Abstract
Nerve signaling within the tumor microenvironment (TME) plays a critical role in the initiation, progression, and metastasis of solid tumors. Due to their highly responsive behavior and activation upon injury and cancer onset, this review specifically focuses on how sympathetic nerves rewire the TME. Within tumors, sympathetic nerves closely interact with various TME components, and their combined signaling often shifts tumor-intrinsic physiology toward tumor-supportive phenotypes. In turn, the TME components, such as myeloid cells, lymphoid cells, extracellular matrix (ECM), endothelial cells, cancer associated fibroblasts (CAFs), and Schwann cells, secrete neurotrophic and axon guidance factors that influence both sympathetic outgrowth and tumor cell behavior, further exacerbating tumor progression and metastasis. Here, we review the current evidence on the multidirectional impacts of sympathetic nerves and both immune and non-immune TME components, the nature of these communication processes, and how exploring these interactions may inform future therapeutics to impair cancer progression and metastasis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10555-025-10241-x.
Keywords: Cancer neuroscience, Tumor microenvironment, Neuro-immune axis, Sympathetic signaling
Introduction: sympathetic nerves in cancers
Tumor-infiltrating nerves were first described in 1897 [1], but substantial scientific investigation into their functions only gained momentum at the turn of the twenty-first century, driven by innovations in imaging technologies. This led to the establishment of cancer neuroscience as a field in 2020 [2]. Many studies have highlighted the critical interactions between intratumor nerves and cancer cells and, more recently, their complex interactions with distinct components of the tumor microenvironment (TME) (Fig. 1).
Fig. 1.
Schematic representation of the tumor microenvironment (TME) with a focus on sympathetic nerve interactions. The diverse cell types and extracellular components that interact within the TME, contributing to cancer progression and neurotrophic signaling, are shown in this figure. Sympathetic nerves (shown in yellow) grow into solid tumors and release neurotransmitters such as norepinephrine (NE), neuropeptide Y (NPY), and adenosine triphosphate (ATP) [7], which influence the surrounding cellular and stromal landscape. Tumors containing enhanced sympathetic innervation and neurotransmitters are typically associated with poor prognosis [8–17]. The interaction between cancer cells and sympathetic nerves contributes to enhanced cell viability, migration, and invasion [4–6], as well as transcriptional changes that affect cancer proliferation and metastasis [18]. Pathways activated include RAF/MEF/ERK, MAPK, CaMK, and AKT [19]. The overall feedback loop also exacerbates tumor angiogenesis and neurogenesis [21]. Intratumor sympathetic interactions modulate immune cell populations (top purple category), including myeloid and lymphoid cells and cancer cells, promoting tumor growth and immune evasion. Additionally, non-immune stromal elements (bottom green category), such as extracellular matrix (ECM), endothelial cells, cancer-associated fibroblasts (CAFs), and Schwann cells, contribute to structural and biochemical support within the TME. Cancer-induced stress amplifies this adrenergic signaling, resulting in altered behavior and signaling of not only cancer cells but also many other immune and non-immune stromal cells within the TME [18, 19]. Understanding these complex interactions can provide insights into targeting sympathetic nerve signaling for therapeutic interventions in cancer
Sympathetic nerves, key mediators of the stress response, have long been associated with carcinogenesis. In normal homeostasis, sympathetic nerves regulate organ development and patterning, injury response, and the physiologic “fight or flight” response. Beyond maintaining homeostasis, sympathetic activation leads to local and systemic upregulation of adrenergic neurotransmitters [3], which have been shown to impact tumor growth and metastasis. Mechanistically, norepinephrine, which binds with high affinity to α- and β-adrenergic receptors [4] expressed on cancer cells, activates downstream signaling pathways such as the RAF/MEK/ERK, MAPK, CaMK, and AKT, driving cancer cell proliferation, migration, and invasion [5–7].
Intratumor sympathetic innervation is prevalent and is typically an adverse prognostic feature in various peripheral cancers, including head-and-neck [8], lung [9], breast [10], gastrointestinal [4], hepatocellular [11], ovarian [12], cervical [13], colorectal [14], pancreatic [15], and prostate cancers [16]. Nerve density increases in the early development of multiple cancer types and is proposed to accumulate through both neurogenesis and axogenesis [17]. The nerve density in the neurovascular bundle of prostate cancer patients and the total area of ganglia in each radical prostatectomy is significantly higher than that in patients without prostate cancer [17]. Two predominant types of neural structures typically observed in the TME are neuronal bundles and neuronal fibers, i.e., axons (Fig. 2). Neuronal bundles comprised of clusters of nerve fibers within and around tumors have historically received significant attention due to their macroscopic visibility. Various types of intratumor innervation have been reported in cancer, including sensory, parasympathetic (cholinergic), and sympathetic (adrenergic) innervation, based on the expression of established cholinergic markers vesicular acetylcholine transporter (VAChT) and choline O-acetyltransferase (ChAT), and adrenergic markers such as tyrosine hydroxylase (TH) and dopamine beta-hydroxylase (DBH) (Fig. 2) [2]. Three-dimensional imaging combined with advanced image segmentation algorithms offers promising tools to profile the proteomic landscape of individual tumor-innervating nerve fibers [18]. The distinct neurotransmitters produced by each nerve and their unique interactions within the TME underscore the complexity of tumor innervation and the intricate role of neuro-signaling in cancer progression.
Fig. 2.
Representative immunofluorescent images of intratumor sympathetic nerve bundles and fibers in pancreatic cancer (left) and prostate cancer (right). The top two images display sympathetic nerve bundles, stained with sympathetic marker tyrosine hydroxylase (TH) (in red), located near pancreatic and prostate tumor cells, which are marked with pan-cytokeratin (pan-CK) (in white). The bottom two images portray representative images of intratumor sympathetic nerve fibers. Nuclei are marked with DAPI (in blue). The remaining unstained areas consist of stromal cells marked by asterisks. These human pancreatic ductal adenocarcinoma and prostate tumor sections were obtained under an IRB-approved protocol and co-stained, using immunohistochemistry, with validated antibodies [anti-tyrosine hydroxylase (EMD Millipore, Ref: AB152), pan-cytokeratin pan type I/II antibody cocktail (AE1/AE3) (Invitrogen, Ref: MA5-13,156)]. These images were captured at 20X with a Zeiss Microscope ApoTome3 and processed and optimized with Zeiss Zen software. In both types of cancer, nerve bundles and fibers are found in close association with tumor and stromal cells, highlighting the spatial organization of nerve-tumor interactions within these distinct microenvironments. Scale bars: 100 µm
Recent studies have further highlighted the role of beta-adrenergic signaling in the TME, where stress-induced norepinephrine alters the function of immune and non-immune stromal cells, promoting angiogenesis or T cell exhaustion [19, 20]. For instance, in prostate cancer, tumor innervating nerves in high-grade prostate tumors show expression of neuronal adhesion molecule Neuroligin 1 (NLGN1), a postsynaptic marker that can be cleaved and released into the TME, which can regulate a diverse array of stromal cell populations, including endothelial and immune cells [21].
These recent studies highlight the critical need for a deeper understanding of how sympathetic nerves interact with immune and non-immune cells within the TME. Defining these interactions and their functional outcomes will provide valuable insights into the role of sympathetic signaling in cancer initiation, progression, and metastasis. This knowledge holds the potential to identify novel therapeutic targets with significant translational value. This review examines current evidence on the complex crosstalk between sympathetic nerves and stromal cells, highlighting their collective contribution to cancer progression and metastasis.
The Crosstalk between sympathetic neurons and immune cells: myeloid and lymphoid cells
The sympathetic nervous system plays nuanced roles in regulating innate and adaptive immune responses. Fundamental catecholamines such as dopamine, norepinephrine, and epinephrine can influence hematopoiesis [22]; however, the direct effect of nerve-immune crosstalk in the TME requires further clarification and investigation. Here, we delve into the multidirectional signaling that occurs between both myeloid and lymphoid cells and in sympathetic nerves (Fig. 3).
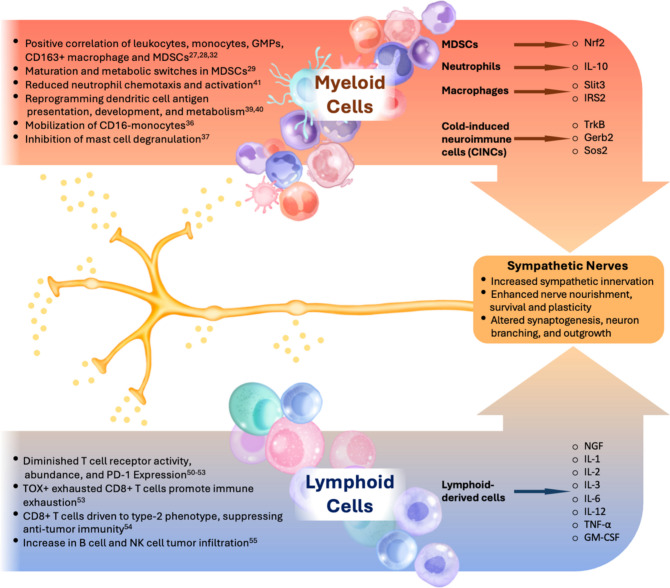
Fig. 3.
