.110078997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM (2003) Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 157(11):1015–1022 [DOI] [PubMed] [Google Scholar]
- Vidyadhara DJ, Yarreiphang H, Raju TR, Alladi PA (2017) Admixing of MPTP-resistant and susceptible mice strains augments nigrostriatal neuronal correlates to resist MPTP-induced neurodegeneration. Mol Neurobiol 54(8):6148–6162. 10.1007/s12035-016-0158-y [DOI] [PubMed] [Google Scholar]
- Vidyadhara DJ, Sasidharan A, Kutty BM, Raju TR, Alladi PA (2019) Admixing MPTP-resistant and MPTP-vulnerable mice enhances striatal field potentials and calbindin-D28K expression to avert motor behaviour deficits. Behav Brain Res 360:216–227. 10.1016/j.bbr.2018.12.015. (Epub 2018 Dec 7) [DOI] [PubMed] [Google Scholar]
- Williams GP, Schonhoff AM, Jurkuvenaite A, Thome AD, Standaert DG, Harms AS (2018) Targeting of the class II transactivator attenuates inflammation and neurodegeneration in an alpha-synuclein model of Parkinson’s disease. J Neuroinflammat 15(1):244. 10.1186/s12974-018-1286-2.PMID:30165873;PMCID:PMC6117927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J (2011) Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol 26(1):S1-58. 10.1007/s10654-011-9581-6 [DOI] [PubMed] [Google Scholar]
- Yarreiphang H, Vidyadhara DJ, Nambisan AK, Raju TR, Sagar BKC, Alladi PA (2023) Apoptotic factors and mitochondrial complexes assist determination of strain-specific susceptibility of mice to parkinsonian neurotoxin MPTP. Mol Neurobiol 60(8):4778–4794. 10.1007/s12035-023-03372-1. (Epub 2023 May 10 PMID: 37162724) [DOI] [PubMed] [Google Scholar]
- Yim N, Ryu SW, Han EC, Yoon J, Choi K, Choi C (2014) Mutant ubiquitin UBB+1 induces mitochondrial fusion by destabilizing mitochondrial fission-specific proteins and confers resistance to oxidative stress-induced cell death in astrocytic cells. PLoS ONE 9(6):e99937. 10.1371/journal.pone.0099937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Du L, Zhang W, Yang Y, Zhou Q, Du G (2017) Therapeutic effects of baicalein on rotenone-induced Parkinson’s disease through protecting mitochondrial function and biogenesis. Sci Rep. 10.1038/s41598-017-07442-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Wang W, Wang C, Siedlak SL, Fujioka H, Tang B, Zhu X (1863) (2017) Mfn2 protects dopaminergic neurons exposed to paraquat both in vitro and in vivo: implications for idiopathic Parkinson’s disease. Biochim Biophys Acta Mol Basis Dis 6:1359–1370. 10.1016/j.bbadis.2017.02.016. (Epub 2017 Feb 16) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.