Abstract
Prostate cancer morbidity and mortality demonstrate a need for more effective targeted therapies. One potential target is EphA2, although paradoxically, pro- and anti-oncogenic effects have been shown to be mediated by EphA2. We demonstrate that unique activating and blocking EphA2-targeting monoclonal antibodies display opposing tumor-suppressive and oncogenic properties in vivo. To further explore this complexity, we performed detailed phosphoproteomic analysis following ligand-induced EphA2 activation. Our analysis identified altered phosphorylation of 73 downstream proteins related to the PI3K/AKT/mTOR and ERK/MAPK pathways, with the majority implicated in cell junction and cytoskeletal organization, cell motility, and tumor metastasis. We demonstrate that the adapter protein SHB is an essential component in mediating the inhibition of the ERK/MAPK pathway in response to EphA2 receptor activation. Furthermore, we identify the adherence junction protein afadin as an EphA2-regulated phosphoprotein which is involved in prostate cancer migration and invasion.
Subject terms: Prostate cancer, Metastasis, Oncogenes, Phosphorylation, Cytoskeleton
Introduction
The Eph family of receptor tyrosine kinases (RTKs) have been implicated in normal development and disease including many human cancers [1]. Numerous studies have shown that Eph receptors can function in either oncogenic or tumor-suppressive capacities [2, 3]. It is now established that oncogenic functions typically occur in a kinase-independent manner, involving Eph receptor crosstalk with known oncogenic and mitogenic receptors. In comparison, Eph tumor-suppressive functions typically occur in a kinase-dependent, ephrin ligand-induced manner. These dual receptor functions add significant complexity to the biology of Eph family RTKs, particularly in the context of cancer. Despite this complexity, Eph and ephrins remain viable cell surface anti-cancer targets [4–6]. EphA2, originally termed ECK [7], was one of the first Eph receptors to be described and has been intensively studied in both development and disease [1]. In particular, EphA2 has been implicated in the pathogenesis of many human cancers, where increasing receptor expression levels act in concert with reduced kinase function to promote tumor progression [8]. Furthermore, EphA2 has been shown to broadly promote oncogenesis by contributing to chemoresistance, self-renewal, and angiogenesis [8–10].
The present study sought to further elucidate the function of EphA2 in the context of prostate cancer, where kinase-dependent and kinase-independent roles have been described, primarily affecting tumor progression and metastasis [11]. Prostate cancer remains a significant disease for which more effective tumor-specific therapies are needed to treat aggressive disease. EphA2 is expressed at high levels in prostate adenocarcinoma compared to benign prostatic epithelium, where its expression is low [12]. In addition, high EphA2 expression has been shown to predict poor prognosis following radical prostatectomy in prostate cancer patients [13, 14]. These studies imply that EphA2 primarily functions as an oncogene in the context of this disease. Mechanistically, EphA2 binding to the high-affinity ligand ephrin-A1 leads to receptor-ligand cluster formation, resulting in kinase activation and downstream signaling events [15, 16]. A seminal study by Wang et al. found that activation of EphA2 inhibited prostate cancer cell migration, whereas receptor overexpression promoted migration in a ligand-independent, non-canonical manner [17]. This migratory, pro-oncogenic effect requires the phosphorylation of EphA2 on Serine 897 via crosstalk with AKT. Functionally, it appears that EphA2 activation inhibits AKT and ERK signaling in a reciprocal loop, diminishing EphA2-S897 phosphorylation and causing receptor-mediated endocytosis and EphA2 degradation [2, 17, 18].
As a first step, to investigate the contribution of EphA2 to prostate cancer tumorigenesis, we employed two in-house developed EphA2-targeting monoclonal antibodies (mAbs) [19]. We show that the IgG1 1F7 mAb, which binds to human and mouse EphA2, actively blocked kinase activation following ephrin-A1 stimulation, whereas the IgG2a 4B3 mAb, which binds exclusively to human EphA2, displayed activating properties. In an orthotopic xenograft model of prostate cancer 1F7 promoted tumor growth, while 4B3 reduced metastatic prostate cancer burden. We further demonstrate that vascular permeability is increased by the EphA2-blocking mAb 1F7, potentially contributing to tumor metastasis. To analyze the complex role of EphA2 in prostate cancer, we performed mass spectrometry (MS) with stable isotope labeling by amino acids in cell culture (SILAC) in prostate cancer cells to uncover interacting protein networks and downstream effector proteins following EphA2 activation. Our analysis identified 73 phosphoproteins that were significantly regulated, predominantly showing reduced phosphorylation upon ephrin-A1 stimulation. Using network analysis, we show that these EphA2-regulated proteins are primarily downstream targets of the PI3K/AKT/mTOR and ERK/MAPK pathways, with the majority implicated in the regulation of cytoskeletal organization, cell motility and tumor metastasis. Furthermore, our detailed phosphoproteomic analysis identified the adherence junction protein afadin (gene name AFDN, also: AF6, MLLT4) and the SH2 domain-containing adapter protein B (SHB) as key phosphoprotein targets downstream of EphA2 receptor tyrosine kinase signaling. Our results identify SHB as an essential component in mediating ERK/MAPK pathway inhibition in response to EphA2 receptor activation. Functional studies identified the AKT substrate afadin as a mediator of prostate cancer migration and invasion.
Results
EphA2 activation inhibits oncogenic signaling and reduces invasion
EphA family receptor mRNA and protein expression were assessed by QPCR and western blotting in a panel of six prostate cancer cell lines. Apart from EphA2 and EphA3, EphA family receptor levels were low with EphA2 being the most consistently elevated; the highest expressing line being PC-3 (Supplementary Fig. 1a, b), thus confirming EphA2 as the predominant EphA receptor expressed in PC-3 cells [20, 21]. Immunofluorescence (IF) analysis of PC-3 cells confirmed elevated EphA2 protein levels in the cell membrane (Supplementary Fig. 1c). Based on these findings, we selected the PC-3 prostate cancer cell line for further functional analysis. The EphA2 kinase was readily activated in PC-3 cells following stimulation with the clustered high-affinity ligand ephrin-A1-Fc, and the results showed inhibition of the AKT, ERK1/2 and FAK/SRC signaling pathways (Fig. 1a and Supplementary Fig. 1d), confirming earlier studies [17, 20, 22]. EphA2 activation rapidly induced transient retraction of cell protrusions and cell rounding as previously reported [20, 23, 24], which was accompanied by increased cortical F-actin staining, consistent with increased actomyosin contractility and concomitant reduction in microtubule polarity (Fig. 1b, d). Consistently, cytochalasin D, an inhibitor of actin dynamics, blocked ephrin-A1-induced cell rounding (Fig. 1c). Cell adhesion to ephrin-A1-Fc-coated culture surfaces was also assessed. PC-3 cells preferentially adhered to ephrin-A1-Fc compared to uncoated and Fc-control-coated surfaces, comparable to integrin-mediated adhesion to Matrigel (Fig. 1e, f). Activation of EphA2 has previously been shown to inhibit cell invasion [17]. We confirmed this finding by embedding PC-3 cells in Matrigel containing clustered ephrin-A1-Fc compared to Fc control protein (Fig. 1g, h and Supplementary Video 1). Reduced invasion in the presence of ephrin-A1-Fc appeared to be due to cell processes repulsion and retraction instead of establishing firm cell-matrix adhesions to pull the cell body forward, as observed in the control (Fig. 1h and Supplementary Video 1). Our data confirm that EphA2 kinase activation causes retraction of cell protrusions, thus promoting a non-invasive phenotype.
Fig. 1. Activation of EphA2 receptors causes rearrangement of the cytoskeleton, retraction of cell protrusions and cell rounding.
a Western blot analysis showing EphA2 receptor phosphorylation and inhibition of AKT, MEK/ERK and FAK/SRC signaling after stimulation with 1 µg/ml clustered ephrin-A1-Fc (efnA1-Fc) for up to 3 h, as indicated, in PC-3 prostate cancer cells. The full 72-h time course is shown in Supplementary Fig. 1d. Confocal microscopy images of the F-actin cytoskeleton labeled with rhodamine-phalloidin (red) in PC-3 cells in response to stimulation with clustered efnA1-Fc compared to Fc control in the absence (b) and the presence (c) of Cytochalasin D (CytoD). Cell nuclei were labeled with DAPI (blue). Incubation times with clustered ephrin-A1-Fc and Fc control, respectively, are indicated in the figure. Cell outlines, based on corresponding brightfield images, are indicated as white lines in the CytoD-treated cells. d Confocal microscopy images of microtubules (α-tubulin, green) in unstimulated and PC-3 cells stimulated for 2 min with clustered efnA1-Fc. Cell outlines and nuclei are visualized using F-actin staining (rhodamine-phalloidin, red) and DAPI (blue), respectively. Anisotropy of microtubules was quantified using the ImageJ plugin FibrilTool. Data represent means ± SE (n > 40 individually analyzed cells). ***p < 0.001 (unpaired t-test) e Quantification of PC-3 cell adhesion to uncoated, fibronectin-, Matrigel-, clustered Fc-control- or efnA1-Fc-coated cell culture surfaces after 30 min. Data represent means ± SE (n = 4 independent experiments). *p < 0.05, ***p < 0.001, ns. not significant (repeated-measure ANOVA with post-hoc Tukey’s multiple comparisons tests). f Fluorescent microscopy images of PC-3 cell adhesion to glass coverslips coated in alternating stripes of clustered efnA1-Fc (10 µg/ml) and either clustered Fc control (10 µg/ml) or Matrigel (1:100) as an alternative substrate for adhesion. Cells were fixed at 1 h and 24 h after plating, then visualized with F-actin (rhodamine-phalloidin, red) and DAPI (blue) labeling. g, h Time-lapse brightfield microscopy images of PC-3 cell invasion into Matrigel containing either 1 ug/ml Fc control or clustered efnA1-Fc. Initial cell-to-Matrigel boundary at t = 0 is indicated as a black line. Colored asterisks and arrow heads follow individual cells and cell protrusions, respectively, over time.
EphA2 antibody characterization
In seeking to better understand the anti- and pro-oncogenic functions of EphA2, we first characterized the binding kinetics and receptor-blocking function of two EphA2-specific mAbs, clones names 4B3 and 1F7. The development of these mAbs has been reported previously [19]. The IgG2a 4B3 mAb binds only to human EphA2, whereas the IgG1 1F7 mAb binds to mouse and human EphA2. Octet analysis was used to define the binding kinetics to biotinylated EphA2-Fc protein alone and followed by ephrin A1-Fc (Supplementary Fig. 2a). The results show that 1F7 and 4B3 bind distinct epitopes and that ephrin-A1 ligand binding does not interfere with 4B3 binding, whereas 1F7 binding to ephrin-saturated EphA2 appears to be reduced, indicating that 1F7 likely binds close to the ephrin binding site. 1F7 and 4B3 binding to PC-3 cells was tested by flow cytometry and confirmed strong equivalent EphA2 receptor binding (Supplementary Fig. 2b). Next, we assessed the ability of 1F7 and 4B3 to activate EphA2 alone or in combination with clustered ephrin A1-Fc. While neither antibody alone induced significant activation, 4B3 permitted ephrinA1-Fc-induced EphA2 kinase phosphorylation, whereas 1F7 effectively blocked receptor phosphorylation on activating sites Y588/Y594 (Fig. 2a and Supplementary Fig. 2c). A clear reduction in phosphorylated ATK levels was observed following ligand-induced EphA2 activation, which was also inhibited following 1F7 treatment (Fig. 2a and Supplementary Fig. 2c). Notably, combination of 1F7 and 4B3 induced receptor activation and downstream signaling, consistent with their ability to bind independent epitopes on EphA2, thus potentially inducing receptor cross-linking. Furthermore, we found that the 4B3 antibody, when crosslinked with a secondary antibody, is capable of inducing EphA2 receptor phosphorylation and inhibiting downstream phosphorylation of AKT on S473. In contrast, clustered 1F7 antibody was not activating (Fig. 2b). Together these data demonstrate contrasting functional effects of 4B3 and 1F7 on EphA2 receptor activation.