Overview of sympathetic nerve interactions with immune cell populations in the TME. Sympathetic nerves modulate the behavior of myeloid and lymphoid cells through neurotransmitter release, leading to varied immunomodulatory effects. In the myeloid cell compartment (orange box, top left), sympathetic signaling enhances the activity of leukocytes, monocytes, granulocyte-monocyte progenitors (GMPs), macrophages, and myeloid-derived suppressor cells (MDSCs), supporting immunosuppressive functions [27, 28, 32]. Furthermore, adrenergic signaling causes the maturation and a metabolic switch in MDSCs [29], reduces neutrophil chemotaxis [41], reprograms dendritic cells [39, 40], mobilizes monocytes [36], and inhibits mast cell degranulation [37]. In contrast, lymphoid cells (blue box, bottom left) show diminished T cell receptor activity and a reduced CD8 + T cell response, with sympathetic nerves further regulating cytokine production, impacting immune tolerance. Adrenergic signaling also drives TOX + exhausted CD8 + T cells immune exhaustion and CD8 + T cells to a type 2 phenotype. B cells and NK cells are encouraged to infiltrate tumors through adrenergic signaling. Key secreted factors and cytokines associated with these interactions are indicated for each immune cell type
Myeloid
Adrenergic influence on myeloid progenitors and immune suppression
Increased plasma norepinephrine in patients and mouse models was found to be positively correlated with higher total leukocyte and inflammatory monocyte prevalence, as well as an enhancement of granulocyte-monocyte progenitors (GMPs) [23]. Blocking the β2-adrenergic receptor reduced splenic GMP proliferation and myelopoiesis [23]. The adrenergic receptors were also shown to play a critical immunosuppressive role by modulating the abundance and behavior of myeloid-derived suppressor cells (MDSCs). A study revealed that disruption of -adrenergic signaling caused MDSC accumulation, resulting in an immunosuppressive TME [24]. The authors functionally attribute this effect to the significantly higher affinity of norepinephrine to -adrenoceptors as compared with β-adrenoceptors, thus resulting in -adrenergic receptor binding, which supports myeloid maturation. Norepinephrine is more likely to engage with the lower affinity β-adrenergic receptors under high levels of norepinephrine, leading to immunosuppressive effects on myeloid cells [24]. Recent findings reveal that the activation of β2-adrenoreceptors leads to metabolic reprogramming in MDSC cells [25]. Through the oxidative stress nuclear factor, erythroid 2–related factor 2 (Nrf2), downstream STAT3 signaling was upregulated, decreasing cell-intrinsic apoptotic pathways and ATP generation [25]. These culminating findings suggest that an increase in local norepinephrine levels can shift the immune TME toward a tumor-suppressive phenotype.
Macrophage polarization and tumor growth
Sympathectomy experiments resulted in the enhanced infiltration of CD163 + M2 macrophages, inhibiting T-cells and promoting tumor growth [26]. In breast cancer, β2-adrenergic signaling was found to regulate macrophage recruitment within tumors, enhancing tumor growth and metastatic spread [27]. Furthermore, adrenergic signaling in ovarian carcinoma enhanced both monocyte and macrophage tumor infiltration and was significantly associated with reduced survival [28]. During lung injury, β2-adrenergic receptors can promote a shift to the anti-inflammatory M2 macrophage phenotype [29], and chronic activation of β2-adrenergic receptors dampens macrophage-mediated inflammation, supporting the widespread immunosuppressive potential of adrenergic signaling [30].
Sympathetic regulation of mast cells, dendritic cells, and neutrophils
Mast cell inflammatory IL-6 cytokine secretion is induced by NGF, causing upregulated prostaglandin D2 expression [31]. This study further indicates that mast cells may be contributing to an inflammatory response through enhanced NGF expression due to injury or another stimulus, such as cancer. In other studies, β-adrenergic signaling was shown to enhance the mobilization of CD16-monocytes [32], but this same signaling also inhibited mast cell degranulation [33], reducing their immunogenic response. In high-grade prostate cancer, recent findings reveal that mast cells exhibit a significantly higher spatial association with nerves compared to low-grade tumors, suggesting that the spatial organization of myeloid cells changes in response to nerve innervation [34].
β2-adrenergic signaling was also shown to reprogram dendritic cell antigen presentation, development, and metabolism [35, 36]. For instance, the activation of the β2-adrenergic receptor reduced CD11c + dendritic cell development, and when this signaling was abrogated, metabolic reprogramming resulted in upregulation of the mammalian target of rapamycin (mTOR) and downregulation of STAT3, leading to an immunogenic response [36].
Norepinephrine treatment of neutrophils in vivo has been shown to modulate the secretion of several cytokines and chemokines, including increased IL-6 expression and reduced INF-y and IL-10 levels, thereby slowing neutrophil chemotaxis and activation [37]. A recent study revealed that stress could induce a pro-metastatic TME through changes in the behavior and signaling of neutrophils. Neutrophils treated with a glucocorticoid agonist experienced increased secretion of IL-10, resulting in suppressed T cell activation [38]. The authors also demonstrated that the formation of neutrophil extracellular traps is associated with stress, demonstrating another mechanism by which adrenergic activation can exacerbate breast cancer metastasis [38]. Altogether, these studies reveal the complexities of the norepinephrine-adrenergic receptor interactions in distinct myeloid cell populations that promote cancer growth through immune evasion.
Bidirectional communication between myeloid cells and sympathetic nerves
Recent evidence suggests that granulocyte colony-stimulating factor (G-CSF), a signaling molecule that facilitates the movement of hematopoietic progenitor stem cells in circulation, promotes adrenergic innervation and prostate cancer progression [39]. Furthermore, M2-like macrophages secrete SLIT3, which binds to the ROBO1 receptor on sympathetic neurons to trigger the protein kinase A (PKA) signaling pathway, resulting in increased release of norepinephrine, as well as enhanced sympathetic nerve growth [40]. In the context of obesity, macrophages that lack insulin receptor substrate 2 (IRS2) become more anti-inflammatory and also support an increase in sympathetic innervation by extracting catecholamines [41]. Adipose tissue macrophages are in a dynamic association with nerve bundles, and these macrophages can potentially facilitate nerve survival and plasticity in cancer. Moreover, macrophages can mediate angiogenesis, increase Schwann cell motility, remodel the ECM, and interact with T and B lymphocytes during nerve repair [42]. Further evidence indicates the ability of sympathetic neuron–associated macrophages (SAMs) to uptake and degrade extracellular norepinephrine, regulating localized adrenergic signaling [43]. It was reported that cold-induced neuroimmune cells (CINCs) harbor brain-derived growth factor (BDNF) and can nourish neurite survival and outgrowth. CINCS can induce a phenotypic switch in SAMs, abrogating their sympathetic-reducing state [42]. Further, RNA-sequencing reveals that CINCs could be regulating other potential growth factors, including TrkB, growth factor receptor bound protein 2 (Gerb2), and SOS Ras/Rho guanine nucleotide exchange factor 2 (Sos2), in addition to several synaptogenesis and neuron outgrowth and branching genes [42]. These recent studies highlight the multidirectional circuits linking the sympathetic nervous system, myeloid cells, and cancer cells, underscoring the need for systemic approaches to map these spatial associations and mechanistic studies to unravel the directionality and functional consequences of each interaction.
Lymphoid
Sympathetic signaling also influences the lymphoid immune response, most prominently T-lymphocytes, in addition to B-lymphocytes and natural killer (NK) cells. Studies show persistent innervation of lymph nodes, exploring potential spatial relationships between lymphoid cells and nerve fibers [44]. β2-adrenergic receptors are prominent on both T and B lymphocytes, and the granular understanding of their functions is still growing [45].
Sympathetic regulation of T cell exhaustion
Norepinephrine signaling through the β2-adrenergic receptor on T cells can diminish T cell receptor activity and, therefore, CD8 + T antiviral response due to decreased tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ) regulation [46]. Upon norepinephrine treatment, T cells can alter secretion of other cytokines, such as interleukin (IL)−6, C–C motif chemokine ligand 2 (CCL2), and C-X-C motif chemokine ligand 1 and 3 (CXCL1, CXCL3) [47]. Adrenergic signaling exerts a stronger inhibitory effect on memory CD8 + T cells compared with naïve CD8 + T cells and CD4 + T cells, likely due to the upregulation of β-adrenoceptors in CD8 + T cells, making them more sensitive to adrenergic signals [47, 48]. Studies blocking the β-adrenergic receptor found an increase in the frequency of intra-tumor CD8 + T cells [49]. These infiltrating T cells suppressed the anti-tumor immune response and decreased programmed cell death protein 1 (PD-1) expression, posing a potential limitation in treatment response [49]. TOX + exhausted CD8 + T cells (TEX) localized near intratumor sympathetic nerves were found to upregulate β-adrenergic receptors. They also found that these β-adrenergic receptors colocalized with several exhaustion markers, including PD-1, TOX, CD39, EOMES, and TBET. Altogether, the authors reason that the upregulation of β-adrenergic receptors on these T cells drives the exhaustion phenotype, reducing T cell activity in the tumor. Other studies reveal that the binding of catecholamines to CD4 + T cells favors the differentiation towards the type-2 immune response phenotype, thus preventing a cytotoxic anti-tumor response [50].
