Abstract
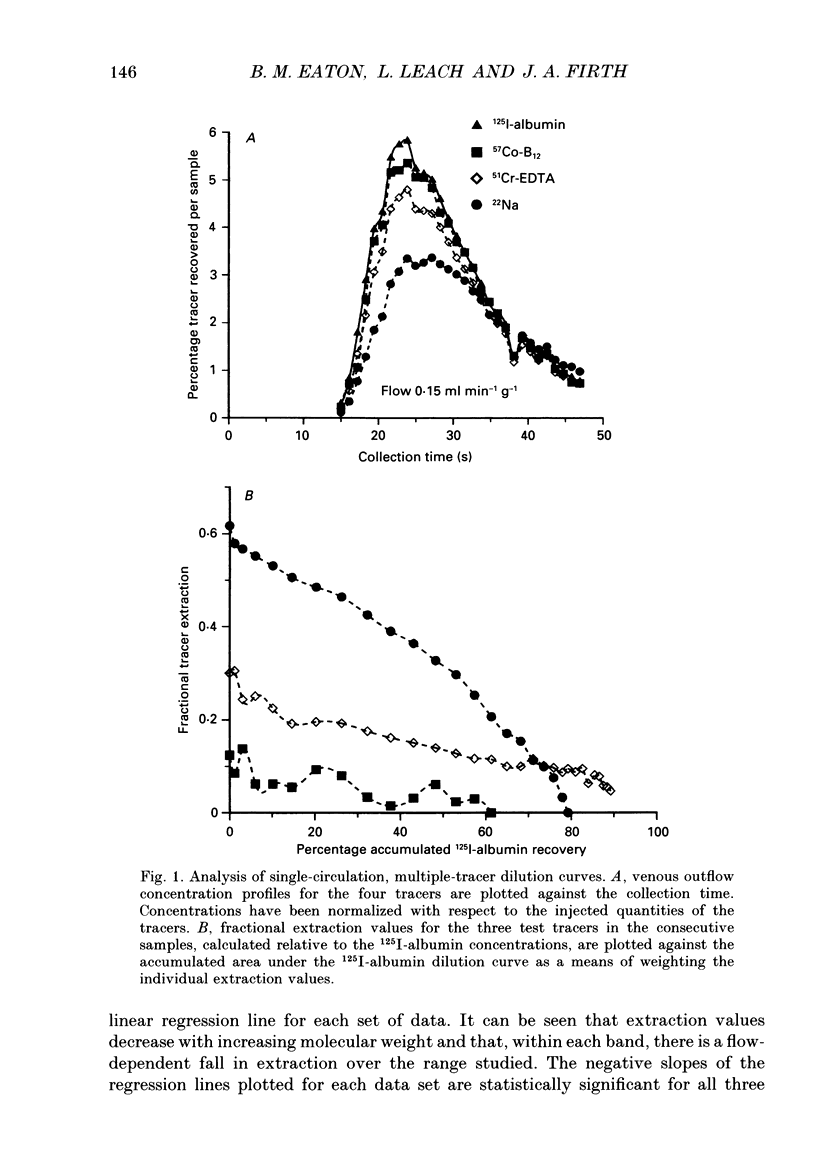
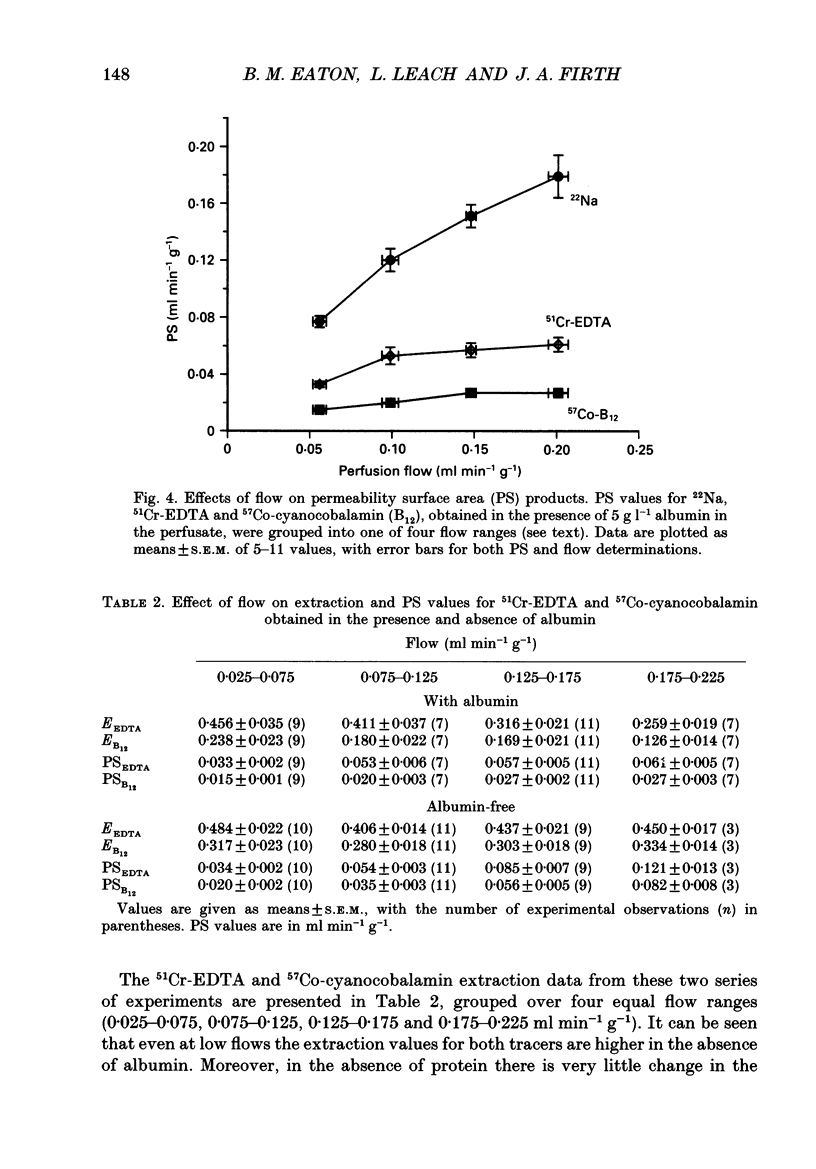
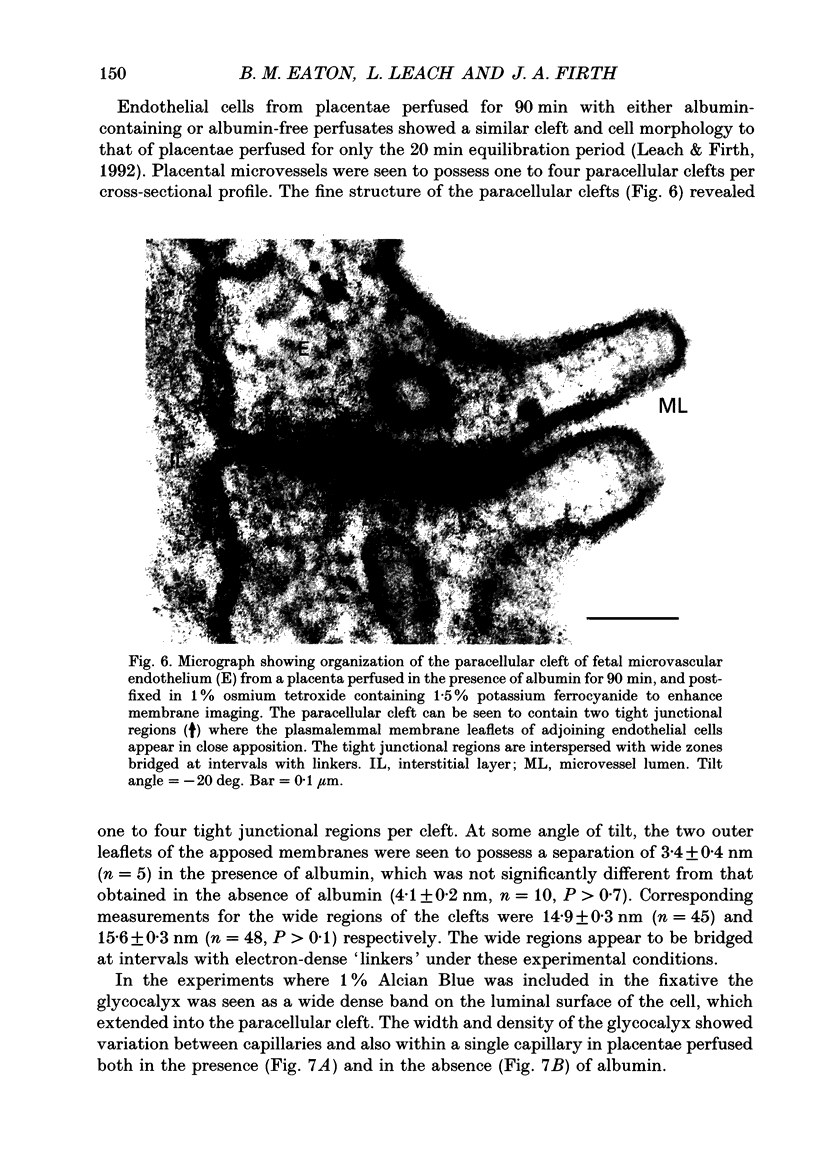
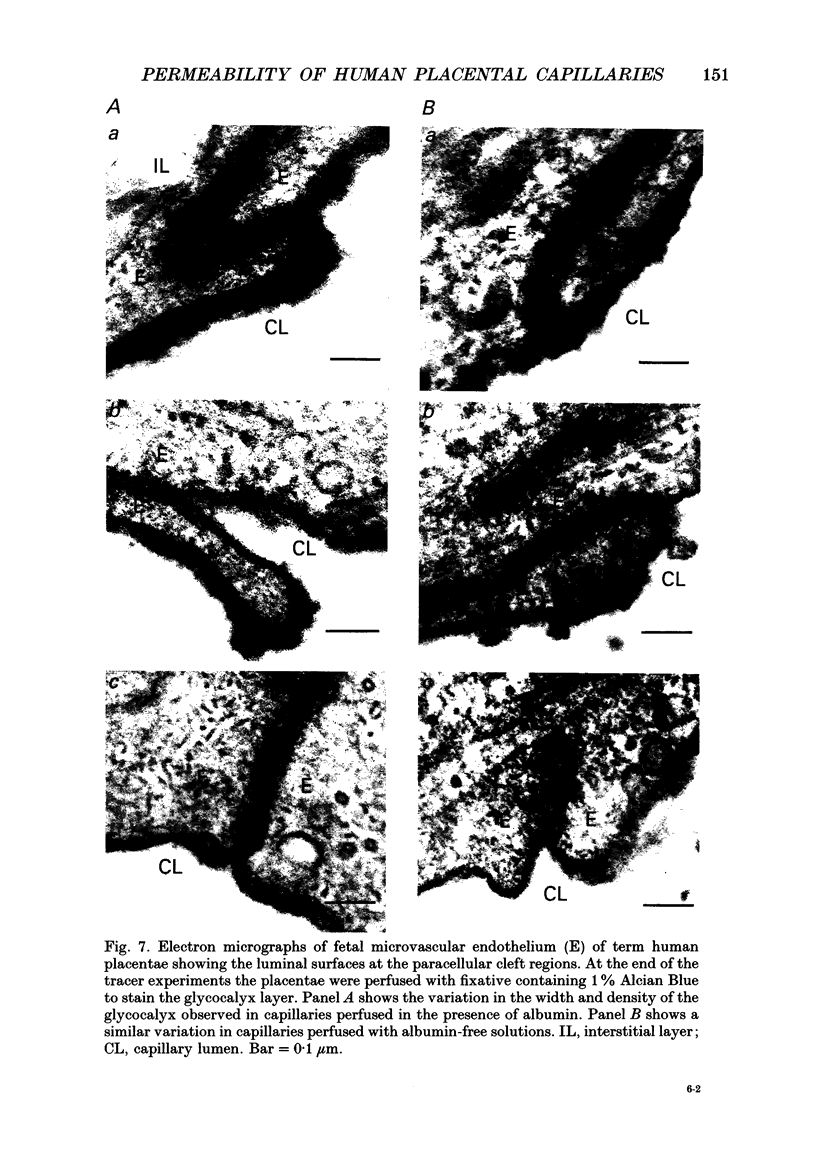
1. Capillary permeability-surface area (PS) products for the low molecular weight radioactive tracers, 22Na, 51Cr-EDTA (relative molecular mass 357) and 57Co-cyanocobalamin (relative molecular mass 1353) were measured in the fetal circulation of isolated dually perfused lobules of normal term human placentae using the single circulation, multiple-tracer dilution technique. 2. In lobules perfused with M199 medium, containing dextran and 5 g l-1 bovine albumin, the extractions of all three tracers decreased as the flow was increased over the range of 2-8 ml min-1, and PS products for 51Cr-EDTA and 57Co-cyanocobalamin, but not for 22Na, reached constant values at flows above 0.1 ml min-1 g-1. 