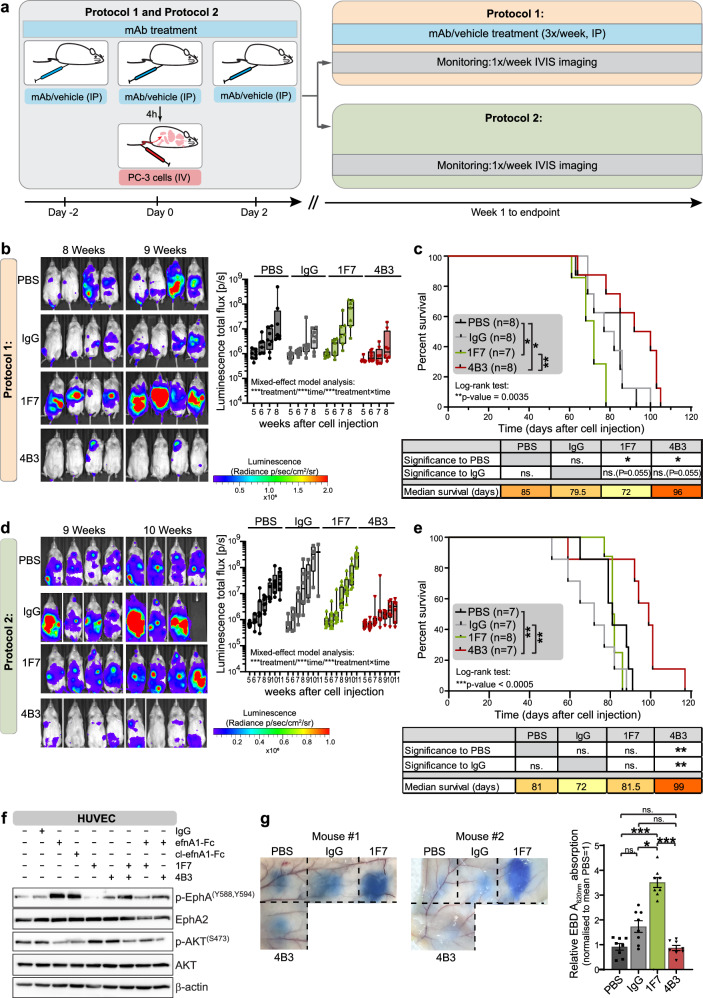
Fig. 2. In vivo efficacy of 1F7 and 4B3 mAbs on tumor growth, survival and metastatic spread in an orthotopic PC-3-luciferase prostate cancer model.
a, b Western blot analysis of EphA receptor activation and AKT inhibition after 20 min-stimulation with ephrin-A1-Fc (efnA1-Fc), clustered ephrin-A1-Fc (cl-efnA1-Fc) and 1F7 and 4B3 alone, in combination and clustered (cl) as indicated. 1F7 blocks efnA1-mediated EphA2 pathway activation (a), while clustered 4B3 induces EphA2 pathway activation (b). β-actin is the loading control. Bar graphs in (b) show the quantification of the relative signal intensities of phospho-EphA and phospho-AKT, with the bars representing the means ± SE (n = 3 independent experiments). c Schematic of experimental design with exemplary histological and macroscopic images (collage of two images to capture the full view) of the orthotopic PC-3 prostate cancer model. NRG mice were orthotopically injected with 2.5 × 105 PC-3-luciferase cells. From day 10, mice received thrice weekly intraperitoneal injections of PBS vehicle control or 1F7 or 4B3 mAbs (8 mg/kg) until endpoint. An IgG isotype control group was included as additional control in later experiments. Tumor burden was imaged weekly by intravital bioluminescence imaging. Tumor (T), bladder (Bl) and seminal vesicles (SV). d Representative intravital bioluminescence images of PC-3-luciferase tumor signals 6 weeks after engraftment showing augmented tumor burden in the 1F7 treatment group. e Chart shows luciferase bioluminescence total flux (pixel/s) over time. Mean ± SE (n = 15 mice per group, pooled from three experiments). Mixed model analysis of log-transformed bioluminescence total flux demonstrated a significant difference between 1F7 and PBS groups over time, which was determined by calculating the fixed effect parameter estimate of treatment (1F7-PBS) × time. f Kaplan–Meier survival analysis (n = 5 mice/group). *p < 0.05 (log-rank (Mantel–Cox) test for pairwise comparisons). g Representative lung ex vivo bioluminescence images of lung metastatic burden 7 weeks after orthotopic PC-3-luciferase engraftment. The box and whisker plot shows the log-scaled median, interquartile range ± maximum/minimum values of ex vivo bioluminescence total flux (pixel/s) of excised lungs; Individual data points represent signals of individual lungs. Data were pooled from three independent animal experiments, except for the isotype control, which had only been included in two of the three experiments. n = 14 lungs (PBS), n = 13 (IgG), n = 12 (1F7), n = 13 (4B3). While lungs from 4B3-treated animals showed a trend towards lower values, one-way ANOVA of log-transformed data with Sidak’s post hoc-test for multiple comparisons found no significant differences due to large variances within each group. h Examples of H&E-stained lung sections comparing metastatic spread from the orthotopic tumor to the lung at 7 weeks after engraftment. Tumor nodules are outlined in blue.
Efficacy of 4B3 and 1F7 on prostate tumor growth and metastasis in vivo
We next assessed the response to 4B3 and 1F7 mAb treatment in vivo using a prostate cancer orthotopic xenograft model. PC-3-luciferase cells reliably formed orthotopic tumors in mice; treatment commenced on day 10 after engraftment consisting of vehicle control, 1F7 and 4B3 (IP, 8 mg/kg, three times weekly) (Fig. 2c). We observed differing human and mouse binding capacities; 4B3 will bind exclusively to EphA2 expressed on the human tumor xenografts, whereas 1F7 is capable of binding EphA2 expressed on both human tumor and mouse host tissues. Imaging showed 1F7 treatment increased tumor burden, leading to a decrease in overall survival (Fig. 2d–f and Supplementary Fig. 2d). Metastatic spread to the lungs was assessed 7 weeks after orthotopic engraftment, showing a trend towards reduced spontaneous lung metastasis in the 4B3-treated cohort (Fig. 2g, h and Supplementary Fig. 2e). However, due to the large variances within groups in the spontaneous metastasis model, these results did not reach significance. To further investigate a possible effect of 4B3 on metastasis, we adopted an experimental metastasis model involving direct injection of PC-3 cells into the periphery via IV tail vein injection (Fig. 3a). Antibodies were administered 2 days and 4 h prior to PC-3 IV injection to have antibodies circulating at the time of PC-3 cell engraftment. Antibodies were subsequently continued three times per week to maintain antibody treatment for the duration of the experiment (Fig. 3a, Protocol 1). 4B3 treatment delayed metastatic spread, significantly increasing overall survival, whereas 1F7 treatment increased metastasis and significantly decreased survival (Fig. 3b, c and Supplementary Fig. 3a). To distinguish between early treatment responses on extravasation and/or tissue invasion at metastatic sites versus later growth once metastatic sites are established, we reduced the 4B3 and 1F7 treatment window. Mabs were injected 2 days prior to, on engraftment day and 2 days following PC-3 IV injection with the aim to have antibody present only during the early phases of metastatic engraftment (Fig. 3a, Protocol 2). Imaging data showed that 1F7 still appeared to promote metastasis, but the response was reduced and did not affect overall survival, suggesting that 1F7 predominantly affects tumor growth/progression. In comparison, the anti-metastatic and significant pro-survival response of 4B3 treatment was maintained (Fig. 3d, e and Supplementary Fig. 3b). This finding suggested that the tumor-suppressive properties of 4B3 occurred early during metastasis formation, affecting tumor cell tissue invasion and/or the establishment of tumors at metastatic sites rather than subsequent tumor growth.
Fig. 3. In vivo efficacy of 1F7 and 4B3 mAb in an intravenous metastatic model of PC-3-luciferase cells.
a Schematic of the experimental design. NRG mice received intraperitoneal injections of 1F7, 4B3 or IgG control mAbs (8 mg/kg) or PBS vehicle controls at 2 days and 4 h prior to and 2 days after tail vein injection with PC-3-luciferase cells. In one experimental setup (Protocol 1) mice continued to receive treatments thrice weekly via intraperitoneal injection until endpoint. For Protocol 2, mice received no further injections of antibody after the initial three doses. Tumor burden was monitored using weekly intravital bioluminescence imaging. Representative bioluminescence images at 8 and 9 weeks and quantification of total luminescence flux (pixel/s) from week 5 to 8 after PC-3 cell engraftment (b) and Kaplan–Meier survival analysis (c) of mice treated according to Protocol 1. The box and whisker plot shows the log-scaled median, interquartile range ± maximum/minimum values of the total bioluminescence flux (pixels/s). Mixed model analysis of log-transformed bioluminescence total flux demonstrated a significant difference between treatment groups, over time and treatments × time. Kaplan–Meier survival curves were analyzed using log-rank test to compare all curves and log-rank (Mantel–Cox) test for pairwise comparisons (n = 7–8 mice per group as indicated), *p < 0.05 and **p < 0.01. Bioluminescence images at 9 and 10 weeks after injection of tumor cells and quantification of total bioluminescence flux (pixel/s) from week 6 to 11 (d) and Kaplan–Meier survival analysis (e) of mice treated according to Protocol 2. The box and whisker plot shows the log-scaled median, interquartile range ± maximum/minimum values of the total bioluminescence flux (pixels/second). Mixed model analysis of log-transformed bioluminescence total flux demonstrated a significant difference between treatment groups, over time and treatments × time. Kaplan–Meier survival curves were analyzed by log-rank test to compare all curves and log-rank (Mantel–Cox) test for pairwise comparisons (n = 7–8 mice per group as indicated), **p < 0.01 and ***p < 0.001. f Western blot analysis of EphA2 receptor activation and AKT inhibition in HUVEC cells after a 20-min stimulation with ephrin-A1-Fc (efnA1-Fc), clustered ephrin-A1-Fc (cl-efnA1-Fc), 1F7 and 4B3 alone and in combination as indicated. 1F7 blocks EphA2 receptor activation by ephrin-A1-Fc. β-actin is the loading control. g Vascular permeability was measured by Miles Assay. Two representative images from the flanks of two mice showing dye effusion in response to 1F7, 4B3 and control IgG mAbs and PBS. Bar graph shows the relative amount of Evans Blue dye extravasation quantified spectroscopically after formamide extraction from tissue biopsy cores. Mean ± SE (n = 8). *p < 0.05, ***p < 0.001, ns. not significant (Repeated measure, one-way ANOVA with post-hoc Tukey’s test for multiple pairwise comparisons).
The 1F7 antibody causes on-target, off-tumor effects impacting endothelial barrier function
EphA2 is expressed on normal endothelium and, together with its ephrin ligands, plays a known role in regulating vascular permeability [25]. In seeking to further understand the pro-metastatic effect of the ephrin-blocking 1F7 antibody, we explored the possibility that 1F7, in addition to any direct effects on PC-3 tumor cells, might also promote metastasis by binding to mouse endothelial EphA2. A model analogous to the pro-metastatic effect of soluble ephrin-A1 in a lung metastasis model [26] would predict that 1F7 induces disruption of the endothelial barrier, causing vascular leak and allowing egress of circulating tumor cells and pro-metastatic stromal elements. We first analyzed the effect of 1F7 on a mouse endothelial cell line (2H-11) and on primary human umbilical vein endothelial cells (HUVECs) and showed that, similar to PC-3 cells, 1F7 was non-activating and indeed blocked the activating effect of ephrin-A1 (Fig. 3f and Supplementary Fig. 3d). To explore the possibility that 1F7 could act like soluble ephrin-A1 and disrupt EphA2-ephrin interactions between endothelial cells [26–28], we utilized the Miles Assay to examine the effects of antibody treatment on vascular permeability (Fig. 3g). 1F7 significantly increased Evans Blue dye extravasation in vivo, consistent with disruption of the endothelial barrier. These data are consistent with the notion that 1F7-induced increase in vascular permeability may allow increased transmigration of tumor cells. However, the results of our xenograft experiments suggest that the dominant pro-tumorigenic effect of 1F7 is due to accelerated tumor growth rather than increased transmigration.
Quantitative phosphoproteomic analysis identifies downstream effector proteins of ligand-induced EphA2 receptor activation
EphA2 activation has been shown to inhibit key oncogenic pathways, including PI3K/AKT, MEK/ERK and FAK/SRC signaling pathways (Fig. 1a and [17, 20, 22]). However, less is known about other downstream effector proteins involved in mediating EphA2 tumor-suppressive functions following kinase activation. We used SILAC in combination with mass spectrometry to measure proteome-wide changes in phosphopeptide abundance following EphA2 activation in PC-3 cells (Fig. 4a). We identified 2735 phosphoproteins with at least 75% localization probability across four biological replicates, comprising two forward- and two reverse-labeled experiments (Supplementary Fig. 4a, b). As expected after TiO2 phosphopeptide enrichment, most of the modified sites were Ser (85%), followed by Thr (13.4%) and Tyr residues (1.6%). We identified 113 phosphopeptides, comprising 128 phosphosites and 73 phosphoproteins that were significantly regulated in at least three out of four biological replicates following EphA2 receptor activation (Fig. 4b and Supplementary Fig. 4a–c; Supplementary Table 1). The majority of these regulated phosphosites (94 of 128) showed reduced phosphorylation, whereas 34, including six from the EphA2 receptor, showed increased phosphorylation. Consistent with our earlier ERK and AKT western blot data, we found reduced phosphorylation of proteins involved in these pathways, including ERK2, p90RSK, JUNB and FOSL downstream of ERK signaling and PRAS40, S6K, eEF2K, and eEF2 downstream of AKT signaling (Supplementary Fig. 4c and Supplementary Table 1).
Fig. 4. Quantitative phosphoproteomics and enrichment analyses show EphA2 modulates phosphorylation of cell-cell adhesion and cytoskeletal proteins.
a Schematic representation of phosphoproteomic workflow. PC-3 cells were differentially labeled with light (12C) and heavy (13C) Arg and Lys isotopes for 6 passages, then activated with clustered ephrin-A1-Fc (efnA1-Fc) or treated with clustered Fc control for 20 min. Following cell lysis and clean-up, equal amounts of lysates from each condition were combined and digested with LysC and trypsin. Phosphopeptides were enriched with TiO2 and analyzed by LC-MS/MS. N = 4 independently SILAC-labeled, stimulated and processed biological replicates. The light and heavy amino acid labeling was alternated between replicates (“label swap”): replicates 1 and 3 were “forward labeled” (Fc control: light, ephrin-A1-Fc: heavy) and replicates 2 and 4 were “reverse labeled”. b Heat map showing the log2-transformed activated:control ratios across the four replicates for the 30 most regulated phosphopeptides. c Regulated phosphoproteins were categorized into protein classes according to function by manually curating information from HRPD, Panther and UniProt databases and the literature. GO enrichment analysis of Cellular Compartment (d) and Biological Process and Molecular Function terms (e). The ClueGo app for Cytoscape was used to calculate enrichment of terms associated with EphA2-regulated proteins compared to our own background dataset and to cluster related and redundant terms. Enrichment was evaluated using one-sided hypergeometric testing. FDR was controlled using the Benjamini–Hochberg correction for multiple hypothesis testing. Node size reflects the number of regulated phosphoproteins linked to a particular term; node color indicates the Benjamini–Hochberg corrected p value as a measure of significance as indicated.