B-lymphocytes and NK cells
Β-adrenergic signaling can also be central to the response of B lymphocytes and NK cells. Inhibition of both β-adrenergic signaling and cyclooxygenase 2 (COX-2) increases B cell tumor infiltration, dampening metastasis-promoting transcription factors, including GATA binding protein 1 and 2 (GATA-1, GATA-2), early-growth-response-3 (EGR3), GRE and STAT3 [51]. In addition, blocking the β-adrenergic receptor also increases circulating NK cells that express CD11a, which could alter the attachment of NK cells to the ECM [36].
Feedback between sympathetic nerves and lymphocytes
Lymphocytes can modulate neuron growth and innervation by secreting NGF. Neurons cocultured with cells isolated from the thymus resulted in more outgrowth of spleen-derived cells, suggesting that mature T cells may also regulate neuronal outgrowth via NGF secretion [52]. Interestingly, a similar coculture setup using sympathetic nerves and thymus cells showed a significant reduction in TH mRNA expression, an enzyme critical for sympathetic catecholamine biosynthesis [53]. Similarly, mRNA coding for NPY was also reduced in response to splenocyte stimulation, suggesting that lymphocytes can alter the expression of sympathetic neurotransmitters [53]. In addition to NGF, cytokines secreted by lymphoid cells such as interleukin IL-1, IL-2, IL-3, IL-6, IL-12, TNF-α, and granulocyte–macrophage colony-stimulating factor (GM-CSF) can stimulate sympathetic nerve growth [54], establishing a positive feedback loop between the two systems.
The crosstalk between sympathetic neurons and non-immune stroma: extracellular matrix (ECM), endothelial cells, cancer-associated fibroblasts (CAFs) and Schwann cells
Tumor-innervating nerves directly interact with the non-immune stroma, including ECM, endothelial cells, CAFs, and Schwann cells. Collectively, these supporting stromal elements exacerbate the growth and progression of primary tumors [55]. However, each type of cancer exhibits a distinct composition of stromal heterogeneity, leading to differing tumor-promoting effects and treatment responses. The understanding of the crosstalk between tumor cells and stroma has accelerated over the years; however, the context-dependent manner in which nerves, and more specifically sympathetic nerves, coordinate with the non-immune stroma to contribute to tumor progression remains understudied [56]. Here, we describe the current studies that evaluate the signaling between ECM, endothelial cells, CAFs, Schwann cells, and sympathetic nerves and how these interactions may exacerbate cancer progression (Fig. 4).
Fig. 4.
The multidirectional crosstalk between sympathetic nerves and the non-immune stromal cells: The TME is composed of various non-immune cell types and ECM that collectively contribute to neuronal development, stability, and invasion within the tumor niche. ECM components (top left green arrow), such as collagens, elastins, laminins, and glycosaminoglycans, provide structural and biomechanical substrates for stabilizing nerve growth and guiding axonal growth [62]. Endothelial cells (red arrow) release neurotrophic factors (e.g., VEGF, NETS, SLIT/ROBO UNC5, and Coa6) which support sympathetic nerve development, homeostasis, survival, and repair response [70–74]. CAFs (purple arrow) secrete molecules, such as NGF, IL-6, and NRG1, that promote neural invasion and repair [88–91]. Schwann cells (blue arrow) form tumor-activated Schwann cell tracks (TASTs) and activate TGF-β and YAP/TAZ pathways, which support cancer metastasis along nerves, known as perineural invasion [101–103]. Bolded components such as VEGF, NETs, NGF, and YAP1, among others, are highlighted as potential targets for therapeutic intervention
Extracellular matrix (ECM)
During organ development and other regulatory physiological responses, ECM glycoproteins and endothelial cells support and manage peripheral nerve growth. ECM is vital in providing structural support to enhance cellular adhesion, survival, proliferation, and migration, especially during an injury response [57]. Collagens and elastins are the most prominent ECM-associated proteins, in addition to other proteins, including laminin, fibronectin, vitronectin, tenascin, proteoglycans, and glycosaminoglycans [57]. ECM deposition and structures are often significantly altered during injury, inflammatory states, or cancers [57]. This acutely altered ECM contributes to reversing the healing response, which further exacerbates cancer growth and survival [59, 60]. Furthermore, the upregulation of ECM-associated proteins may be intimately linked to enhanced tumor innervation, as nerves favorably utilize ECM to direct axon outgrowth. For example, collagens, laminins, and fibronectins provide scaffolding and axon guidance cues, which can facilitate axon sprouting, growth, and navigation [58]. Furthermore, ECM poses biomechanical forces by altering surface topography, electrical properties, and stiffness, modulating sympathetic nerve growth and guidance throughout solid tumors [59]. A recent study highlights transcriptional program differences in cancer cells within the perineural space, which shows a marked reduction in collagen fiber thickness [60]. The authors propose that the decrease in ECM stiffness within the perineural space, as opposed to the enhanced stiffness around the neuronal bundles, may facilitate cancer cell plasticity and adaptation to the intraneural environment [60]. This finding underscores a potential mechanism by which the ECM remodeling could directly support perineural invasion, promoting metastatic progression.
Endothelial cells
It is well established that endothelial cells and neurons rely on each other throughout development, as well as for growth, maintenance, and injury repair [61]. Organized into neurovascular bundles, blood vessels supply nerves with the necessary oxygen and nutrients, while nerves, in turn, regulate vascular tone and blood flow. Furthermore, as sprouting and branching capillaries navigate the microenvironment, they rely on spatial guidance cues similar to those used in peripheral nerve axon branching, including shared molecular pathways and signaling molecules [61]. Common endothelial and pericyte cues, such as vascular endothelial growth factor (VEGF), netrins (NETs), and platelet-derived growth factor-B (PDGFB) [62], influence both nerve growth and cancer progression. VEGF, a critical neurotrophic factor, supports neuronal survival by stabilizing neuronal microtubes and promoting vascularization [63]. To regulate neuronal growth, VEGF competes with the axon guidance semaphorin (SEMA) family proteins to bind neuropilin (NRP) receptors. Notably, VEGF-NRP1 binding enhances sympathetic nerve sprouting in the myocardium [64]. Overexpression of NRP1 in endothelial cells has also been linked to endothelial-to-mesenchymal transition, promoting pancreatic cancer cell growth and fibrosis [65]. Additional axon guidance molecules which are frequently upregulated in solid tumors, including SLIT/ROBO [66] and NETs [67], and UNC5 [68], contribute to both angiogenesis and axon guidance. The neurotrophic receptor TRKA on endothelial cells further promotes neuro-vascular alignment and sympathetic nerve sprouting in thermogenic adipose tissue, with implications for regulating tumor metabolism [69]. In prostate cancer, β2-adrenergic activation has been shown to induce an angio-metabolic switch [19], where deletion of β-adrenergic receptors in endothelial cells led to increased vascularity and oxidative phosphorylation, marked by heightened mitochondrial cytochrome C oxidase assembly factor (Coa6) [19]. These studies show how this essential homeostatic relationship between endothelial cells and nerves is coopted during tumor formation.
Cancer-associated fibroblasts (CAFs)
CAFs are a heterogeneous group of cells that can adopt either tumor-supportive or tumor-suppressive phenotypes. A subset of CAFs, originating from resident stellate cells in both hepatic and pancreatic cancers [70], was originally discovered with the autonomic neuron-marking method of gold chloride [71] and was therefore thought to be of neural crest origin [72]. However, subsequent studies did not support this finding [73]. Nonetheless, the reason why stellate cells express several neuronal markers, including fibrillary acid protein (GFAP) [74] and nestin (P75) [71], remains unclear.
CAFs contribute substantially to tumor desmoplasia, a process often correlated with neural invasion [75]. This underscores the need to better understand the complex interactions between CAFs, nerves, and cancer cells, which collectively contribute to tumor progression and metastasis. However, the influence of sympathetic nerves on CAF phenotypes and their role in cancer progression remains controversial. For example, one in vitro study found no significant changes in tumor cell proliferation or collagen expression following norepinephrine treatment despite its clear effects on calcium spikes and proinflammatory responses in isolated hepatic stellate cells through activation of the nuclear factor kappa B (NF-kB) signaling [76]. Conversely, another study showed that norepinephrine, in conjunction with NPY, promoted the proliferation of activated myofibroblastic hepatic stellate cells, with accompanying morphological changes and altered collagen gene expression [77]. This proliferative response was later shown to be mediated through p38 MAPK, phosphoinositide 3‐kinase (PI3K), and MEK signaling pathways [78]. These contrasting findings suggest that sympathetic nerve signaling can modulate CAF phenotypes, with effects that likely depend on the specific context of the TME.
Mechanisms of CAF, sympathetic nerve, and cancer interactions
A key mechanism through which CAFs influence cancer progression is by releasing nerve growth factors and axon guidance molecules, which facilitate neural growth within the TME and accelerate tumor spread. CAFs are prominent sources of NGF, a pan-neuronal neurotrophin that has also been closely linked to increased cancer cell proliferation and migration [79, 80]. NGF binds with high-affinity to TRKA receptors on tumor cells, activating the PI3K/AKT pathway, which in turn activates glycogen synthase kinase-3 (GSK-3), leading to increased cell survival, proliferation, and motility [80, 81], particularly in pancreatic and head-and-neck cancers.