3. Flow-independent PS products in the presence of albumin were 0.025 +/- 0.002 ml min-1 g-1 (mean +/- S.E.M., n = 25) for 57Co-cyanocobalamin and 0.057 +/- 0.003 ml min-1 g-1 (n = 25) for 51Cr-EDTA. The ratio of PS values (51Cr-EDTA/57Co-cyanocobalamin) was 2.28, while the ratio of the corresponding free diffusion coefficients was 1.79, indicating substantial restriction to the diffusion of the 57Co-cyanocobalamin. 4. In another series of lobules perfused in the absence of albumin, extraction values for all three test tracers were constant over the same flow range. Values at high flow rates were therefore about twice those measured in the presence of albumin, and PS products for all three tracers failed to reach diffusion-limited values. 6. Lobules perfused with and without albumin were fixed using a glutaraldehyde fixative containing 1% Alcian Blue dye. An ultrastructural examination of the endothelium showed no significant changes in cell or cleft morphology, or in the glycocalyx, in the absence of albumin which might account for the observed permeability change. 7. These data are the first physiological measurements specifically characterizing fetal microvascular permeability in the human placenta. The results suggest that permeability resembles that found in skeletal muscle and, as such, the endothelium presents a significant barrier to the diffusion of large solutes. The observed 'protein effect' indicates that albumin can interact with elements of the solute pathway to increase its restrictiveness.