Functional enrichment analyses of EphA2 downstream signaling molecules
Next, we performed enrichment analyses to gain insight into the functional relevance of the identified EphA2-regulated phosphoproteins and hence the role of EphA2 receptor activation in prostate cancer. Regulated phosphoproteins were individually categorized into functional protein classes by manually curating data collated from the HPRD [29], GeneCards [30], Uniprot [31] and Panther [32] databases and the literature (Fig. 4c and Supplementary Table 2). A large proportion of proteins belong to protein classes related to cell signaling and cytoskeletal regulation, including actin-binding adapters and actin-dynamics regulators. Gene Ontology (GO) enrichment analysis of EphA2-regulated proteins revealed enrichment of cellular compartments describing cell-cell and cell-substrate junctions, the actin cytoskeleton, contractile structures, the TORC1 complex, and membrane compartments, including rafts and endosomes (Fig. 4d and Supplementary Table 3). Similarly, GO annotations for biological processes and molecular functions showed significant enrichment of a large cluster of terms related to cytoarchitecture, including cell-cell adhesion, cell junction assembly, actin cytoskeleton binding and small GTPases (Fig. 4e and Supplementary Table 4). Terms related to axon guidance, chemotaxis, and plasma membrane localization were also significantly over-represented (Fig. 4e and Supplementary Table 4). Consistent with EphA2 being a member of the RTK family, we also found a large cluster of enriched annotations describing signaling responses to stimuli, such as hormones and growth factors. Similar functions were confirmed by the enrichment analysis of KEGG and Reactome pathways (Supplementary Fig. 5a and Supplementary Table 5). Furthermore, a comparison of our EphA2-regulated dataset to proteomic analyses of the E-cadherin [33] and integrin [34] adhesomes showed that 26 and 31 of our 73 regulated phosphoproteins were in common with these datasets, respectively. Together, these results suggest that EphA2, upon stimulation, regulates the phosphorylation of proteins involved in the regulation of cytoarchitecture, cell adhesion, and motility.
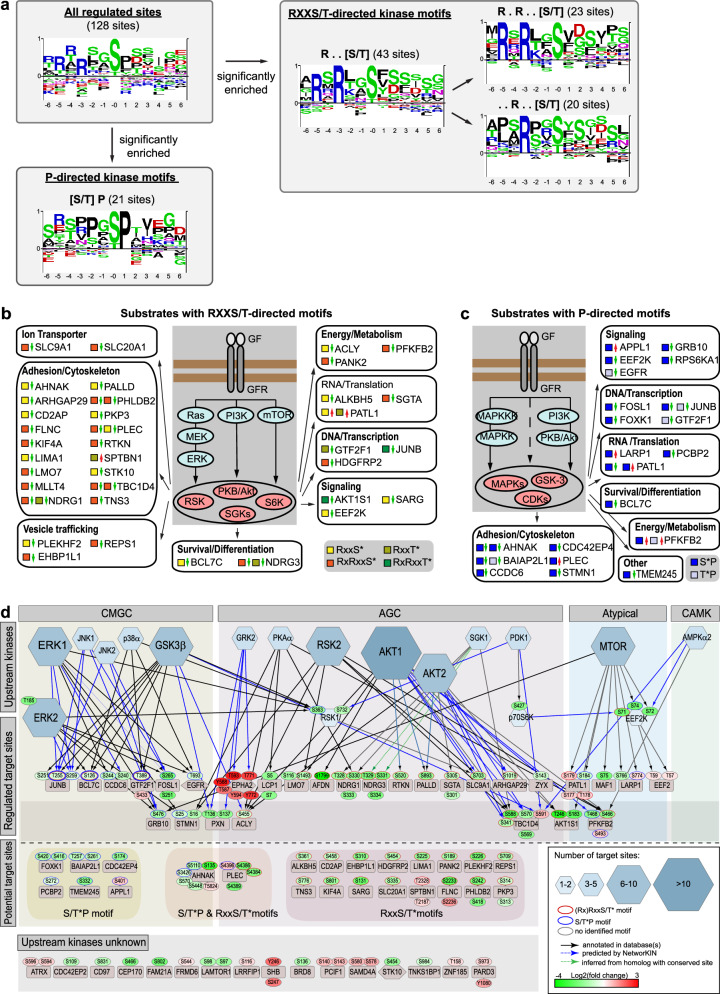
Motif and upstream kinase analyses confirm inhibition of the AKT/mTOR/GSK3 and MEK/ERK/RSK pathways as key mediators of EphA2 downstream signaling
To better understand the networks involved in mediating EphA2 downstream signaling, we performed motif enrichment analysis of the regulated phosphorylation sites (Fig. 5a–c and Supplementary Fig. 5b). This demonstrated an overrepresentation of the RXX[S/T*] and the extended RXRXX[S/T*] motifs, which are the preferred sites of AGC-kinase family members, including AKT, RSKs, S6K and SGKs. Also overrepresented in our dataset is the [S/T*]P proline-directed kinase motif, which is the preferred motif of CMGC kinases, including MAPKs, CDKs and GSK3. To further elucidate which kinases are the most likely intermediaries between activated EphA2 kinase and the phosphorylation of the identified downstream effector proteins, we performed an upstream kinase analysis (Fig. 5d and Supplementary Fig. 5c). To model the EphA2 phosphorylation network, we assigned potential upstream kinases to the EphA2-regulated phosphorylation sites identified in our phosphoproteomic analysis based on known (black edges) and predicted (blue edges) kinase-substrate relationships by querying the PhosphoSitePlus [35], HRPD [29], Uniprot [31], and NetworKIN [36] databases. In addition, candidate AGC kinase and CMGC kinase substrates were assigned and organized according to the regulated site motifs. Upstream kinase node sizes are indicative of the number of connected substrates and hence the likelihood of a particular kinase to be a relevant kinase, mediating signals downstream of EphA2 and upstream of the identified EphA2-regulated effector phosphoproteins. Based on this analysis, AKT, ERK1/2, mTOR, GSK-3β and RSK2 are the most likely candidate kinases involved in the phosphorylation of effector proteins, which are regulated downstream of activated EphA2 kinase. The vast majority of the sites regulated by these kinases show reduced phosphorylation after EphA2 activation, suggesting that these kinases are inhibited in response to EphA2 activation. This notion is consistent with the literature [17, 20, 22] and was confirmed by Proteome Profiler Array and western blot analysis demonstrating strong inhibition of the mTOR/AKT/p70S6K/GSK3 and MEK/ERK/RSK pathways (Fig. 6a and Supplementary Fig. 6).
Fig. 5. Motif and upstream kinase analysis of regulated phosphosites identifies RXXS/T-directed AGC kinases and proline-directed CMGC kinases as mediators of EphA2 signaling.
a Enriched motifs were identified using the Motifs Analysis Tool from PhosphoSitePlus. 13-mer phosphosite-centered peptide sequences of regulated phosphosites were analyzed using the Motif-All algorithm (significance threshold of 1e−05, support threshold of 0.05). A minimum motif occurrence threshold of five was manually applied to significantly overrepresented motifs. Motif logos were generated from the sequences of all 128 regulated phosphosites and from the phosphosites conforming with a particular enriched motif as indicated. Schematics showing proteins with an EphA2-regulated (RX)RXX[S/T]* AGC kinase motif (b) and [S/T]*P proline-directed CMGC kinase motif (c) organized by cellular functions. Phosphopeptide motifs are indicated by colored squares; regulation (up/down) is indicated by red and green arrows. d For the analysis of kinase-substrate relationship the PhosphoSitePlus, HRPD, Uniprot and NetworKIN databases were queried for known and predicted upstream kinases of regulated phosphorylation sites. Resulting kinase-substrate relationships are shown as black edges for known connections and as blue edges for connections predicted by the NetworKIN database. Kinases are organized according to kinase families. Only kinases with at least 3 substrate connections are shown. For the full network see Supplementary Fig. 5c. CMGC kinases are primarily proline-directed kinases. AGC kinases comprise kinases with a substrate specificity for RXX[S/T]* motifs. Phosphorylation sites with an RXX[S/T]* or an [S/T]*P motif are indicated by red and blue outlines around phosphosite nodes, respectively. Kinase node sizes reflect the number of regulated phosphosite connected to a kinase. mTOR, AKT, ERK, GSK-3β and RSK are the most likely candidate kinases for mediating signaling downstream of EphA2.
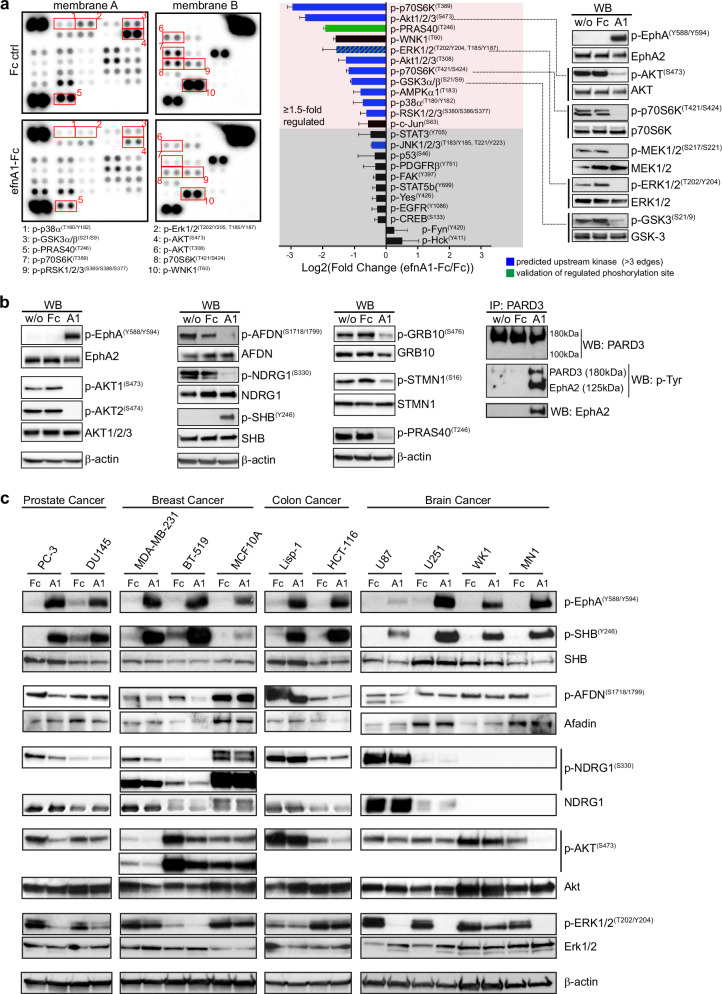
Fig. 6. Validation of phosphoproteomic data.
a, b Proteome Profiler Human Phospho-Kinase Array and western blot analysis validating pathway regulation downstream of EphA2 kinase activation in PC-3 cells. PC-3 cells were left untreated or incubated with clustered Fc control (Fc) or clustered ephrin-A1-Fc (A1) for 20 min to activate the EphA2 receptor. Representative images of array and western blot membranes are shown. β-actin is the western blot loading control. Increased PARD3 tyrosine phosphorylation was shown by immunoprecipitating PARD3 and subsequent probing with an anti-phospho-tyrosine antibody. Bar graph shows the quantification of the Proteome Profiler array as log2(fold change (efnA1-Fc:Fc control)). Mean ± SE, (n = 4 biological replicates). c Western blot analysis assessing EphA2-signaling in a panel of prostate, breast, colon and brain cancer cell lines. Cell lines were incubated with clustered Fc control (Fc) or clustered ephrin-A1-Fc (A1) for 20 min. SHB phosphorylation on Y246 is consistently upregulated by EphA2 receptor activation across all tested cell lines. Downregulation of Afadin (S1718/S1799) and NDRG1 was observed only in cell lines which responded with a robust downregulation of Akt phosphorylation on S473 (PC-3, MDA-MB-231, BT-519, HCT-116 and MN1).