In prostate cancer, crosstalk between Trk receptors and the androgen receptor (AR) has been shown to facilitate interactions between CAFs, nerves, and cancer cells [81]. Notably, recent studies have uncovered a mechanism in which CAFs enhance the expression of transcriptional regulator yes-associated protein 1 (YAP1), which activates TEA domain family member 1 (TEAD1) signaling. This signaling cascade promotes further NGF secretion from CAFs, supporting nerve growth and remodeling within the TME [82]. The elevated NGF levels, in turn, drive further metastatic behavior, known as perineural invasion. Neural-derived CCL2, a chemokine secreted by nerve cells, binds to C–C motif chemokine receptor 2 (CCR2) on prostate cancer cells. This interaction activates PI3K/AKT signaling and induces EMT, thereby enhancing the metastatic potential of cancer cells. Overall, this study reveals a feedback loop in which CAFs induce YAP1 overexpression in prostate cancer cells, leading to increased NGF and CCL2 secretion, further promoting nerve growth and metastatic spread [82]. Importantly, recent findings in colorectal cancer provide additional support for the role of YAP signaling in tumor progression, showing that activation of β2-adrenergic receptors can mediate YAP signaling. Furthermore, the use of Trk inhibitors in these models effectively abrogated the tumor-promoting effects of this pathway [14]. Collectively, these studies underscore the therapeutic potential of targeting nerve-stromal interactions, specifically through the inhibition of NGF-TrkA signaling, YAP1/TEAD1 pathways, and adrenergic receptor-mediated crosstalk among more quickly emerging interactions, as a strategy to disrupt cancer progression and improve patient outcomes.
CAFs express axon guidance molecules
CAFs express axon guidance and neuro-modulating cues that contribute to tumor progression by guiding axon growth and branching, as well as influencing cancer cell tumor-promoting phenotypes. Axon guidance cue, SLIT2, is produced by CAFs in a ROBO1 dependent manner, which activates NIMA-related kinase 9 (NEK9), resulting in cytoskeletal reorganization and an enhanced gastric cancer metastatic potential [83]. In pancreatic cancer tissue coculture systems, another common axon guidance cue, netrin G1 (NetG1), is overexpressed by CAFs and was shown to support tumor growth by significantly increasing glutamate/glutamine metabolism in cancer cells. In vivo NetG1 knockout models showed that reducing glutamate/glutamine lessened the activation and function of NK cells, leading to a more immunosuppressive environment, further demonstrating the importance of elucidating the complex interplay between immune, stromal, and neuronal communications in the context of cancer [84]. Activated CAFs were shown to upregulate netrin-1 (NET1) and Unc-5 netrin receptor B (UNC5B), enhancing both colon and lung cancer cell stemness [85]. Interfering with the NET1 signaling by pharmacologically inhibiting NET1 reduced CAF expression of IL-6 and decreased both stemness markers and clonogenicity of the cancer cells, further supporting a mechanism that can lead to disease progression [85]. In prostate cancer, antiandrogen therapy upregulates the secretion of neuregulin 1 (NRG1) in CAFs, which plays a major role in neuroprotection during axon injury and Schwann cell migration [86]. NRG1 was subsequently shown to activate human epidermal growth factor receptor 3 (HER3) signaling in prostate cancer cells, significantly enhancing cancer cell survival and proliferation. Blocking the NRG1-HER3 signaling axis completely reverses this phenotype, further validating that CAFs could directly promote hormone therapy resistance through NRG1 regulation [87].
Schwann cells
Schwann cells, a type of glial cell, are best known for providing myelin sheaths for the peripheral nervous system, but they have also been found to directly support and stimulate cancer growth [88]. Originating from the neural crest, Schwann precursors can either transition to become myelin Schwann cells, non-myelin Schwann cells, or repair (Bungner bands) Schwann cells and play critical roles in sympathetic nervous system development, maintenance, and nerve damage response [89, 90]. The Bungner bands are particularly responsible for guiding nerves by creating tracks that neurons can travel along. Due to their dynamic roles in the sympathetic nervous system, they are highly plastic cells and can play several roles in facilitating malignant progression [90]. A recently published study demonstrated that Schwann cell density increased throughout pancreatic cancer progression and that Schwann cells have several compensatory mechanisms to regulate their density in response to sudden changes [91]. Intratumor Schwann cells morphologically change upon inflammation, as well as contribute to intratumor sympathetic nerve remodeling [91].
In the TME, Schwann cells are activated by TNF-α [92] and IL-6 [93] signaling and chemo attracted to tumor cell-secreted NGF, artemin [94], and CXCL12 [95]. Primarily interrogated in pancreatic and prostate cancers, Schwann cells have been found to promote malignant phenotypes in cancer cells, partially through the activation of transforming growth factor beta (TGF-β) signaling [96], and were positively associated with perineural invasion [97]. Schwann cells were also found to create dynamic guiding tracks, named tumor-activated Schwann cell tracks (TASTs), which physically align and facilitate cancer cell mobility and migration [98]. Schwann cells are also responsible for facilitating components in the TME. In vitro, Schwann cells can attract MDSC myeloid regulators through the increased expression of IL-1Ra, TNF-α, CCL3, CCL4, CXCL2, CXCL12, and CXCL13 when cocultured with cancer cells in vitro [99]. Further in vitro findings suggest that combined factors can exert a strong chemo-attractive force on bone marrow-derived MDSCs [99]. Recently, Schwann cells were also found to induce a phenotypic switch to activate EMT in pancreatic cancer through the promotion of an inflammatory CAF (iCAF) state [88]. In lung cancer, in response to chemotherapeutics, Schwann cells have been recently shown to activate the YAP/TAZ signaling pathway, demonstrating a role for Schwann cells in chemoresistance [100]. Altogether, in addition to their usual role in supporting and maintaining nerves, Schwann cells have more recently been found to acquire remarkable plasticity to promote metastasis and contribute to therapy resistance.
Mediation of communication within the TME: paracrine signaling through extracellular vesicles
Dynamic paracrine signaling is one of the major lines of short-distance intercellular messaging that mediates this multidirectional intercellular crosstalk. Tumor-associated extracellular vesicles (EVs), including microvesicles, exosomes, ectosomes, oncosomes, and cytoplasts, can package cargo such as proteins, RNA, microRNA (miRNA), long non-coding RNAs (lncRNA) DNA, and lipids [101]. A growing literature is showing that EVs are essential directors of tumor progression and metastasis [102] and can facilitate neurite extension into solid tumors [103]. Sympathetic axon structures intrinsically facilitate paracrine signaling by releasing neurotransmitters from varicosities, as well as axon-ending boutons (shown in Figs. 3 and 4) [104]. Varicosities are swellings along the axons of sympathetic nerves where neurotransmitters can be released, allowing for signaling effects to be distributed throughout the extension of the sympathetic nerve [105]. This contributes to rapid and broad responses in surrounding cells in the TME [105]. Ultrastructural vesicles within neurons have been identified, and neuron-derived exosomes are known to be released from damaged neurons in the central nervous system (CNS) [106]. However, the majority of neuron-derived EV studies are currently in the CNS, and evidence of EV communication directly released from nerves in the sympathetic nervous system remains limited [107].
Recent studies have shown cancer cell-released EVs promote neurite outgrowth into the TME. In head-and-neck cancer cells, EVs contain miRNA-34a and miRNA-141, and reducing these miRNAs suppressed axon outgrowth [108]. While this effect can be attributed to the NGF content of EVs [109], another study showed that exosome-induced neurite outgrowth was potentiated by axon guidance cargo EphrinB1, independent of NGF [103]. This aligns with findings showing that pancreatic cancer stem cell-released EVs are significantly enriched for a variety of neuro-modulating proteins, including HSPA8, AGRN, COL6A3, CNTN1, CAP1, MSN, TUBA3E, and TUBB6, which may directly contribute to intratumor innervation [110]. Interestingly, during spinal cord injury, macrophage-derived EVs containing miRNA-23a-3p induced M2 polarization [111], suggesting that EVs could mediate communication between immune cells in response to nerve damage. These findings on EVs and their diverse cargo highlight the need for further studies to examine their specific compositions and the responses they elicit in target cells.
Non-immune stroma cells such as CAFs and Schwann cells have also been found to mediate cancer invasion through their EV cargo. A study in salivary adenoid cystic carcinoma showed that exosome-educated fibroblasts containing CXC chemokine receptor 4 (CXCR4), NGF, TRKA, and BDNF promoted cancer cell invasion [112]. They demonstrated that exosomes containing enhanced NGF stimulated cancer cell invasion, leading to increased perineural invasion, specifically through downstream signaling of TRKA on the cancer cells. Notably, they demonstrated that inhibiting these exosomes containing NGF, which blocked the activation of the NGF-TRKA pathway, could be a possible point of novel therapeutic intervention [112]. Another recent study in a pancreatic cancer model demonstrated that CAF-derived EVs contain perineural invasion-associated transcripts, further enabling metastasis [113]. They demonstrated that the cargo, Y-box binding protein 1 (YBX1), stabilizes m5C modification in TRKA, early growth response 1 (EGR1), and SMAD family receptor 7 (SMAD7), indicating that lncRNA can mediate epigenetic switches that contribute to perineural invasion [113]. Other studies showed that repair Schwann cell-derived EVs (rSC-EVs) can carry protein and miRNA cargos that regulate cell adhesion, angiogenesis, and stimulation of immunosuppressive responses [114]. The neuroprotective functions of these rSC-EVs are likely hijacked by tumor cells to promote tumorigenesis. Upon tumor innervation, rSC-EVs can be taken up directly by cancerous cells to further disease progression. In prostate cancer, SC-EVs stimulate prostate-specific antigen (PSA) secretion, which promotes prostate cancer cell proliferation [114].