The adapter protein SHB is required to mediate EphA2-induced inhibition of the ERK/MAPK pathway
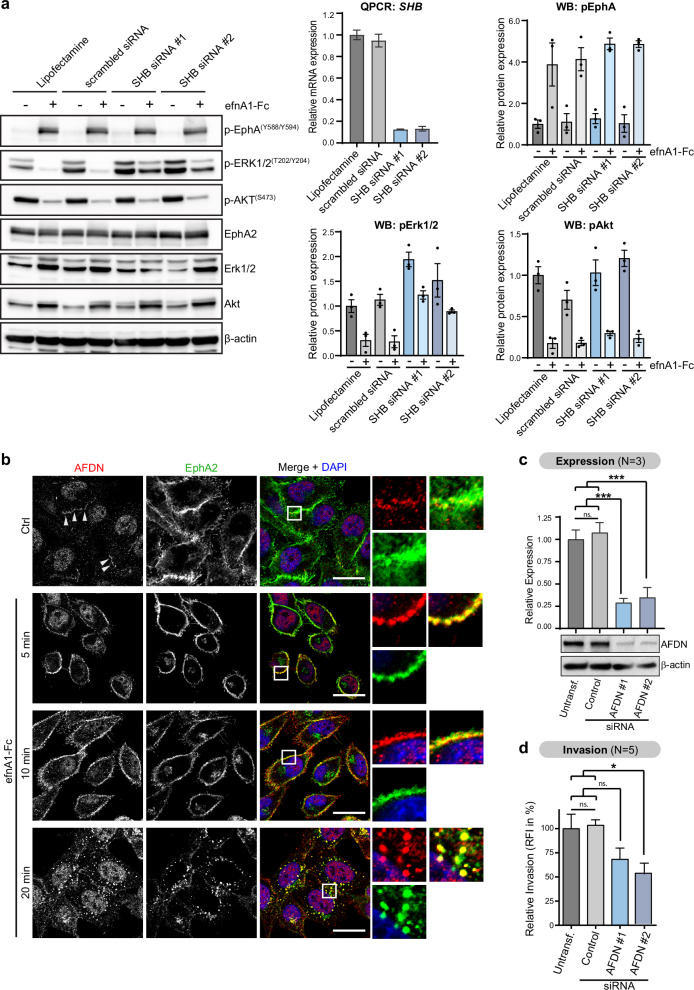
To further validate our mass spectrometry findings and prioritize phosphoproteins for further studies, we performed western blot analysis for proteins with some of the largest fold-changes in phosphorylation. Reassuringly, western blot analysis of all the sites that we tested showed strong regulation and confirmed our phosphoproteomic data: The adapter proteins SHB (pY246) and PARD3 (pY—not site-specific) showed increased phosphorylation, whereas the adapter protein GRB10 showed a strong reduction in phosphorylation at S476 (Fig. 6b). The downstream effector proteins afadin (AFDN, S1718/S1799), NDRG1 (S330), stathmin (STMN1, S16) and PRAS40 (T246), consistent with the phosphoproteomic data, all showed reduced phosphorylation (Fig. 6b). The afadin and PRAS40 sites, for example, are known sites of AKT kinases. Consistently, we observed concurrent near ablation of S473 phosphorylation of AKT isoforms 1 and 2 after stimulation, which is indicative of the inhibition of kinase activity (Fig. 6b). To extend our findings beyond PC-3 prostate cancer cells, we validated several of the phosphoprotein targets in a panel of prostate, breast, colorectal and brain cancer cell lines (Fig. 6c). Inhibition of phosphorylation of Afadin (S1718/1799) and NDRG1(S333) in response to ephrin-A1 stimulation was observed in some, but not all the cell lines. Inhibition, consistent with these sites conforming with the AKT consensus motif, was observed only in cell lines which responded to EphA2 stimulation with robust inhibition of Akt phosphorylation on S473 (PC-3, MDA-MB-231, BT-519, HCT-116 and MN1). The adapter protein SHB, on the other hand, showed consistent and strong upregulation of Y246 phosphorylation across all tested cell lines when stimulated with clustered ephrin-A1-Fc for 20 min. SHB is a ubiquitously expressed SH2 domain-containing adapter protein, which has been implicated in the signal transduction of several tyrosine kinase receptors [37–40]. To test a possible role of SHB in EphA2 receptor tyrosine kinase signal transduction, we transiently knocked down SHB using two distinct siRNA sequences before stimulating cells with ephrin-A1-Fc. Western blot analysis of AKT (S473) and Erk1/2 (T202/204) phosphorylation show that SHB knockdown increased basal ERK1/2 phosphorylation levels and diminished inhibition of ERK1/2 phosphorylation in response to EphA2 receptor activation, while AKT signaling responses remained unchanged (Fig. 7a). These data demonstrate a role for the adapter protein SHB in the signal transduction of activated EphA2 via the Erk1/2 MAPK pathway.
Fig. 7. Functional validation identifies SHB as a mediator of EphA2-induced Erk-pathway inhibition and afadin as an EphA2-regulated phosphoprotein that mediates cell invasion in PC-3 prostate cancer cells.
a Western blot analysis of the effect of transient SHB siRNA knockdown on Akt and Erk pathway inhibition in response to EphA2 activation. Cell lines were incubated with clustered Fc control (Fc) or clustered ephrin-A1-Fc (efnA1-Fc) for 20 min. A representative western botting image is shown. β-actin is the loading control. Relative phosphorylated protein levels of phospho-EphA, phospho-Erk and phospho-AKT were quantified and are shown as bar charts. Mean ± SE (n = 3 biological replicates shown as individual data points). SHB Knockdown was confirmed by quantitative QPCR and relative mRNA expression levels determined using the 2−ΔΔCt method with β-actin as the housekeeping gene and the Lipofectamine control as the reference sample. Mean ± SE (n = 3 biological replicates). Knockdown of SHB diminished the ligand-induced EphA2-mediated Erk1/2 pathway inhibition, while the inhibitory effect on the Akt pathway remained intact. b Representative confocal immunofluorescence images of afadin (AFDN, red) and EphA2 (green) cellular localization in response to EphA2 stimulation with clustered ephrin-A1-Fc (efnA1-Fc). EphA2 activation regulates the intracellular localization of afadin. Cell nuclei are labeled with DAPI (blue). Scale bars are 20 µm. c Transient knockdown of afadin (AFDN) with two different siRNA sequences was confirmed by western blot analysis. β-actin is the loading control. Bar chart shows knockdown efficiency as determined by densitometry of western blotting results. Mean ± SE (n = 3 biological replicates). ***p value < 0.001, ns. not significant (One-way ANOVA with post-hoc Šidák test for multiple pairwise comparisons). d Transwell invasion assay comparing invasion of afadin (AFDN) knockdown cells compared to untransfected and negative control siRNA-transfected PC-3 cells. Mean ± SE (n = 5 biological replicates). *p value < 0.05, ns. not significant (One-way ANOVA with post-hoc Šidák test for multiple pairwise comparisons).
The adherens junction protein afadin is an EphA2-regulated mediator of cell invasion
Phosphorylation of the adherens junction protein Afadin on serine S1799 (the same site has been referred to as S1718 based on another isoform [41]) was the most strongly reduced phosphorylation site after EphA2 activation. The same site was described by Elloul et al. as an AKT substrate that, in its phosphorylated form, promotes cell migration/invasion in breast cancer [41]. Thus, afadin was considered a candidate mediator of EphA2-regulated cell migration and invasion. Co-staining of afadin and EphA2 (Fig. 7b) showed that afadin (red) in unstimulated cells localizes predominantly to the nucleus (blue) and to a lesser extent to distinct plasma membrane regions. Upon stimulation with ephrin-A1, afadin is increasingly recruited to the plasma membrane, where it colocalizes with EphA2 (green). At later time-points (20 min), we observed that afadin had been internalized together with EphA2 into punctate intracellular compartments, consistent with receptor-mediated endocytosis of EphA2 into endosomes. We were unable to demonstrate interaction of EphA2 and afadin by co-immunoprecipitation of endogenous proteins (not shown). However, a proximity ligation assay showed increased signals indicative of protein-protein interactions/very close proximity (<40 nm) of EphA2 and afadin after 5 min of stimulation (Supplementary Fig. 7b). These data demonstrate that EphA2 signaling is involved in regulating both the phosphorylation state and the cellular distribution of afadin, although direct molecular interaction could not be conclusively demonstrated. Furthermore, transient siRNA-mediated knockdown of afadin with two distinct siRNA sequences (Fig. 7c) demonstrated a significant reduction in the invasion of PC-3 cells in transwell invasion assays (Fig. 7d), highlighting afadin as an important mediator of cell invasion in prostate cancer cells, similar to findings in breast cancer [41]. Taken together, these findings demonstrate that afadin is an EphA2-regulated phosphoprotein, which is involved in mediating invasion of prostate cancer cells.
Discussion
In the first part of the study, we characterize two EphA2 mAbs in the context of prostate cancer: 1F7, which actively blocks ephrin-induced kinase activation, and 4B3, which has activating properties, a process well-described to be tumor-suppressive [2, 3]. Notably, 1F7 can bind to both human and mouse EphA2, while 4B3 only binds to human EphA2 on the tumor xenograft and does not interact with mouse host tissues. We tested 1F7 and 4B3 antibodies in both orthotopic and intravenous PC-3 xenograft models of human prostate cancer. In support of EphA2 receptor activation being tumor-suppressive, we found that the 4B3 mAb slowed metastatic spread. In contrast, the 1F7 mAb accelerated tumor progression at primary and metastatic sites.
The pro-tumorigenic effect of 1F7, which inhibits EphA2 kinase activation, is consistent with 1F7 blocking any activating signals by ephrin ligands expressed on stromal or endothelial cells, thus countering any effect of EphA2 canonical signaling in reducing tumorigenesis and thus promoting kinase-independent pro-oncogenic functions of EphA2 [2, 3, 17]. In the intravenous PC-3 metastasis model, 1F7-treated animals also displayed accelerated tumor progression and decreased survival. This effect was reduced when 1F7 administration ceased shortly after tumor cell engraftment, implying that 1F7, as in the orthotopic xenograft tumors, primarily promotes tumor growth rather than tumor dissemination. In addition, our data show that 1F7, which binds both human and mouse EphA2, induces vascular leak in the normal mouse endothelium, an effect that may contribute to tumor infiltration and the egress of pro-tumorigenic stromal elements into the metastatic sites. A recent review highlighted the expression of EphA2 in normal endothelium and the capacity of cytokines and other pathologies to potently upregulate the expression of both EphA2 and its ephrin ligands, resulting in vascular leakage [25]. The ability of Eph/ephrin interaction to switch from pro-adhesive to de-adhesive effects is a well-described phenomenon [42–44]. Our results demonstrate that 1F7 inhibits ephrin-mediated receptor activation in both PC-3 prostate cancer cells and mouse and human endothelial cells. That 1F7 blockade of ephrin interaction with EphA2 induces vascular leak is consistent with studies demonstrating EphA2-ephrin-A1 interaction, under normal homeostatic conditions, contributes to the stability of cell junctions and promotes endothelial cell-cell adhesion and barrier function, while disruption causes vascular leak. In EphA2-null mice, the loss of EphA2 disrupts endothelial-pericyte interactions, resulting in leaky vessels [26, 45]. Similarly, soluble ephrin-A1 has been shown to increase vascular permeability by disrupting adherens and tight junctions between endothelial cells, and both soluble ephrin-A1 and EphA2 on exosomes were found to disrupt the endothelial barrier and promote metastasis [26, 28, 46]. Conversely, treatment with EphA4-Fc as a pan-ephrin inhibitor has recently been shown to counteract vascular leak in a sepsis model by removing soluble ephrin-A1 released during inflammation, thereby preventing breakdown of endothelial junctions and maintaining endothelial barrier integrity [47]. However, it is important to note, that while disruption of the endothelial barrier might lead to increased extravasation of PC-3 tumor cells, the dominant effect of 1F7 in our study appeared to be increased tumor growth.
In contrast to 1F7, the 4B3 mAb permitted ephrin-induced EphA2 receptor activation and, if clustered, was able to activate EphA2. This suggests that, in the presence of 4B3, PC-3 tumor cells, which themselves do not express significant levels of ephrin-A ligands, continue to receive activating, anti-oncogenic signals by ephrin ligands expressed on or shed by stromal and endothelial cells. In addition, in contrast to IF7 IgG1 mAb, the 4B3 IgG2a mAb, would bind with high affinity to mouse FcγRI, FcRγIV and FcRn receptors which, in the absence of endogenous immunoglobulins in NRG mice, would decorate the surface of myelomonocytic and endothelial cells, effectively clustering the 4B3 antibody, thus enabling it to activate human EphA2 receptors in vivo. In our study, 4B3 showed efficacy in reducing metastatic spread and improved survival in the intravenous animal experiments. This effect was maintained when 4B3 was administered only in the days prior to and shortly after intravenous injection of PC-3 cells, suggesting an early anti-metastatic effect of EphA2 kinase activation. These results are consistent with the 4B3 antibody functioning in an agonistic capacity, similar to other studies demonstrating anti-tumor effects of agonistic EphA2 mAbs in xenograft models [48–50]. In these studies, EphA2 kinase activation, followed by receptor internalization and degradation, implied that the underlying mechanisms involve induction of anti-oncogenic, canonical signaling and decreased EphA2 cell surface expression. Furthermore, EphA2 kinase activation diminishes the phosphorylation of EphA2 at S897, thereby countering the pro-invasive and pro-metastatic effects of non-canonical EphA2 signaling [17, 51–54]. An alternative explanation for the anti-oncogenic effects of 4B3 in vivo, is the possibility that the interaction of 4B3 with the extracellular domain of EphA2 alters its ability to engage in multimeric complexes in the absence of ligand binding. The presence of ligand-independent multimeric complexes of EphA2, which promote EphA2 S897 oncogenic signaling, have recently been described by Wang et al. [55]. The extracellular domain was shown to play a key role in this atypical, ligand-independent multimerization and disruption of this process was proposed by the authors as a possible avenue for therapeutic intervention to block ligand-independent, oncogenic EphA2 signaling. Regardless of the mechanism, it is important to note that the 4B3 mAb is human-specific and highlights direct effects on tumor cells without any indirect effects on host stromal and vascular elements. This has clinical implications, as shown by a human-specific EphA2 antibody-drug conjugate MEDI-547, which was well tolerated and demonstrated anti-tumor efficacy in animal models but caused significant vascular toxicity in a phase I human trial that was halted [56, 57].