Significance and considerations of sympathetic crosstalk as a therapeutic target
The Cancer Neuroscience field provides critical insights into the supportive role of tumor-innervating nerves in cancer progression and metastasis. However, a better understanding of the multi-directional interactions between nerves and other cells in the TME is needed to facilitate the development of novel efficacious therapeutics.
Sympathetic-specific surgical, pharmacological, or genetic denervation studies show variable, but promising results in certain cancers. For example, in a gastric cancer model, sympathetic denervation by vagotomy or botulinum toxin type A treatment not only reduced tumorigenesis, but the sympathectomized animals also measured smaller tumors and reduced dysplasia [115]. In prostate cancer pre-clinical models, both surgical and chemical denervation reduced tumor initiation and growth [16]. However, surgical sympathectomies in head-and-neck cancer revealed no significant changes in tumor growth [108]. In an in vivo pancreatic cancer model, chemical sympathectomy utilizing the highly oxidative chemical 6-hydroxydopamine (6-OHDA), entering through the sympathetic-expressed dopamine active transporter (DAT) and causing mitochondrial dysfunction leading to sympathetic cell death, reduced survival by accelerating both tumor growth and metastasis [26]. These variable findings highlight the importance of improving organ-specific sympathetic denervation techniques to reduce off-target effects and investigating the impact of denervation during different stages of organ and tumor growth.
Multiple studies and clinical trials have attempted to reduce the general effects of NGF, stress response, and adrenergic signaling during cancer treatments and have revealed some promising results. Two phase 3 clinical trials tested tanezumab (NCT00545129 & NCT02609828), a monoclonal antibody that blocks NGF, in cancer patients and met safety and efficacy requirements. However, these trials did not yield any cancer-related outcomes such as progression or metastasis and served primarily to test administration [116].
The diagnosis of cancer and the financial burden associated with medical expenses can trigger a stress response mediated by the sympathetic nervous system and the hypothalamic–pituitary–adrenal (HPA) axis [27]. There is strong evidence that patients who effectively manage stress have better prognoses compared with those experiencing higher levels of stress [117]. Building on the evidence that broad-scale psychologic stress supports cancer growth and metastasis, several clinical trials are evaluating stress management (NCT02939612), and some other promising studies show that stress management can result in significantly reduced cancer risk and progression. A pharmacological approach in alleviating general stress symptoms with beta-blockers has shown benefits, including reduced cancer risk and improved survival in both human and murine models of breast [118], ovarian [119], pancreatic [120], and prostate cancers [121]. Furthermore, phase 1–3 clinical trials supplementing ovarian and breast cancer patients with the non-selective beta antagonist, propranolol (NCT01308944, ACTRN12615000889550), have initially resulted in improved quality of life and reduced metastatic features. These highlight efforts in the cancer neuroscience community to rethink commonly used drugs that may provide an additional benefit as a combination treatment for patients.
Although the described clinical trials are focused on sympathetic-associated signaling, harnessing insights into specific sympathetic-TME interactions could further enhance treatment efficacy. For example, we previously discussed that co-inhibition of β-adrenergic and COX-2 signaling alters immune infiltration and dampens metastasis-promoting factors [51]. Low-dose aspirin, which blocks COX activity as an anti-inflammatory, has been shown to reduce ovarian cancer risk [122]. Interestingly, an ongoing clinical trial is investigating the combined use of propranolol and a COX-2 inhibitor (NCT03838029), which may further clarify the efficacy of targeting these pathways. Targeting NRP receptors on various cells in the TME also presents a promising therapeutic strategy. For example, targeting NRP1 on endothelial cells may suppress tumor-promoting phenotypes [65], while inhibiting NRP1 on T Reg cells could act as a checkpoint inhibitor, reinvigorating the anti-cancer immune response [123]. Another potential approach involves blocking NETs, which can be overexpressed by CAFs. The anti-netrin-1-mAB (NP137) [124] is currently being evaluated in a phase 1 clinical trial (NCT02977195).
When generating and testing therapies, it will also be important to consider that changes in the TME and cancer cell states are also communicated back to the brain through specific mechanisms, including neural pathways and humoral factors [125, 126]. A recent study showed that vagal neurons are activated and responsive to inflammatory signals and in turn can also control inflammation [127]. Another recent study in head-and-neck tumors showed that infiltrating nerves undergo transcriptomic changes linked to nerve injury [128]. They found that these nerves extend directly back to the CNS and that cancer-bearing animals experience enhanced activation of this tumor-brain circuit [128]. To further improve patient outcomes, it is crucial to deepen our understanding of the mechanisms underlying these long-distance interactions between peripheral tumors and the CNS, elucidating their specific impact on tumor biology and patient prognosis.
Conclusion
Altogether, the studies reviewed here underscore the potential of targeting sympathetic neuronal signaling as a strategy for improving cancer management. However, to translate these insights into effective therapies, a deeper systemic and molecular understanding of tumor-nerve interactions within the TME is essential. For instance, knowing that β-adrenergic signaling can induce T cell exhaustion, mitigating the immunosuppressive effects of sympathetic signaling could enhance anti-tumor immunity. This opens the potential for synergistic treatment strategies combining immunotherapies with drugs that specifically target β-adrenergic signaling, ultimately leading to more effective treatment options. In-depth studies on how β-adrenergic signaling influences the expression of immune checkpoint molecules on T cells, macrophages, and MDSCs will open avenues for combining sympathetic nerve-targeting drugs with immune checkpoint inhibitors. Similarly, exploring whether other aspects of the sympathetic nervous system, such as the effects of neuroglial cells in the TME, could further enrich our understanding of immune modulation in cancer. Furthermore, more precisely quantifying the extent of sympathetic nervous system involvement in individual tumors could identify patients that are most likely to respond to these combined treatment approaches.
The conflicting results regarding β-adrenergic signaling in myeloid cells also highlight the need to investigate the dose-dependent effects of norepinephrine on MDSC accumulation in the TME. This underscores the importance of better understanding the physiological context of β-adrenergic signaling in patients undergoing cancer therapies. Future studies should leverage preclinical cancer models to explore how varying levels of β-adrenergic signaling impact tumor progression and metastasis. This will also help define the therapeutic windows for β-adrenergic blockers to optimize their clinical application.
Importantly, these studies emphasize the need to map the spatial profiles of multidirectional interactions within the TME, including the distance-dependent effects of sympathetic signaling. Such quantitative insights will be pivotal in developing computational models to predict the molecular and phenotypic changes resulting from combinatorial treatments. As the field advances and uncovers further evidence of the role of tumor innervation, these findings will be crucial for designing therapies that can effectively disrupt sympathetic signaling pathways, ultimately improving clinical outcomes for cancer patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- 6-OHDA
6-Hydroxydopamine
- AKT
Protein kinase B
- AR
Androgen receptor
- Arg-1
Arginase 1
- ATP
Adenosine triphosphate
- BDNF
Brain-derived growth factor
- BRAF
B-Raf proto-oncogene
- CAFs
Cancer-associated fibroblasts
- CCL
C-C motif chemokine ligand
- CCR
C-C motif chemokine receptor
- CD14
Cluster of differentiation 14
- ChAT
Choline O-acetyltransferase
- CINCs
Cold-induced neuroimmune cells
- CNS
Central nervous system
- Coa6
Cytochrome C oxidase assembly factor
- COX-2
Cyclooxygenase 2
- CXCR4
CXC chemokine receptor 4
- DAT
Dopamine active transporter
- DBH
Dopamine beta-hydroxylase
- DCC
Netrin receptor DCC
- ECM
Extracellular matrix
- EGR3
Early-growth-response-3
- EGR1
Early growth response 1
- EMT
Mesenchymal transition
- EVs
Extracellular vesicles
- G-CSF
Granulocyte colony-stimulating factor
- GATA-1, GATA-2
GATA-binding proteins 1 and 2
- Gerb2
Growth factor receptor bound protein 2
- GFAP
Fibrillary acid protein
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- GMP
Granulocyte-monocyte progenitors
- GSK
Glycogen synthase kinase-3
- HER3
Epidermal growth factor receptor 3
- HPA
Hypothalamic-pituitary-adrenal
- iCAF
Inflammatory CAF
- IFN-y
Interferon gamma
- IL
Interleukin
- IRS2
Insulin receptor substrate 2
- LIF
LIF interleukin 6 family cytokine
- MAP2
Microtubule-associated protein 2
- MDSCs
Myeloid-derived suppressor cells
- miRNA
MicroRNA
- mTOR
Mammalian target of rapamycin
- NE
Norepinephrine
- NEK9
NIMA-related kinase 9
- NET1
Netrin-1
- NetG1
Netrin G1
- NETs
Netrins
- NF-kB
Nuclear factor kappa B
- NF1
Neurofibromatosis 1
- NGF
Nerve growth factor
- NK
Natural killer
- NP137
Anti-netrin-1-mAB
- NPY
Neuropeptide Y
- NRAS
Neuroblastoma RAS
- NRG1
Neuregulin 1
- NRP
Neuropilin
- NRXN1
Neurexin 1
- p75
Neurotrophin receptor p75
- PD-1
Programmed cell death protein 1
- PDGFB
Platelet-derived growth factor-B
- PI3K
Phosphoinositide 3‐kinase
- PKA
Protein kinase A
- PLXN
Plexin
- PSA
Prostate-specific antigen
- ROBO
Roundabout
- rSC-EVs
Schwann cell-derived EVs
- SAMs
Sympathetic neuron–associated macrophages
- SEMA
Semaphoring
- SMAD7
SMAD family receptor 7
- Sos2
SOS Ras/Rho guanine nucleotide exchange factor 2
- STAT3
Transcription 3
- TASTs
Tumor-activated Schwann cell tracks
- TEAD1
TEA domain family member 1
- TEX
TOX + exhausted CD8 + T cells
- TGF-B
Transforming growth factor beta
- TH
Tyrosine hydroxylase
- TME
Tumor microenvironment
- TNF-α
Tumor necrosis factor alpha
- TP73
Tumor protein 73
- TRKA
Tropomyosin receptor kinase A
- TRKB
Tropomyosin receptor kinase B
- TSP1
Thrombospondin type-1
- UNC5
UNC-5 netrin receptor C
- UNC5B
Unc-5 netrin receptor B
- VAChT
Vesicular acetylcholine transporter
- VEGF
Vascular endothelial growth factor
- YAP1
Yes-associated protein 1
- YBX1
Y-box binding protein 1
Author contribution
AS wrote the main manuscript text with help from TK, KG, PD, NK, and SEE. TK generated original drawings for the manuscript. AS, NK, and SEE conceived of the manuscript content. All authors contributed critical review and revisions to the manuscript and have approved the final version.