In seeking to better understand the observed anti-tumor effects of EphA2 receptor activation, we performed quantitative phosphoproteomics to investigate downstream signaling in response to stimulation with ephrin-A1 (Fig. 8). We and others have shown that PC-3 cells express high levels of EphA2 but negligible levels of other EphA receptors or ephrin-A ligands, making this a “clean” model to specifically assess EphA2-mediated downstream kinase events [17, 20–22]. Previous studies have demonstrated that the activation of EphA2 induces inhibition of the PI3K/AKT, ERK/MAPK and FAK/SRC pathways [17, 18, 20, 22], however downstream effector proteins remained largely understudied. We found that EphA2 kinase activation led to changes in the phosphorylation of proteins predominantly involved in cell-cell and cell-matrix adhesion and cytoskeletal regulation. Consistently, these changes coincide with increased contractile forces, retraction of cell protrusions, and inhibition of locomotion, as previously reported [17]. Motif and upstream kinase analyses of regulated phosphosites are consistent with inhibition of AKT/mTOR and MEK/ERK pathways as key mediators of EphA2 signaling. Furthermore, our study identified the adapter protein SHB, which became phosphorylated on Y246 in response to EphA2 activation, as a necessary component in the signal transduction of ligand-activated EphA2 receptor. SHB siRNA knockdown demonstrated its role in mediating the inhibition of the ERK/MAPK pathway in response to EphA2 activation but did not impact on the PI3K/AKT pathway. SHB, a ubiquitously expressed adapter protein, has previously been implicated in the signal transduction of several other receptor tyrosine kinases, including PDGFR, FGFR, VEGFR and EphB2 [37–40]. These studies demonstrated that receptor activation induces SHB tyrosine phosphorylation on several tyrosine residues, including Y246, a site that has been implicated in the binding of RasGAP, a negative regulator of the Ras/ERK/MAPK signaling cascade [40]. Thus, our results suggest a potential mechanism for EphA2-mediated inhibition of ERK/MAPK signaling. While our manuscript, in line with previous reports [17, 18, 20, 22], demonstrates inhibition of AKT, ERK1/2 and GSK3B kinases, it is possible that EphA2 signaling also stimulates phosphatase activities that may contribute to the observed inhibition of protein phosphorylation of AKT and ERK1/2 substrates.
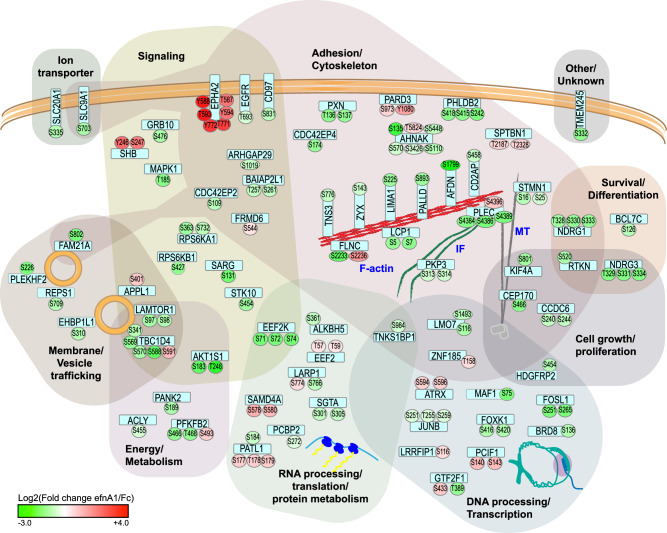
Fig. 8. Summary of EphA2-regulated phosphoproteins.
Schematic showing EphA2-regulated phosphoproteins as rounded rectangular nodes (light blue) and regulated phosphorylation sites as circular nodes. Proteins are grouped based on known functions and are depicted as a modified Venn-style diagram to allow for multiple functions. The color and intensity of phosphosite nodes indicates the log2-transformed fold-change (efnA1-Fc:Fc control) values for the significantly EphA2-regulated phosphopeptides as determined by quantitative phosphoproteomics.
The AKT/mTOR and ERK/MAPK pathways relay signals by phosphorylating numerous substrates that regulate a multitude of biological functions, including cell growth, survival, metabolism, and invasion. Intriguingly, our phosphoproteomic dataset indicated that EphA2 predominantly inhibits the phosphorylation of AKT and ERK substrates involved in cytoskeletal regulation, cell adhesion and invasion in prostate cancer cells. The selectivity of downstream substrates may, at least in part, be due to the subcellular localization of EphA2, which determines its protein interactions and the recruitment and translocation of substrates. A study by Perez White et al. identified EphA2-associated proteins in healthy keratinocytes using a proximity ligation assay in combination with mass spectrometry [58]. Of our list of EphA2-regulated phosphoproteins, approximately one third (25 out of 73 proteins) were either identical (12 proteins) or paralogs (13 proteins) of proteins identified in this study.
Consistently, we observed that afadin, which is associated with EphA2 in keratinocytes [58], is recruited to and colocalizes with EphA2 at the plasma membrane upon EphA2 activation. This coincided with afadin showing the strongest decrease in phosphorylation in our dataset of EphA2-regulated phosphoproteins. Afadin is a cell junction scaffolding protein. Early studies described two isoforms [59]: a short splice variant, s-afadin, expressed in neural tissues, and a ubiquitously expressed, long splice variant, l-afadin, which contains an F-actin binding domain that connects the cell adhesion molecule nectin to the actin cytoskeleton [59, 60]. While the Uniprot database [31] lists six isoforms of human afadin (Uniprot ID P55196), the literature appears to refer to l-afadin more broadly as any of the larger/ubiquitously expressed isoforms. We chose the canonical sequence (Uniprot isoform 4) for the numbering of the EphA2-regulated afadin phosphorylation site (S1799). The same phosphorylation site (KERQRLFS*QG) is also found in isoforms 3 (S1718) and 5 (S1809). Elloul et al. identified the S1718/S1799/S1809 site as an AKT substrate and demonstrated that the function of afadin can be switched from cell-cell adhesion to an invasion-promoting protein in breast cancer by regulating the phosphorylation of this site [41]. Similarly, our study highlights afadin as an important regulator of cell invasion in prostate cancer cells and further demonstrates that activated EphA2 alters the localization and leads to dephosphorylation of afadin at its AKT substrate site (S1718/S1799/S1809), implying a similar mechanism and identifying EphA2 as an upstream regulator. Regulation of afadin by Eph receptors at cell junctions may indeed be a more common theme; afadin has been shown to interact with EphB2 and EphB3 in a kinase activation-dependent manner at specialized sites of cell-cell contact in the brain, leading to increased EphB-mediated tyrosine phosphorylation of afadin [61, 62]. More broadly, phosphorylation of cell junction proteins has been described as a common switch to regulate protein interactions and, consequently, function [63]. Our dataset of EphA2-regulated phosphoproteins was significantly enriched for adherence junction and cytoskeletal proteins, some of which are known to be able to switch between adhesive and invasion-promoting functions, including afadin, plakophilin, PARD3 and palladin among others [41, 64, 65].
In summary, our study demonstrates that EphA2-ephrin-A interactions induce downstream signaling on targets that promote cell adhesion and inhibit the formation of membrane protrusions and cell motility, thus acting in a tumor-suppressive manner and contributing to epithelial and endothelial integrity. Indeed, EphA2 has been shown to regulate adherens and tight junctions and vice versa in epithelial and endothelial cells [66–68]. Furthermore, our ephrin-blocking 1F7 antibody interfered with the EphA2-ephrin linkage, which acts to maintain endothelial barrier function, and induced vascular leak, an effect that potentially contributes to metastasis. Tumor cells often show a concomitant loss of ephrin-A expression when EphA2 acts in a ligand-independent, pro-oncogenic manner [2, 8]. Thus, restoring kinase activation has been proposed as a therapeutic avenue for targeting EphA2-expressing human cancers [6]. Consistent with the dichotomous role of this receptor, our EphA2-targeting mAbs with distinct activating and blocking functions demonstrated opposing tumor-suppressive and oncogenic properties. Our in vivo antibody treatment studies indicate that the addition of an activating EphA2 mAb can restore EphA2 signaling in ephrin-A-negative aggressive epithelial cancers and inhibit pro-tumorigenic effects. Notably, the 1F7 mAb, which blocks ephrin binding, thus preventing EphA2 activation, increased prostate cancer aggressiveness.
Methods
Cell culture
PC-3 (ATCC #CRL-1435), LNCaP (ATCC #CRL-1740), DU145 (ATCC #HTB-81), MDA-MB-231 (ATCC #HTB-26), BT-519 (ATCC #HTB-122), MCF10A (ATCC #CRL-10317), Lisp-1 (RRID:CVCL_9U26), HCT-116 (ATCC #CCL-247), U-87MG (ATCC #HTB-14) and U251MG (ECACC #09063001) cell lines were maintained in RPMI 1640 (in-house media service) and 2H-11 (ATCC #CRL-2163) in DMEM media (Thermo Fisher Scientific), containing 10% fetal bovine serum (FBS), 2 mM L-Glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin (all Thermo Fisher Scientific). PC-3M and PC-3MM2, metastatic sublines of PC-3 cells [69], were kind gifts from Prof. C. A. Pettaway (The University of Texas MD Anderson Cancer Center). Human umbilical vein endothelial cells (HUVEC; Thermo Fisher Scientific #C0035C) were maintained in 200PRF medium containing low serum growth supplement (both Thermo Fisher Scientific). The primary glioblastoma cell lines WK1 and MN1 were generated in-house [70, 71] and were maintained as glioma neural stem cell (GNS) cultures using StemPro NSC SFM (Thermo Fisher Scientific) as per manufacturer’s guidelines. Cell line identity of human cell lines and mycoplasma-free status of cell cultures were confirmed by in-house short tandem repeat (STR) profiling and mycoplasma diagnostic testing services, respectively. PC-3 cells expressing luciferase (PC-3-luciferase) were generated using Firefly Luciferase Lentifect Purified Lentiviral Particles (GeneCopoeia). Briefly, 70–80% confluent cells in a 6-well were transduced with 2 µl of Lentifect particles in 2 ml of antibiotic-free media containing 8 µg/ml polybrene (Merck). After centrifugation (500 × g, 45 min), the cells were incubated for 24 h and medium replaced. Selection medium containing 1 µg/ml puromycin (Sigma-Aldrich) was added 72 h after transduction. For stimulation experiments, human ephrin-A1-Fc (recombinant soluble ephrin-A1 fused to human IgG1Fc; custom production by CSL) or Fc control proteins (human IgG; Thermo Fisher Scientific #02-7102) were clustered with Fc fragment-specific rabbit anti-human secondary antibodies (Jackson ImmunoResearch #309005008) at a 2:1 ratio in serum-free RPMI media (1 h, 4 °C) before stimulating serum-starved cells at a final concentration of 1 µg/ml. For stimulation experiments with clustered anti-EphA2 antibodies, mouse anti-EphA2 antibodies were clustered with Fc fragment-specific goat anti-mouse secondary antibodies (Jackson ImmunoResearch #115-005-071) at a 2:1 ratio in serum-free RPMI media (1 h, 4 °C) before stimulating serum-starved cells at a final concentration of 10 and 30 µg/ml, respectively. Cytochalasin D (Merck), was used at a final concentration of 1 µM.
Development of EphA2 monoclonal antibodies
The development of the EphA2 monoclonal antibodies (mAbs) used in this study has been reported previously [19]. Briefly, the 4B3 hybridoma was derived from BALB/c mice and the 1F7 hybridoma from EphA2 placental alkaline phosphatase (PLAP) reporter knockout mice [72] immunized with EphA2-Fc protein, generated using an EphA2-Fc expression vector (kindly provided by Prof. B. Wang, Case Western Reserve University), as the immunogen.
Bio-layer interferometry
The antibody binding kinetics of EphA2-specific mAbs to human EphA2-Fc-loaded biosensors were compared by bio-layer interferometry using the Octet RED system (Sartorius). Recombinant EphA2-Fc protein was conjugated to biotin (1:1 molar ratio) using EZ-Link Sulfo NHS-LC-LC-Biotin (Thermo Fisher Scientific) following the manufacturer’s instructions. Excess biotin was removed using Zeba Spin desalting columns 7K MWCO (Thermo Fisher Scientific). Biotinylated EphA2-Fc protein (50 µg/ml) was loaded onto Octet Streptavidin Biosensors (Sartorius) according to the manufacturer’s instructions, and the binding of EphA2-specific antibodies and human ephrin-A1-Fc to individual sensors was recorded. Upon saturation, the probes were exchanged into 1× Kinetics Buffer (1 mM phosphate, 15 mM NaCl, 0.005% Tween 20 and 0.1 mg/ml BSA) to allow dissociation.
Western blotting, immunoprecipitation and Proteome Profiler Array
Cells were lysed in lysis buffer A (20 mM Tris/HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, containing PhosSTOP Phosphatase and cOmplete, and Mini, EDTA-free Protease Inhibitor Cocktails (both Merck)). Crude lysates were centrifuged (16,000 × g), supernatants collected, and protein concentrations determined using the Bio-Rad protein assay (Bio-Rad Laboratories). Equal amounts of denatured protein samples were separated using SDS-PAGE and transferred onto PVDF membranes. Blocked membranes (5% BSA/PBS-Tween) were analyzed using the following primary antibodies: Anti-phospho-EphA2/A3/A4 (Y588 + Y596) (#ab62256), anti-phospho-SHB (Y246) (#ab138388), anti-SHB (#ab129190) and anti-FAK (#ab40794) antibodies were purchased from Abcam. Phospho-EphA2 (S897) (#6347), anti-EphA2 (#6997), anti-phospho-AKT (S473) (#4060), anti-AKT (#2920), anti-phospho-ERK1/2 (T202/T204) (#4370), anti-ERK1/2 (#4695), anti-phospho-MEK1/2 (S217/221) (#9154), anti-MEK1/2 (#9122), anti-phospho-FAK (Y397) (#8556), anti-phospho-SRC (Y416) (#2101), anti-SRC (#2110), anti-phospho-p70 S6 Kinase (T421/S424) (#9204), anti-p70 S6 Kinase (#2708), anti-phospho-PRAS40 (T246) (#13175), anti-phospho-GSK-3α/β (S21/S9) (#9327), anti-GSK-3α/β (#5676), anti-phospho-afadin (S1718) (#5485), anti-afadin (#D1Y3Z), anti-phospho-NDRG1 (S330) (#11899), anti-NDRG1 (#9485), anti-phospho-tyrosine (P-Tyr-100) (#9411), anti-β-actin (#4970) and anti-β-actin (#3700) antibodies were purchased from Cell Signaling Technology. The anti-PARD3 (#11085-1-AP) antibody was purchased from Thermo Fisher Scientific. Blots were developed using goat anti-rabbit and goat anti-mouse IgG-HRP antibodies (both Agilent: #P044801-2, #P044701-2) and Clarity Western ECL Substrate (Bio-Rad Laboratories). For immunoprecipitation experiments, equal amounts of cell lysates were pre-cleared with 20 µl of 50%-slurry Protein G Sepharose beads (Abcam #ab193259). Anti-PARD3 antibody (Thermo Fisher Scientific #11085-1-AP) was bound to Protein G Sepharose beads (1 h, 4 °C) and the unbound antibody was removed by washing with lysis buffer. 40 µl of antibody-coated beads (50%-slurry) were added to pre-cleared lysates and incubated for at least 2 h at 4 °C. Beads were washed with lysis buffer and immunoprecipitated proteins were analyzed by western blotting. The Proteome Profiler Human Phospho-Kinase Array Kit (R&D Systems, #ARY003) was used according to the manufacturer’s instructions.