Funding
This project was supported by funding from the Cancer Early Detection Advanced Research Center (CEDAR) (CEDAR Project ID # Full 2022–1494) at Oregon Health & Science University, Knight Cancer Institute.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Young, H. H. (1897). On the presence of nerves in tumors and of other structures in them as revealed by a modification of Ehrlich’s method of ‘vital staining’ with methylene blue. Journal of Experimental Medicine,2, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monje, M., et al. (2020). Roadmap for the emerging field of cancer neuroscience. Cell,181, 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva, D., Quintas, C., Gonçalves, J., & Fresco, P. (2022). Contribution of adrenergic mechanisms for the stress-induced breast cancer carcinogenesis. Journal of Cellular Physiology,237, 2107–2127. [DOI] [PubMed] [Google Scholar]
- 4.Petrescu, M., et al. (2024). Sympathetic nervous influences are negative prognostic factors in stomach cancer. Life Basel Switz.,14, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbieri, A., et al. (2015). The stress hormone norepinephrine increases migration of prostate cancer cells in vitro and in vivo. International Journal of Oncology,47, 527–534. [DOI] [PubMed] [Google Scholar]
- 6.Zhang, B., et al. (2020). The stress hormone norepinephrine promotes tumor progression through β2-adrenoreceptors in oral cancer. Archives of Oral Biology,113, 104712. [DOI] [PubMed] [Google Scholar]
- 7.Qian, W., et al. (2018). Norepinephrine enhances cell viability and invasion, and inhibits apoptosis of pancreatic cancer cells in a Notch-1-dependent manner. Oncology Reports,40, 3015–3023. [DOI] [PubMed] [Google Scholar]
- 8.Schmitd, L. B., Scanlon, C. S., & D’Silva, N. J. (2018). Perineural invasion in head and neck cancer. Journal of Dental Research,97, 742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao, J.-X., et al. (2016). Autonomic nervous infiltration positively correlates with pathological risk grading and poor prognosis in patients with lung adenocarcinoma. Thorac. Cancer,7, 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao, Q., et al. (2014). The clinicopathological significance of neurogenesis in breast cancer. BMC Cancer,14, 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang, L., et al. (2017). Sympathetic and parasympathetic innervation in hepatocellular carcinoma. Neoplasma,64, 840–846. [DOI] [PubMed] [Google Scholar]
- 12.Barr, J. L., et al. (2021). Intra-tumoral nerve-tracing in a novel syngeneic model of high-grade serous ovarian carcinoma. Cells,10, 3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu, Y., et al. (2018). Perineural invasion in early-stage cervical cancer and its relevance following surgery. Oncology Letters,15, 6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi, H. et al. (2024). Neuro-mesenchymal interaction mediated by a β2 adrenergic-nerve growth factor feedforward loop promotes colorectal cancer progression. Cancer Discov.10.1158/2159-8290.CD-24-0287. [DOI] [PMC free article] [PubMed]
- 15.Ceyhan, G. O., et al. (2009). Pancreatic neuropathy results in “neural remodeling” and altered pancreatic innervation in chronic pancreatitis and pancreatic cancer. Off. J. Am. Coll. Gastroenterol. ACG,104, 2555. [DOI] [PubMed] [Google Scholar]
- 16.Magnon, C., et al. (2013). Autonomic nerve development contributes to prostate cancer progression. Science,341, 1236361. [DOI] [PubMed] [Google Scholar]
- 17.Ayala, G. E., et al. (2008). Cancer-related axonogenesis and neurogenesis in prostate cancer. Clinical Cancer Research,14, 7593–7603. [DOI] [PubMed] [Google Scholar]
- 18.Ait-Ahmad, K., Ak, C., Thibault, G., Chang, Y. H. & Eksi, E. S. Axonfinder: automated segmentation of tumor innervating neuronal fibers. SSRN Scholarly Paper at https://papers.ssrn.com/abstract=4931895 (2024). [DOI] [PMC free article] [PubMed]
- 19.Zahalka, A. H., et al. (2017). Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science,358, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Globig, A.-M., et al. (2023). The β1-adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature,622, 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eksi, S. E., et al. (2021). Epigenetic loss of heterogeneity from low to high grade localized prostate tumours. Nature Communications,12, 7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maestroni, G. J., et al. (1998). Neural and endogenous catecholamines in the bone marrow Circadian association of norepinephrine with hematopoiesis? Experimental Hematology,26, 1172–1177. [PubMed] [Google Scholar]
- 23.Vasamsetti, S. B., et al. (2018). Sympathetic neuronal activation triggers myeloid progenitor proliferation and differentiation. Immunity,49, 93-106.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nevin, J. T., Moussa, M., Corwin, W. L., Mandoiu, I. I., & Srivastava, P. K. (2020). Sympathetic nervous tone limits the development of myeloid-derived suppressor cells. Science Immunology,5, 9368. [DOI] [PubMed] [Google Scholar]
- 25.Daneshmandi, S., et al. (2024). Myeloid-derived suppressor cell mitochondrial fitness governs chemotherapeutic efficacy in hematologic malignancies. Nature Communications,15, 2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillot, J., et al. (2022). Sympathetic axonal sprouting induces changes in macrophage populations and protects against pancreatic cancer. Nature Communications,13, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sloan, E. K., et al. (2010). The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Research,70, 7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armaiz-Pena, G. N., et al. (2014). Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget,6, 4266–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin, J., et al. (2015). Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Reports,48, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma, M., Patterson, L., Chapman, E., & Flood, P. M. (2017). Salmeterol, a long-acting β2-adrenergic receptor agonist, inhibits macrophage activation by lipopolysaccharide from Porphyromonas gingivalis. Journal of Periodontology,88, 681–692. [DOI] [PubMed] [Google Scholar]
- 31.Marshall, J. S., Gomi, K., Blennerhassett, M. G., & Bienenstock, J. (1999). Nerve growth factor modifies the expression of inflammatory cytokines by mast cells via a prostanoid-dependent mechanism1. The Journal of Immunology,162, 4271–4276. [PubMed] [Google Scholar]
- 32.Wang, W., & Cao, X. (2019). Beta-adrenergic signaling in tumor immunology and immunotherapy. Critical Reviews in Immunology,39, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong, L. K., Morice, A. H., Yeo, W. W., Schleimer, R. P., & Peachell, P. T. (1995). Functional desensitization of beta agonist responses in human lung mast cells. American Journal of Respiratory Cell and Molecular Biology,13, 540–546. [DOI] [PubMed] [Google Scholar]
- 34.Ak, Ç., et al. (2024). Multiplex imaging of localized prostate tumors reveals altered spatial organization of AR-positive cells in the microenvironment. iScience,27, 110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hervé, J., et al. (2013). β2-Adrenoreceptor agonist inhibits antigen cross-presentation by dendritic cells. The Journal of Immunology,1950(190), 3163–3171. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadpour, H., et al. (2018). Blockade of host β2-adrenergic receptor enhances graft-versus-tumor effect through modulating antigen presenting cells. The Journal of Immunology,200, 2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholls, A. J., Wen, S. W., Hall, P., Hickey, M. J., & Wong, C. H. Y. (2018). Activation of the sympathetic nervous system modulates neutrophil function. Journal of Leukocyte Biology,103, 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He, X.-Y., et al. (2024). Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment. Cancer Cell,42, 474-486.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobrenis, K., Gauthier, L. R., Barroca, V., & Magnon, C. (2015). Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development. International Journal of Cancer,136, 982–988. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Y.-N., et al. (2021). Slit3 secreted from M2-like macrophages increases sympathetic activity and thermogenesis in adipose tissue. Nature Metabolism,3, 1536–1551. [DOI] [PubMed] [Google Scholar]
- 41.Rached, M.-T., et al. (2019). Deletion of myeloid IRS2 enhances adipose tissue sympathetic nerve function and limits obesity. Molecular Metabolism,20, 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blaszkiewicz, M., et al. (2022). Adipose tissue myeloid-lineage neuroimmune cells express genes important for neural plasticity and regulate adipose innervation. Frontiers in Endocrinology,13, 864925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pirzgalska, R. M., et al. (2017). Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nature Medicine,23, 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cleypool, C. G. J., Mackaaij, C., LotgerinkBruinenberg, D., Schurink, B., & Bleys, R. L. A. W. (2021). Sympathetic nerve distribution in human lymph nodes. Journal of Anatomy,239, 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanders, V. M. (2012). The Beta2-adrenergic receptor on T and B lymphocytes: Do we understand it yet? Brain, Behavior, and Immunity,26, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Estrada, L. D., Ağaç, D., & Farrar, J. D. (2016). Sympathetic neural signaling via the β2-adrenergic receptor suppresses T-cell receptor-mediated human and mouse CD8(+) T-cell effector function. European Journal of Immunology,46, 1948–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slota, C., Shi, A., Chen, G., Bevans, M., & Weng, N. (2015). Norepinephrine preferentially modulates memory CD8 T cell function inducing inflammatory cytokine production and reducing proliferation in response to activation. Brain, Behavior, and Immunity,46, 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zalli, A., et al. (2015). Targeting ß2 adrenergic receptors regulate human T cell function directly and indirectly. Brain, Behavior, and Immunity,45, 211–218. [DOI] [PubMed] [Google Scholar]
- 49.Bucsek, M. J., et al. (2017). β-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Research,77, 5639–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Angelis, E., Pecoraro, M., Rusciano, M. R., Ciccarelli, M., & Popolo, A. (2019). Cross-talk between neurohormonal pathways and the immune system in heart failure: A review of the literature. International Journal of Molecular Sciences,20, 1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaashua, L., et al. (2017). Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clinical Cancer Research,23, 4651–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kannan, Y., Stead, R. H., Goldsmith, C. H., & Bienenstock, J. (1994). Lymphoid tissues induce NGF-dependent and NGF-independent neurite outgrowth from rat superior cervical ganglia explants in culture. Journal of Neuroscience Research,37, 374–383. [DOI] [PubMed] [Google Scholar]
- 53.Barbany, G., Friedman, W. J., & Persson, H. (1991). Lymphocyte-mediated regulation of neurotransmitter gene expression in rat sympathetic ganglia. Journal of neuroimmunology, 32(2), 97–104. 10.1016/0165-5728(91)90001-n [DOI] [PubMed]
- 54.Kannan-Hayashi, Y., Moriyama, M., & Nakamura, Y. (2008). Lymphocytes and adrenergic sympathetic nerves: The role of cytokines. In NeuroImmune Biology,6, 305–336. Elsevier. [Google Scholar]
- 55.Hanahan, D., & Monje, M. (2023). Cancer hallmarks intersect with neuroscience in the tumor microenvironment. Cancer Cell,41, 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vickman, R. E., et al. (2020). Deconstructing tumor heterogeneity: The stromal perspective. Oncotarget,11, 3621–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diller, R. B., & Tabor, A. J. (2022). The role of the extracellular matrix (ECM) in wound healing: A review. Biomimetics,7, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koopmans, G., Hasse, B., & Sinis, N. (2009). Chapter: The role of collagen in peripheral nerve repair. International Review of Neurobiology,87, 363–379. [DOI] [PubMed] [Google Scholar]
- 59.Yu, L. M. Y., Leipzig, N. D., & Shoichet, M. S. (2008). Promoting neuron adhesion and growth. Materials Today,11, 36–43. [Google Scholar]
- 60.Chiaro, P. D., et al. (2024). Mapping functional to morphological variation reveals the basis of regional extracellular matrix subversion and nerve invasion in pancreatic cancer. Cancer Cell,42, 662-681.e10. [DOI] [PubMed] [Google Scholar]
- 61.Larrivée, B., Freitas, C., Suchting, S., Brunet, I., & Eichmann, A. (2009). Guidance of vascular development. Circulation Research,104, 428–441. [DOI] [PubMed] [Google Scholar]
- 62.Lu, X., et al. (2004). The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature,432, 179–186. [DOI] [PubMed] [Google Scholar]
- 63.Rosenstein, J. M., Mani, N., Khaibullina, A., & Krum, J. M. (2003). Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. Journal of Neuroscience,23, 11036–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lähteenvuo, J., et al. (2020). Susceptibility to cardiac arrhythmias and sympathetic nerve growth in VEGF-B overexpressing myocardium. Molecular Therapy,28, 1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matkar, P. N., et al. (2016). Novel regulatory role of neuropilin-1 in endothelial-to-mesenchymal transition and fibrosis in pancreatic ductal adenocarcinoma. Oncotarget,7, 69489–69506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang, Z., et al. (2019). Targeting the SLIT/ROBO pathway in tumor progression: Molecular mechanisms and therapeutic perspectives. Therapeutic Advances in Medical Oncology,11, 1758835919855238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villanueva, A. A., et al. (2018). The Netrin-4/Laminin γ1/Neogenin-1 complex mediates migration in SK-N-SH neuroblastoma cells. Cell Adhesion & Migration,13, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pradella, D., et al. (2021). A ligand-insensitive UNC5B splicing isoform regulates angiogenesis by promoting apoptosis. Nature Communications,12, 4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daquinag, A. C., Gao, Z., Yu, Y., & Kolonin, M. G. (2022). Endothelial TrkA coordinates vascularization and innervation in thermogenic adipose tissue and can be targeted to control metabolism. Molecular Metabolism,63, 101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Helms, E. J., et al. (2021). Mesenchymal lineage heterogeneity underlies nonredundant functions of pancreatic cancer–associated fibroblasts. Cancer Discovery. 10.1158/2159-8290.CD-21-0601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sherman, M. H. (2018). Stellate cells in tissue repair, inflammation, and cancer. Annual review of cell and developmental biology, 34(1), 333–355. 10.1146/annurev-cellbio-100617-062855 [DOI] [PubMed]
- 72.Sato, M., Suzuki, S., & Senoo, H. (2003). Hepatic stellate cells: Unique characteristics in cell biology and phenotype. Cell Structure and Function,28, 105–112. [DOI] [PubMed] [Google Scholar]
- 73.Cassiman, D., Barlow, A., Vander Borght, S., Libbrecht, L., & Pachnis, V. (2006). Hepatic stellate cells do not derive from the neural crest. Journal of Hepatology,44, 1098–1104. [DOI] [PubMed] [Google Scholar]
- 74.Gard, A. L., White, F. P., & Dutton, G. R. (1985). Extra-neural glial fibrillary acidic protein (GFAP) immunoreactivity in perisinusoidal stellate cells of rat liver. Journal of Neuroimmunology,8, 359–375. [DOI] [PubMed] [Google Scholar]
- 75.Demir, I. E., Friess, H., & Ceyhan, G. O. (2012). Nerve-cancer interactions in the stromal biology of pancreatic cancer. Frontiers in Physiology,3, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sancho-Bru, P., et al. (2006). Norepinephrine induces calcium spikes and proinflammatory actions in human hepatic stellate cells. American Journal of Physiology. Gastrointestinal and Liver Physiology,291, G877-884. [DOI] [PubMed] [Google Scholar]
- 77.Oben, J. A., Yang, S., Lin, H., Ono, M., & Diehl, A. M. (2003). Norepinephrine and neuropeptide Y promote proliferation and collagen gene expression of hepatic myofibroblastic stellate cells. Biochemical and Biophysical Research Communications,302, 685–690. [DOI] [PubMed] [Google Scholar]
- 78.Sigala, B., et al. (2013). Sympathetic nervous system catecholamines and neuropeptide Y neurotransmitters are upregulated in human NAFLD and modulate the fibrogenic function of hepatic stellate cells. PLoS ONE,8, e72928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Descamps, S., et al. (2001). Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. Journal of Biological Chemistry,276, 17864–17870. [DOI] [PubMed] [Google Scholar]
- 80.Jiang, J., Bai, J., Qin, T., Wang, Z., & Han, L. (2020). NGF from pancreatic stellate cells induces pancreatic cancer proliferation and invasion by PI3K/AKT/GSK signal pathway. Journal of Cellular and Molecular Medicine,24, 5901–5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Donato, M., Giovannelli, P., Migliaccio, A., & Castoria, G. (2023). The nerve growth factor-delivered signals in prostate cancer and its associated microenvironment: When the dialogue replaces the monologue. Cell & Bioscience,13, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen, T., et al. (2022). YAP1-TEAD1 mediates the perineural invasion of prostate cancer cells induced by cancer-associated fibroblasts. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease,1868, 166540. [DOI] [PubMed] [Google Scholar]
- 83.Lu, G., et al. (2023). Cancer associated fibroblast derived SLIT2 drives gastric cancer cell metastasis by activating NEK9. Cell Death & Disease,14, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Francescone, R., et al. (2021). Netrin G1 promotes pancreatic tumorigenesis through cancer associated fibroblast driven nutritional support and immunosuppression. Cancer Discovery,11, 446–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sung, P.-J., et al. (2019). Cancer-associated fibroblasts produce netrin-1 to control cancer cell plasticity. Cancer Research,79, 3651–3661. [DOI] [PubMed] [Google Scholar]
- 86.Wu, L., Walas, S. J., Leung, W., Lo, E. H. & Lok, J. (2015). Neuregulin-1 and neurovascular protection. in Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects (ed. Kobeissy, F. H.) (CRC Press/Taylor & Francis, Boca Raton (FL)).