Quantitative PCR
Total RNA was extracted and purified using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. First-strand cDNA was synthesized using oligo(dT) primers in conjunction with Superscript IV Reverse Transcriptase (Thermo Fisher Scientific) according to the manufacturer’s instructions. Quantitative real-time PCR was carried out using SYBR Green PCR Master Mix (Applied Biosystems) following the manufacturer’s instructions. The PCR primers used for SHB were F: 5’ GAT CCC TTT GAT GCC AAG AA and R: 5’ CTC TCC GAG TCC GAG TCA AC and have been reported previously [73]. The following PCR conditions were used: 40 cycles of denaturation at 95 °C for 30 s, followed by annealing and extension for 30 s at 63 °C. All reactions were performed in technical triplicate for each of the biological triplicates on an ABI Viia 7 real-time PCR system (Applied Biosystems). Cycle thresholds (Ct) were determined and exported using ABI QuantStudio 5 software (Applied Biosystems). The fold change in SHB mRNA transcripts levels between groups was determined using the 2−ΔΔCT-method with β-actin as the reference house-keeping gene.
Flow cytometry analysis
The cells were detached with 5 mM EDTA/PBS and washed with FACS buffer (2% FCS/PBS). 0.5–1 × 106 cells were incubated in 5–10 µg/ml primary antibodies (1F7 or 4B3 mAbs) or human EphA3-Fc (in-house), washed, then incubated with 5 µg/ml secondary antibodies donkey anti-mouse Alexa Fluor 647 (#A-31571) or goat anti-human Alexa Fluor 488 (#A-11013, both Thermo Fisher Scientific). Data were acquired on an LSR Fortessa flow cytometer (BD Biosciences) and analyzed using FlowJo software (FlowJo, LLC).
Immunofluorescence microscopy
For the stripe assay, glass coverslips were coated in a stripe-like pattern using silicone matrices (Prof. Bastmeyer, Karlsruher Institute of Technology) by adapting the method of Knoll et al. [74]. Briefly, 10 µg/ml clustered ephrin-A1-Fc was used to fill the channels of the silicone matrices covered with a glass coverslip. To generate a binary substrate choice, alternating stripes were subsequently coated with 10 µg/ml clustered Fc control or 1:100 Matrigel Basement Membrane Matrix (Corning, #354234). For immunofluorescence staining, PFA-fixed cells were permeabilized with 0.1% Triton X-100/PBS, blocked with 0.25% BSA/PBS and immunolabelled with the following primary antibodies: anti-EphA2 (1F7 mAb), anti-afadin/AF-6 (Novus Biochemicals, #NBP1-90219), and anti-α-tubulin (Cell Signaling Technology, #2125). Rhodamine-phalloidin (#R415). Alexa Fluor 488- and Alexa Fluor 647-conjugated secondary antibodies were purchased from Thermo Fisher Scientific. Ephrin-A1-Fc-coated stripes were visualized using donkey anti-rabbit IgG Alexa Fluor 488 (Thermo Fisher Scientific, #R37118). Coverslips were mounted using ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific). Images were acquired with a Zeiss LSM780-NLO confocal microscope. The anisotropy of microtubules was analyzed using the FibrilTool plugin [75] in ImageJ [76].
Proximity ligation assay
For the proximity ligation assay (PLA), cells were plated on coverslips, allowed to adhere overnight, serum-starved for 3 h, then stimulated with 1 ug/ml clustered ephrin-A1-Fc for 5 min or left untreated. PFA-fixed cells were permeabilized with 0.1% Triton X-100/PBS prior to Duolink PLA. Duolink Fluorescent PLA (Merck) was carried out according to the manufacturer’s instructions using the following reagents: Duolink In Situ PLA Probe Anti-Mouse PLUS (# DUO92001), Duolink In Situ PLA Probe Anti-Rabbit MINUS (# DUO92005), Duolink In Situ Wash Buffers, Fluorescence (#DUO82049) and Duolink In Situ Detection Reagents Green (#DUO92014). Briefly, coverslips were blocked with Duolink Blocking Solution for 60 min, then incubated for 60 min with the anti-afadin antibody (Novus Biochemicals #NBP1-90219) and anti-EphA2 1F7 mAb (in-house), both diluted 1:50 in Duolink Antibody Diluent. Coverslips incubated with anti-afadin antibody alone or anti-EphA2 antibody alone or in the absence of antibody serve as negative technical PLA controls. Coverslips were washed in Duolink Wash Buffer A, then incubated with the Duolink PLUS and MINUS PLA probes for 60 min. After washing in Duolink Wash Buffer A, coverslips were incubated with Duolink ligation solution for 90 min, again washed in Duolink Wash Buffer A and incubated with Duolink amplification solution for 100 min, then washed with Duolink Wash Buffer B. All incubation steps were carried out in a humidity chamber at 37 °C. Slides were mounted in ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific). Images were acquired with a Zeiss LSM780-NLO confocal microscope.
Live cell brightfield imaging
PC-3 cells were plated in one well of a silicone culture insert (Ibidi, #IBI81176). Once confluent, the insert was removed, and cells were overlaid with Matrigel Basement Membrane Matrix (Corning #354234) diluted 1:1.2 in CO2-independent medium (Thermo Fisher Scientific), containing a final concentration of 1 µg/ml clustered ephrin-A1-Fc or Fc-control. Matrigel was allowed to gel before CO2-independent medium containing 1 µg/ml ephrin-A1-Fc or Fc control was overlaid. Time-lapse brightfield microscopy images were acquired on an IX81 inverted microscope (Olympus) with a motorized stage and environmental chamber using xCellence Software (Olympus) and processed using ImageJ [76].
siRNA silencing and transwell invasion assay
Lipofectamine2000 (Thermo Fisher Scientific) was used according to the manufacturer’s instructions. Ambion Silencer Select siRNA oligonucleotides were purchased from Thermo Fisher Scientific: Afadin siRNA sequence #1: 5’-GGAUCACACUGGAUGCUCAtt-3’ and sequence #2: 5’-GCGUGUUACACGUUCCCAAtt-3’, SHB siRNA sequence #1 5’-GCAAAUAUGGUAUCACGGAtt-3’ and sequence #2: 5’-GGUAUCCAGUUAUAUGACAtt-3’, Grb10 siRNA sequence #1: 5’-GAAUGCUCCUUUACCAGAAtt-3’ and sequence #2 5’-GGCUUUUUCUCCUCCGUGAtt-3’, and Negative Control #1 siRNA (#4390843). Per well of a 6-well plate 4 µl transfection reagent and 12.5 pmol siRNA were prepared in OptiMEM (Thermo Fisher Scientific). The cells were cultured under antibiotic-free conditions. For transwell invasion experiments, cells were transfected, incubated for 30 h, serum-starved for 18 h, then set up using the QCM ECMatrix Cell Invasion Assay, 24-well (8 µm), fluorimetric (Merck #ECM 554) according to the manufacturer’s instructions. Briefly, cells were harvested using 5 mM EDTA/PBS. 2.5 × 105 cells in serum-free RPMI media were added to each transwell insert. FBS/RPMI medium (10%) was added to the lower chamber, and cells were incubated for 24 h.
Animal studies
All experimental protocols for the animal studies were approved by the QIMR Berghofer Animal Ethics Committee (A0304-620M). No sample size calculations were performed prior to the study. The investigators were not blinded to the group allocation. Intravital bioluminescence imaging was performed to objectively monitor and quantify tumor burden, supporting the validity of the survival data. At endpoint, animals were euthanised by cervical dislocation. Tissues samples were fixed in 10% neutral-buffered formalin, paraffin-embedded, and stained with hematoxylin and eosin (H&E).
Prostate orthotopic xenografts
PC-3-luciferase cells were orthotopically engrafted into the prostates of 6–8 week-old male NRG mice (NOD.Cg-Rag1tm1MOM IL2rgtm1Wjl/SzJ [77], The Jackson Laboratory, #007799). Mice received the analgesic Temgesic IP (0.03 mg/kg in PBS) prior to surgery and a top-up dose (0.06 mg/kg, SC) after surgery. Mice were anesthetized IP with 100 mg/kg ketamine and 10 mg/kg xylazine. The prostate was accessed through a lower midline abdominal incision by gently lifting the bladder through the incision. PC-3-luciferase cells (2.5 × 105) in 10 µl PBS were injected using a 27 G micro-injection needle and Hamilton syringe. The needle was left in place for an additional minute. The bladder was placed back in the abdominal cavity, and muscle and skin layers were individually sutured using lyconate monofilament absorbable 5/0 sutures and sutures secured with Vetbond tissue adhesive (3 M Animal Care Products). Mice were randomized into treatment groups before treatment commenced on day 10 after engraftment. The mice received thrice weekly IP injections of 8 mg/kg 1F7 mAb, 4B3 mAb, or IgG1 (#BE0083)/IgG2a (#BE0085) InVivoMAb isotype control antibodies (1:1 ratio, both BioXCell) in PBS or PBS vehicle control until endpoint.
Intravenous metastatic xenografts
Male NRG Mice from multiple litters were randomized into treatment groups prior to the start of the experiment. PC-3-luciferase cells (5 × 105) in 200 µl PBS were injected via the lateral tail vein. Mice received 8 mg/kg 1F7 mAb, 4B3 mAb, IgG1/IgG2a isotype control mAbs or PBS vehicle control 2 days and 4 h prior to tumor cell engraftment. Mice either continued treatment thrice weekly until endpoint (“Protocol 1”) or received one final dose on day 2 after cell injection (“Protocol 2”). Mice were excluded from the experiment if humane endpoint was reached without demonstrating detectable tumor burden by intravital bioluminescence imaging.
Intravital and ex vivo bioluminescence imaging
Tumor burden was monitored using intravital bioluminescence imaging. Mice were anesthetized by isoflurane inhalation, injected IP with 100 µl of 5 mg/ml D-luciferin (Gold Biotechnology, #eLUCK-3G) in PBS and imaged using the IVIS Spectrum in vivo imaging system (PerkinElmer). For ex vivo imaging of lung metastatic burden, mice were euthanized 5 min after luciferin injection, and the lungs were dissected and imaged 5 min after euthanasia. Signals were quantified using Living Image Software (PerkinElmer).
Vascular permeability studies
Vascular permeability was measured using the Miles Assay. Male NRG mice were injected with 200 µl of filter-sterilized 0.5% Evans Blue dye (Sigma-Aldrich) in PBS via the lateral tail vein. Mice were anesthetized with ketamine/xylazine as described above. Ten minutes after dye injection each mouse received 30 µl of 1F7, 4B3 and isotype control mAbs (2 µg/ml each) and PBS control via intradermal injection into discrete areas of the flank. The mice were euthanized 20 min later. Evans Blue dye effusion into the dermis was photographed and tissue biopsy cores were collected using 6 mm biopsy punches (Kai-Group). Evans Blue dye was extracted in 400 µl formamide per core (48 h, 55 °C) and quantified by spectrophotometry (A620nm).