- 87.Zhang, Z., et al. (2020). Tumor microenvironment-derived NRG1 promotes antiandrogen resistance in prostate cancer. Cancer Cell,38, 279-296.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xue, M., et al. (2023). Schwann cells regulate tumor cells and cancer-associated fibroblasts in the pancreatic ductal adenocarcinoma microenvironment. Nature Communications,14, 4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mapps, A. A., Boehm, E., Beier, C., Keenan, W. T., Langel, J., Liu, M., Thomsen M. B., Hattar, S., Zhao, H., Tampakakis, E., & Kuruvilla, R. (2022). Satellite glia modulate sympathetic neuron survival, activity, and autonomic function. Elife, 11, e74295. 10.7554/eLife.74295 [DOI] [PMC free article] [PubMed]
- 90.Jessen, K. R., Mirsky, R., & Lloyd, A. C. (2015). Schwann cells: Development and role in nerve repair. Cold Spring Harbor Perspectives in Biology,7, a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rangel-Sosa, M. M., Mann, F., & Chauvet, S. (2024). Pancreatic Schwann cell reprogramming supports cancer-associated neuronal remodeling. Glia,72, 1840–1861. [DOI] [PubMed] [Google Scholar]
- 92.Salvo, E., et al. (2021). TNFα promotes oral cancer growth, pain, and Schwann cell activation. Science and Reports,11, 1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Demir, I. E., et al. (2016). Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut,65, 1001–1014. [DOI] [PubMed] [Google Scholar]
- 94.Ceyhan, G. O., et al. (2010). Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Annals of Surgery,251, 923–931. [DOI] [PubMed] [Google Scholar]
- 95.Demir, I. E., et al. (2017). Early pancreatic cancer lesions suppress pain through CXCL12-mediated chemoattraction of Schwann cells. Proceedings of the National Academy of Sciences. U. S. A.,114, E85–E94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sroka, I. C., et al. (2016). Schwann cells increase prostate and pancreatic tumor cell invasion using laminin binding A6 integrin. Journal of Cellular Biochemistry,117, 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang, W., et al. (2022). Autophagic Schwann cells promote perineural invasion mediated by the NGF/ATG7 paracrine pathway in pancreatic cancer. Journal of Experimental & Clinical Cancer Research CR,41, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deborde, S., et al. (2022). Reprogrammed Schwann Cells Organize into Dynamic Tracks that Promote Pancreatic Cancer Invasion. Cancer Discovery,12, 2454–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang, Y., et al. (2023). Schwann cell-derived CXCL2 contributes to cancer pain by modulating macrophage infiltration in a mouse breast cancer model. Brain, Behavior, and Immunity,109, 308–320. [DOI] [PubMed] [Google Scholar]
- 100.Otani, Y., et al. (2024). Adrenergic microenvironment driven by cancer-associated Schwann cells contributes to chemoresistance in patients with lung cancer. Cancer Science,115, 2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iraci, N., Leonardi, T., Gessler, F., Vega, B., & Pluchino, S. (2016). Focus on extracellular vesicles: Physiological role and signalling properties of extracellular membrane vesicles. International Journal of Molecular Sciences,17, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Becker, A., et al. (2016). Extracellular vesicles in cancer: Cell-to-cell mediators of metastasis. Cancer Cell,30, 836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Madeo, M., et al. (2018). Cancer exosomes induce tumor innervation. Nature Communications,9, 4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burnstock, G. (2008). Non-synaptic transmission at autonomic neuroeffector junctions. Neurochemistry International,52, 14–25. [DOI] [PubMed] [Google Scholar]
- 105.Gu, C. (2021). Rapid and reversible development of axonal varicosities: a new form of neural plasticity. Frontiers in Molecular Neuroscience, 14, 610857. 10.3389/fnmol.2021.610857 [DOI] [PMC free article] [PubMed]
- 106.Pineles, B., et al. (2022). Neuronal exosome proteins: Novel biomarkers for predicting neonatal response to therapeutic hypothermia. Archives of Disease in Childhood. Fetal and Neonatal Edition,107, 60–64. [DOI] [PubMed] [Google Scholar]
- 107.Hutchings, C., Phillips, J. A., & Djamgoz, M. B. A. (2020). Nerve input to tumours: Pathophysiological consequences of a dynamic relationship. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer,1874, 188411. [DOI] [PubMed] [Google Scholar]
- 108.Amit, M., et al. (2020). Loss of p53 drives neuron reprogramming in head and neck cancer. Nature,578, 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lucido, C. T., et al. (2019). Innervation of cervical carcinoma is mediated by cancer-derived exosomes. Gynecologic Oncology,154, 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ruivo, C. F., et al. (2022). Extracellular vesicles from pancreatic cancer stem cells lead an intratumor communication network (EVNet) to fuel tumour progression. Gut,71, 2043–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peng, P., et al. (2021). Exosomes-mediated phenotypic switch of macrophages in the immune microenvironment after spinal cord injury. Biomedicine & Pharmacotherapy,144, 112311. [DOI] [PubMed] [Google Scholar]
- 112.Xu, Z., Zheng, X., & Zheng, J. (2019). Tumor-derived exosomes educate fibroblasts to promote salivary adenoid cystic carcinoma metastasis via NGF-NTRK1 pathway. Oncology Letters,18, 4082–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zheng, S., et al. (2024). Extracellular vesicle–packaged PIAT from cancer-associated fibroblasts drives neural remodeling by mediating m5C modification in pancreatic cancer mouse models. Science Translational Medicine,16, eadi0178. [DOI] [PubMed] [Google Scholar]
- 114.Guo, Y., & Gil, Z. (2022). The role of extracellular vesicles in cancer-nerve crosstalk of the peripheral nervous system. Cells,11, 1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao, C.-M., et al. (2014). Denervation suppresses gastric tumorigenesis. Science translational medicine,6, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nair, A. S. (2018). Tanezumab: Finally a monoclonal antibody for pain relief. Indian Journal of Palliative Care,24, 384–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lutgendorf, S. K., et al. (2012). Social influences on clinical outcomes of patients with ovarian cancer. Journal of Clinical Oncology,30, 2885–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Melhem-Bertrandt, A., et al. (2011). Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. Journal of Clinical Oncology,29, 2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Watkins, J. L., et al. (2015). Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer,121, 3444–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Renz, B. W., et al. (2018). β2 Adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell,33, 75-90.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu, H., et al. (2015). Impact of beta-blockers on prostate cancer mortality: A meta-analysis of 16,825 patients. OncoTargets and Therapy,8, 985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hurwitz, L. M., et al. (2022). Modification of the association between frequent aspirin use and ovarian cancer risk: A meta-analysis using individual-level data from two ovarian cancer consortia. Journal of Clinical Oncology,40, 4207–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chuckran, C. A., Liu, C., Bruno, T. C., Workman, C. J., & Vignali, D. A. (2020). Neuropilin-1: A checkpoint target with unique implications for cancer immunology and immunotherapy. Journal for Immunotherapy of Cancer,8, e000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grandin, M., et al. (2016). Inhibition of DNA methylation promotes breast tumor sensitivity to netrin-1 interference. EMBO Molecular Medicine,8, 863–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dantzer, R., Konsman, J.-P., Bluthé, R.-M., & Kelley, K. W. (2000). Neural and humoral pathways of communication from the immune system to the brain: Parallel or convergent? Autonomic Neuroscience,85, 60–65. [DOI] [PubMed] [Google Scholar]
- 126.Jiang, S.-H., et al. (2020). Systemic regulation of cancer development by neuro-endocrine-immune signaling network at multiple levels. Frontiers in Cell and Developmental Biology,8, 586757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jin, H., Li, M., Jeong, E., Castro-Martinez, F., & Zuker, C. S. (2024). A body–brain circuit that regulates body inflammatory responses. Nature,630, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barr, J., Walz, A., Restaino, A. C., Amit, M., Barclay, S. M., Vichaya, E. G., Spanos, W. C., Dantzer, R., Talbot, S., & Vermeer, P. D. (2024). Tumor-infiltrating nerves functionally alter brain circuits and modulate behavior in a mouse model of head-and-neck cancer. Elife 13:RP97916. 10.7554/eLife.97916 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.