Sample preparation for phosphoproteomic mass spectrometry analysis
PC-3 cells were grown in RPMI 1640 for SILAC medium containing 10% dialyzed FBS, 100 U/ml Penicillin, 100 µg/ml Streptomycin (all Thermo Fisher Scientific). The medium was supplemented with 200 mg/l L-proline to prevent amino acid conversion [78] and either 48 mg/l unlabeled “light” L-12C6-arginine (Arg) and 200 mg/l L-12C6-lysine (Lys) or equimolar amounts of the isotopic “heavy” L-13C6-Arg and L-13C6-Lys (all Cambridge Isotope Laboratories). After six passages cells showed >95% labeling efficiency, which did not increase further with subsequent passages. PC-3 cells grown under “heavy” and “light” conditions for 6 passages were serum-starved overnight, then incubated for 20 min with 1 µg/ml clustered ephrinA1-Fc and control Fc, respectively (“forwards experiment”). The experiment was done in biological quadruplet and labels were exchanged between conditions in replicates two and four (“reverse experiment”). Cells were washed thrice with ice-cold PBS and lysed on ice with RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific) containing Roche PhosSTOP and cOmplete, Mini, EDTA-free Protease Inhibitor Cocktails (both Merck). Lysates were centrifuged (16,000 × g, 20 min, 4 °C) and the supernatants were collected. Equal amounts (800 µg) of cell lysates from “heavy” and “light” conditions were pooled and proteins enriched using a 2D clean-up kit (GE Healthcare) and quantitated using the 2D Quant kit (GE Healthcare). Samples (a total of 1.5 mg) were digested using a double digest with Lys-C and trypsin endoproteases as previously described [79]. Briefly, proteins were precipitated using −20 °C methanol. Protein pellets were washed thrice in 1 ml of −20 °C 90% (v/v) methanol, centrifuged at 16,000 × g for 20 min at 4 °C and pellets resuspended in 8 M urea/100 mM NH4HCO3 and sonicated for 5 min. The urea concentration was adjusted to 6 M using 50 mM NH4HCO3 before incubation with Lys-C (Wako) at an enzyme-to-substrate ratio of 1:100 at 37 °C for 6 h. The samples were then diluted with 50 mM NH4HCO3 to adjust the urea concentration to 1.6 M before trypsin (Promega) was added at an enzyme-to-substrate ratio of 1:50, followed by incubation at 37 °C for 18 h. Phosphopeptides were enriched with TiO2 beads (GL Science). Briefly, 800 µg of digested peptides were dried and resuspended in 1 M glycolic acid solution prepared in 5% (v/v) TFA, containing 80% (v/v) acetonitrile and 1% (v/v) SDS. Subsequently, the solutions were diluted to 0.2% (v/v) SDS and loaded on to a TiO2 column (peptide:TiO2 beads ratio of 1:4). The column was washed twice with 1% (v/v) and 0.1% TFA containing 80% (v/v) and 20% (v/v) acetonitrile, respectively. Phosphopeptides were eluted with 25% ammonia and immediately acidified with formic acid. The eluates were dried and resuspended in 0.1% TFA containing 2% (v/v) acetonitrile.
Mass spectrometry and data processing
All mass spectrometric analyses were performed using a Nano Ultra-High Performance Liquid Chromatograph (nUHPLC) (Waters NanoAcquity) interfaced with a high-resolution LTQ Orbitrap-VelosPro mass spectrometer (Thermo Fisher Scientific). Quantitative proteomic data (for normalization of phosphoproteomic data) were obtained by running a single shot 300 min LC-MS analysis on a 2 μg aliquot of the LysC/tryptic digest (four replicates). Four elutions from TiO2 column (phosphopeptide-enriched elutions) for each biological replicate were analyzed by LC-MS using a 180 min LC gradient and the top 20 CID-MSA (multistage activation) method on the LTQ-Orbitrap-VelosPro. A Waters C18 BEH, 130 Å, 1.7 μm particle size, 75 μm × 200 μm analytical column and Waters 2G-V/M C18 Symmetry trap, 100 Å, 5 μm particle size, 180 μm × 20 mm trap were used for all analysis. A flow rate of 300 nl/min and a column temperature of 35 °C were used.
RAW MS data files were analyzed using the MaxQuant computational platform [80] (version 1.4.0.8). Proteins and peptides were identified using the Andromeda search engine [81] by querying concatenated forward and reverse sequences from the complete human Uniprot database [31] downloaded on 31/01/2013. To search for precursor and fragment ions, the mass tolerance was set to 6 ppm and 20 ppm, respectively. The enzyme specificity was set to trypsin/P and Lys-C/P, allowing a maximum of two missed cleavages. The minimum peptide length was set to seven amino acids. Carboxamidomethyl-cysteine was defined as a fixed modification, whereas phosphorylation of serine/threonine/tyrosine, acetylation of protein N-termini, deamidation of asparagine/glutamine, and oxidation of methionine were defined as variable modifications. Peptides and proteins were identified with a maximum false discovery rate (FDR) of 1%. Reverse and contaminant hits were removed from the MaxQuant output file. Only phosphopeptides with class I phosphorylation sites, which had been assigned with a localization probability of ≥0.75 [82], were considered for further analysis. To assess the significance of outlier ratios from the bulk of the distribution “Significance B” (calculated by MaxQuant version 1.4.0.8) was obtained by grouping protein subsets into intensity bins and correcting for multiple hypothesis testing with a Benjamini–Hochberg corrected p value threshold of 0.05. Q-value estimation was computed using the “qvalue” package in R (version 3.1.2). Phosphopeptides were considered to be regulated at a Benjamini–Hochberg corrected p value threshold of <0.05. Phosphopeptides had to be significantly regulated in at least three out of four biological replicates to be included in the list of EphA2-regulated phosphopeptides for downstream bioinformatics analysis.
Downstream bioinformatics analysis
Gene ontology (GO) and pathway enrichment analysis
Enrichment analyses of GO annotations [83], KEGG [84], and REACTOME [85] pathways were performed using the ClueGO plugin [86] (version 2.3.4) in Cytoscape [87] (version 3.2.1). Precompiled annotation files for QuickGO [88], KEGG and REACTOME Pathways were downloaded on 21/09/2017. All evidence codes except “Inferred from Electronic Annotation” were allowed. GO levels 2 to 8 were included in the analysis. The minimum number of genes for each term was set to 2 (GO Cellular Component) and 3 (other GO annotations, KEGG and REACTOME Pathways), and the minimum percent coverage of terms was specified as 7.5% (GO annotations) or 5% (KEGG and REACTOME Pathways). EphA2-regulated proteins were queried for enriched terms against our own background dataset (all detected phosphoproteins with class I phosphorylation sites; contaminant and reverse sequences removed). Enrichment was evaluated using a one-sided hypergeometric test. FDR was controlled using the Benjamini–Hochberg correction for multiple hypothesis testing. Significantly overrepresented terms (corrected p value < 0.05) were visualized, and related and redundant terms clustered with the ClueGO plugin in Cytoscape (kappa score threshold of 0.3 for GO enrichment and 0.4 for KEGG and REACTOME Pathway analyses). Clusters were manually annotated to summarize cluster themes.
Functional classification
Proteins were manually curated into protein classes based on information collated from the UniProt [31], GeneCards [30], Human Protein Reference Database (HPRD) [29], and Panther [32] databases. Protein functions were assigned based on the protein class, GO analysis and information collated from the above databases. Proteins and associated phosphorylation sites were visualized using the Phosphopath App [89] in Cytoscape [87] and imported into Adobe Illustrator for function overlay and layout editing.
Motif enrichment
The PhosphositePlus [35] Motif Analysis Tool was used to query EphA2-regulated phosphosites for enriched linear phosphoserine and phosphothreonine motifs. 13-mer phosphosite-centered peptide sequences of regulated phosphosites were analyzed using the Motif-All algorithm (significance threshold of 1e−05, support threshold of 0.05). A motif occurrence threshold of five was manually applied to significantly overrepresented motifs. Motif logos were generated using the PSP Production Algorithm of the PhosphositePlus Sequence Logo Tool.
Upstream kinase analysis
Known kinase-substrate relationships were extracted from PhosphositePlus [35] (“putative in vivo kinases”), HPRD [29], Uniprot [31] and KEA2 (http://www.maayanlab.net/KEA2/index.html; library: “Literature Based Kinase-Substrate Library with Phosphosites”). Upstream kinase prediction analysis was performed using the NetworKIN database [36]. Kinases with a minimum score of three and a maximal score difference of four from the top scoring kinase were considered predicted upstream kinases. Kinase-substrate relationships were visualized in Cytoscape in conjunction with the Phosphopath App to visualize phosphosites.
Statistical analysis and reproducibility
Statistical analyses, unless specified otherwise in the respective method sections, were performed using Prism 9 (GraphPad) and JMP (SAS Institute) software. Appropriate statistical tests for group comparisons were determined in consultation with the Statistics Unit at QIMR Berghofer Medical Research Institute. Applied tests include two-tailed unpaired Student’s t test, repeated measure ANOVA with post hoc Tukey’s test, one-way ANOVA with post hoc Sidak’s test, mixed model analysis and mixed-effect regression analysis. For survival analyses, the log-rank (Mantel–Cox) test was used to determine the significance between experimental groups. Applied statistical tests, sample sizes and p values are indicated in the figure legends. QQ plots and homoscedasticity plots were examined to assess normal distribution and equal variances of the data, respectively. Where appropriate, data were log-transformed prior to statistical analysis. No sample size calculations were performed prior to the study. For in vitro experiment the sample size represents the number of biological replicates generated independently and on separate day. For in vivo experiments sample size represents the number of mice per treatment group from the same experiment or pooled across multiple identical experiments as indicated in the figure legends. The investigators were not blinded to the group allocations. A p value of <0.05 was considered statistically significant. Unless specified otherwise in the figure legends, bar graphs represent the mean ± standard error (SE); box and whisker plots represent the median and interquartile range ± minimum/maximum values. Statistical significance is indicated by asterisks: *p < 0.05, **p < 0.01, and ***p < 0.001.
Supplementary information
Acknowledgements
The authors thank Christine Hoogland for bioinformatics support and the staff of the Animal, Microscopy, Flow Cytometry and Histology Facilities and the Statistical Unit at QIMR Berghofer Medical Research Institute for technical support with this work. This work was supported by two Rio Tinto Ride to Conquer Cancer Grants, a Cancer Council Queensland Research Project Grant (APP1067384) and Christine Sadler and The Sid Faithfull Group.
Author contributions
CO contributed to conception, experimental design, acquisition and analysis, interpretation and manuscript drafting. KAD contributed to conception, experimental design, acquisition and analysis and interpretation. BWD contributed to conception, interpretation and manuscript drafting. AWB contributed to conception, experimental design, interpretation and manuscript drafting. JJG contributed to conception, experimental design and interpretation of the work. JKM contributed to the conception, experimental design and acquisition and analysis. KJB, FMS, BAJ, LTC, HAY, KB and YL contributed to acquisition and analysis. FA-E contributed to the analysis and interpretation. JKC contributed to experimental design and analysis. MGC contributed to the interpretation of the work.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository [90] with the dataset identifier PXD053835. All other data generated and analyzed as part of the current study are available from the corresponding authors on reasonable request.
Competing interests
Provisional patent applications claiming the anti-EphA2 monoclonal antibodies 1F7 and 4B3 that are described in this manuscript have been filed by the QIMR Berghofer Medical Research Institute.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. All experimental protocols for the animal studies were approved by the QIMR Berghofer Animal Ethics Committee (Approval number: A0304-620M). The primary human glioblastoma cell lines WK1 and MN1 were generated in-house and have been reported previously [70, 71]. Patient tumor tissues to generate these cell lines were collected following informed consent and with approval from the Royal Brisbane Women’s Hospital and QIMR Berghofer Human Research Ethics Committees (Approval number: P3420, HREC/17/QRBW/577 Novel Therapies for Brain Cancer).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jeffrey J. Gorman, Andrew W. Boyd, Bryan W. Day.
Contributor Information
Carolin Offenhäuser, Email: Carolin.Offenhauser@qimrberghofer.edu.au.
Bryan W. Day, Email: Bryan.Day@qimrberghofer.edu.au
Supplementary information
The online version contains supplementary material available at 10.1038/s41388-024-03206-x.
References
- 1.Kania A, Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol. 2016;17:240–56. [DOI] [PubMed] [Google Scholar]
- 2.Miao H, Wang B. EphA receptor signaling-complexity and emerging themes. Semin Cell Dev Biol. 2012;23:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd AW, Bartlett PF, Lackmann M. Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov. 2014;13:39–62. [DOI] [PubMed] [Google Scholar]
- 5.Charmsaz S, Boyd AW. Eph receptors as oncotargets. Oncotarget. 2017;8:81727–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barquilla A, Pasquale EB. Eph receptors and ephrins: therapeutic opportunities. Annu Rev Pharm Toxicol. 2015;55:465–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindberg RA, Hunter T. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Mol Cell Biol. 1990;10:6316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wykosky J, Debinski W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res. 2008;6:1795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brantley-Sieders DM, Chen J. Eph receptor tyrosine kinases in angiogenesis: from development to disease. Angiogenesis. 2004;7:17–28. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Song W, Amato K. Eph receptor tyrosine kinases in cancer stem cells. Cytokine Growth Factor Rev. 2015;26:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taddei ML, Parri M, Angelucci A, Onnis B, Bianchini F, Giannoni E, et al. Kinase-dependent and -independent roles of EphA2 in the regulation of prostate cancer invasion and metastasis. Am J Pathol. 2009;174:1492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker-Daniels J, Coffman K, Azimi M, Rhim JS, Bostwick DG, Snyder P, et al. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275–80. [DOI] [PubMed] [Google Scholar]
- 13.Kurose H, Ueda K, Kondo R, Ogasawara S, Kusano H, Sanada S, et al. Elevated expression of EPHA2 is associated with poor prognosis after radical prostatectomy in prostate cancer. Anticancer Res. 2019;39:6249–57. [DOI] [PubMed] [Google Scholar]
- 14.Sachdeva A, Hart CA, Kim K, Tawadros T, Oliveira P, Shanks J, et al. Non-canonical EphA2 activation underpins PTEN-mediated metastatic migration and poor clinical outcome in prostate cancer. Br J Cancer. 2022;127:1254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartley TD, Hunt RW, Welcher AA, Boyle WJ, Parker VP, Lindberg RA, et al. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature. 1994;368:558–60. [DOI] [PubMed] [Google Scholar]
- 16.Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, et al. Architecture of Eph receptor clusters. Proc Natl Acad Sci USA. 2010;107:10860–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang NY, Fernandez C, Richter M, Xiao Z, Valencia F, Tice DA, et al. Crosstalk of the EphA2 receptor with a serine/threonine phosphatase suppresses the Akt-mTORC1 pathway in cancer cells. Cell Signal. 2011;23:201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herath NI, Spanevello MD, Doecke JD, Smith FM, Pouponnot C, Boyd AW. Complex expression patterns of Eph receptor tyrosine kinases and their ephrin ligands in colorectal carcinogenesis. Eur J Cancer. 2012;48:753–62. [DOI] [PubMed] [Google Scholar]
- 20.Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–9. [DOI] [PubMed] [Google Scholar]
- 21.Noberini R, Rubio de la Torre E, Pasquale EB. Profiling Eph receptor expression in cells and tissues: a targeted mass spectrometry approach. Cell Adh Migr. 2012;6:102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao H, Wei BR, Peehl DM, Li Q, Alexandrou T, Schelling JR, et al. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527–30. [DOI] [PubMed] [Google Scholar]
- 23.Huang X, Wu D, Jin H, Stupack D, Wang JY. Induction of cell retraction by the combined actions of Abl-CrkII and Rho-ROCK1 signaling. J Cell Biol. 2008;183:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter N, Nakamoto T, Hirai H, Hunter T. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas). Nat Cell Biol. 2002;4:565–73. [DOI] [PubMed] [Google Scholar]
- 25.Vreeken D, Zhang H, van Zonneveld AJ, van Gils JM. Ephs and Ephrins in adult endothelial biology. Int J Mol Sci. 2020;21:5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ieguchi K, Tomita T, Omori T, Komatsu A, Deguchi A, Masuda J, et al. ADAM12-cleaved ephrin-A1 contributes to lung metastasis. Oncogene. 2014;33:2179–90. [DOI] [PubMed] [Google Scholar]
- 27.Ieguchi K, Maru Y. Roles of EphA1/A2 and ephrin-A1 in cancer. Cancer Sci. 2019;110:841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larson J, Schomberg S, Schroeder W, Carpenter TC. Endothelial EphA receptor stimulation increases lung vascular permeability. Am J Physiol Lung Cell Mol Physiol. 2008;295:L431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasad TS, Kandasamy K, Pandey A. Human Protein Reference Database and Human Proteinpedia as discovery tools for systems biology. Methods Mol Biol. 2009;577:67–79. [DOI] [PubMed] [Google Scholar]
- 30.Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, et al. The GeneCards Suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinforma. 2016;54:1.30.31–1.30.33. [DOI] [PubMed] [Google Scholar]
- 31.UniProt C. UniProt: the Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023;51:D523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas PD, Ebert D, Muruganujan A, Mushayahama T, Albou LP, Mi H. PANTHER: making genome-scale phylogenetics accessible to all. Protein Sci. 2022;31:8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Z, Neilson LJ, Zhong H, Murray PS, Zanivan S, Zaidel-Bar R. E-cadherin interactome complexity and robustness resolved by quantitative proteomics. Sci Signal. 2014;7:rs7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horton ER, Byron A, Askari JA, Ng DHJ, Millon-Fremillon A, Robertson J, et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol. 2015;17:1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, et al. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 2012;40:D261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linding R, Jensen LJ, Pasculescu A, Olhovsky M, Colwill K, Bork P, et al. NetworKIN: a resource for exploring cellular phosphorylation networks. Nucleic Acids Res. 2008;36:D695–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cross MJ, Lu L, Magnusson P, Nyqvist D, Holmqvist K, Welsh M, et al. The Shb adaptor protein binds to tyrosine 766 in the FGFR-1 and regulates the Ras/MEK/MAPK pathway via FRS2 phosphorylation in endothelial cells. Mol Biol Cell. 2002;13:2881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karlsson T, Kullander K, Welsh M. The Src homology 2 domain protein Shb transmits basic fibroblast growth factor- and nerve growth factor-dependent differentiation signals in PC12 cells. Cell Growth Differ. 1998;9:757–66. [PubMed] [Google Scholar]
- 39.Karlsson T, Songyang Z, Landgren E, Lavergne C, Di Fiore PP, Anafi M, et al. Molecular interactions of the Src homology 2 domain protein Shb with phosphotyrosine residues, tyrosine kinase receptors and Src homology 3 domain proteins. Oncogene. 1995;10:1475–83. [PubMed] [Google Scholar]
- 40.Wagner MJ, Hsiung MS, Gish GD, Bagshaw RD, Doodnauth SA, Soliman MA, et al. The Shb scaffold binds the Nck adaptor protein, p120 RasGAP, and Chimaerins and thereby facilitates heterotypic cell segregation by the receptor EphB2. J Biol Chem. 2020;295:3932–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elloul S, Kedrin D, Knoblauch NW, Beck AH, Toker A. The adherens junction protein afadin is an AKT substrate that regulates breast cancer cell migration. Mol Cancer Res. 2014;12:464–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmberg J, Frisén J. Ephrins are not only unattractive. Trends Neurosci. 2002;25:239–43. [DOI] [PubMed] [Google Scholar]
- 43.Boyd AW, Lackmann M. Signals from Eph and ephrin proteins: a developmental tool kit. Sci STKE. 2001;2001:re20. [DOI] [PubMed] [Google Scholar]
- 44.Klein R. Eph/ephrin signalling during development. Development. 2012;139:4105–9. [DOI] [PubMed] [Google Scholar]
- 45.Okazaki T, Ni A, Baluk P, Ayeni OA, Kearley J, Coyle AJ, et al. Capillary defects and exaggerated inflammatory response in the airways of EphA2-deficient mice. Am J Pathol. 2009;174:2388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X, Li Y, Chen C, Dong J, Zhou J, Tong D, et al. Exosomal EphA2 promotes tumor metastasis of triple-negative breast cancer by damaging endothelial barrier. Clin Exp Metastasis. 2023;40:105–16. [DOI] [PubMed] [Google Scholar]
- 47.Khan N, Kumar V, Li P, RAPIDS Study Group, Schlapbach LJ, Boyd AW, et al. Inhibiting Eph/ephrin signaling reduces vascular leak and endothelial cell dysfunction in mice with sepsis. Sci Transl Med. 2024;16:eadg5768. [DOI] [PubMed]
- 48.Carles-Kinch K, Kilpatrick KE, Stewart JC, Kinch MS. Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res. 2002;62:2840–7. [PubMed] [Google Scholar]
- 49.Bruckheimer EM, Fazenbaker CA, Gallagher S, Mulgrew K, Fuhrmann S, Coffman KT, et al. Antibody-dependent cell-mediated cytotoxicity effector-enhanced EphA2 agonist monoclonal antibody demonstrates potent activity against human tumors. Neoplasia. 2009;11:509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landen CN Jr., Lu C, Han LY, Coffman KT, Bruckheimer E, Halder J, et al. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–70. [DOI] [PubMed] [Google Scholar]
- 51.Volz C, Breid S, Selenz C, Zaplatina A, Golfmann K, Meder L, et al. Inhibition of tumor VEGFR2 induces serine 897 EphA2-dependent tumor cell invasion and metastasis in NSCLC. Cell Rep. 2020;31:107568. [DOI] [PubMed] [Google Scholar]
- 52.Li JY, Xiao T, Yi HM, Yi H, Feng J, Zhu JF, et al. S897 phosphorylation of EphA2 is indispensable for EphA2-dependent nasopharyngeal carcinoma cell invasion, metastasis and stem properties. Cancer Lett. 2019;444:162–74. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Monclus S, Lopez-Alemany R, Almacellas-Rabaiget O, Herrero-Martin D, Huertas-Martinez J, Lagares-Tena L, et al. EphA2 receptor is a key player in the metastatic onset of Ewing sarcoma. Int J Cancer. 2018;143:1188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang C, Smalley I, Emmons MF, Sharma R, Izumi V, Messina J, et al. Noncanonical EphA2 signaling is a driver of tumor-endothelial cell interactions and metastatic dissemination in BRAF inhibitor‒resistant melanoma. J Invest Dermatol. 2021;141:840–51.e844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi X, Lingerak R, Herting CJ, Ge Y, Kim S, Toth P, et al. Time-resolved live-cell spectroscopy reveals EphA2 multimeric assembly. Science. 2023;382:1042–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson D, Gooya J, Mao S, Kinneer K, Xu L, Camara M, et al. A human antibody-drug conjugate targeting EphA2 inhibits tumor growth in vivo. Cancer Res. 2008;68:9367–74. [DOI] [PubMed] [Google Scholar]
- 57.Annunziata CM, Kohn EC, LoRusso P, Houston ND, Coleman RL, Buzoianu M, et al. Phase 1, open-label study of MEDI-547 in patients with relapsed or refractory solid tumors. Invest N Drugs. 2013;31:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perez White BE, Ventrella R, Kaplan N, Cable CJ, Thomas PM, Getsios S. EphA2 proteomics in human keratinocytes reveals a novel association with afadin and epidermal tight junctions. J Cell Sci. 2017;130:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, et al. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, et al. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145:539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hock B, Bohme B, Karn T, Yamamoto T, Kaibuchi K, Holtrich U, et al. PDZ-domain-mediated interaction of the Eph-related receptor tyrosine kinase EphB3 and the ras-binding protein AF6 depends on the kinase activity of the receptor. Proc Natl Acad Sci USA. 1998;95:9779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buchert M, Schneider S, Meskenaite V, Adams MT, Canaani E, Baechi T, et al. The junction-associated protein AF-6 interacts and clusters with specific Eph receptor tyrosine kinases at specialized sites of cell-cell contact in the brain. J Cell Biol. 1999;144:361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bertocchi C, Vaman Rao M, Zaidel-Bar R. Regulation of adherens junction dynamics by phosphorylation switches. J Signal Transduct. 2012;2012:125295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fearnley GW, Young KA, Edgar JR, Antrobus R, Hay IM, Liang WC, et al. The homophilic receptor PTPRK selectively dephosphorylates multiple junctional regulators to promote cell-cell adhesion. Elife. 2019;8:e44597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asano E, Maeda M, Hasegawa H, Ito S, Hyodo T, Yuan H, et al. Role of palladin phosphorylation by extracellular signal-regulated kinase in cell migration. PLoS ONE. 2011;6:e29338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang WB, Ireton RC, Zhuang G, Takahashi T, Reynolds A, Chen J. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci. 2008;121:358–68. [DOI] [PubMed] [Google Scholar]
- 67.Orsulic S, Kemler R. Expression of Eph receptors and ephrins is differentially regulated by E-cadherin. J Cell Sci. 2000;113:1793–802. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka M, Kamata R, Sakai R. EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J Biol Chem. 2005;280:42375–82. [DOI] [PubMed] [Google Scholar]
- 69.Kozlowski JM, Fidler IJ, Campbell D, Xu ZL, Kaighn ME, Hart IR. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984;44:3522–9. [PubMed] [Google Scholar]
- 70.Stringer BW, Day BW, D’Souza RCJ, Jamieson PR, Ensbey KS, Bruce ZC, et al. A reference collection of patient-derived cell line and xenograft models of proneural, classical and mesenchymal glioblastoma. Sci Rep. 2019;9:4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D'Souza RCJ, Offenhauser C, Straube J, Baumgartner U, Kordowski A, Li Y, et al. Q-cell glioblastoma resource: proteomics analysis reveals unique cell-states are maintained in 3D culture. Cells. 2020;9:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naruse-Nakajima C, Asano M, Iwakura Y. Involvement of EphA2 in the formation of the tail notochord via interaction with ephrinA1. Mech Dev. 2001;102:95–105. [DOI] [PubMed] [Google Scholar]
- 73.Jamalpour M, Li X, Cavelier L, Gustafsson K, Mostoslavsky G, Hoglund M, et al. Tumor SHB gene expression affects disease characteristics in human acute myeloid leukemia. Tumour Biol. 2017;39:1010428317720643. [DOI] [PubMed] [Google Scholar]
- 74.Knoll B, Weinl C, Nordheim A, Bonhoeffer F. Stripe assay to examine axonal guidance and cell migration. Nat Protoc. 2007;2:1216–24. [DOI] [PubMed] [Google Scholar]
- 75.Boudaoud A, Burian A, Borowska-Wykret D, Uyttewaal M, Wrzalik R, Kwiatkowska D, et al. FibrilTool, an ImageJ plug-in to quantify fibrillar structures in raw microscopy images. Nat Protoc. 2014;9:457–63. [DOI] [PubMed] [Google Scholar]
- 76.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pearson T, Shultz LD, Miller D, King M, Laning J, Fodor W, et al. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin Exp Immunol. 2008;154:270–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bendall SC, Hughes C, Stewart MH, Doble B, Bhatia M, Lajoie GA. Prevention of amino acid conversion in SILAC experiments with embryonic stem cells. Mol Cell Proteom. 2008;7:1587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dave KA, Norris EL, Bukreyev AA, Headlam MJ, Buchholz UJ, Singh T, et al. A comprehensive proteomic view of responses of A549 type II alveolar epithelial cells to human respiratory syncytial virus infection. Mol Cell Proteom. 2014;13:3250–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–72. [DOI] [PubMed] [Google Scholar]
- 81.Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10:1794–805. [DOI] [PubMed] [Google Scholar]
- 82.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–48. [DOI] [PubMed] [Google Scholar]
- 83.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fabregat A, Jupe S, Matthews L, Sidiropoulos K, Gillespie M, Garapati P, et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018;46:D649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25:1091–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Binns D, Dimmer E, Huntley R, Barrell D, O’Donovan C, Apweiler R. QuickGO: a web-based tool for Gene Ontology searching. Bioinformatics. 2009;25:3045–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raaijmakers LM, Giansanti P, Possik PA, Mueller J, Peeper DS, Heck AJ, et al. PhosphoPath: visualization of phosphosite-centric dynamics in temporal molecular networks. J Proteome Res. 2015;14:4332–41. [DOI] [PubMed] [Google Scholar]
- 90.Perez-Riverol Y, Bai J, Bandla C, Garcia-Seisdedos D, Hewapathirana S, Kamatchinathan S, et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50:D543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository [90] with the dataset identifier PXD053835. All other data generated and analyzed as part of the current study are available from the corresponding authors on reasonable request.