Full text
PDF




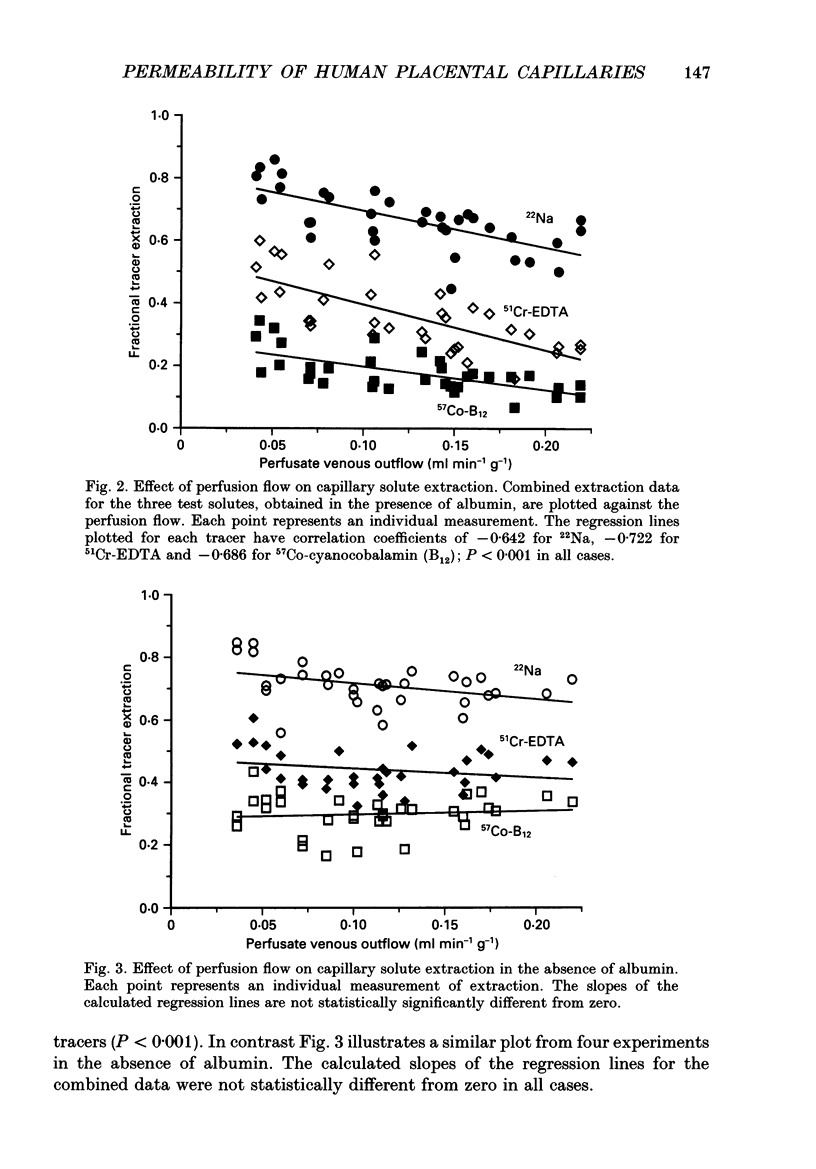
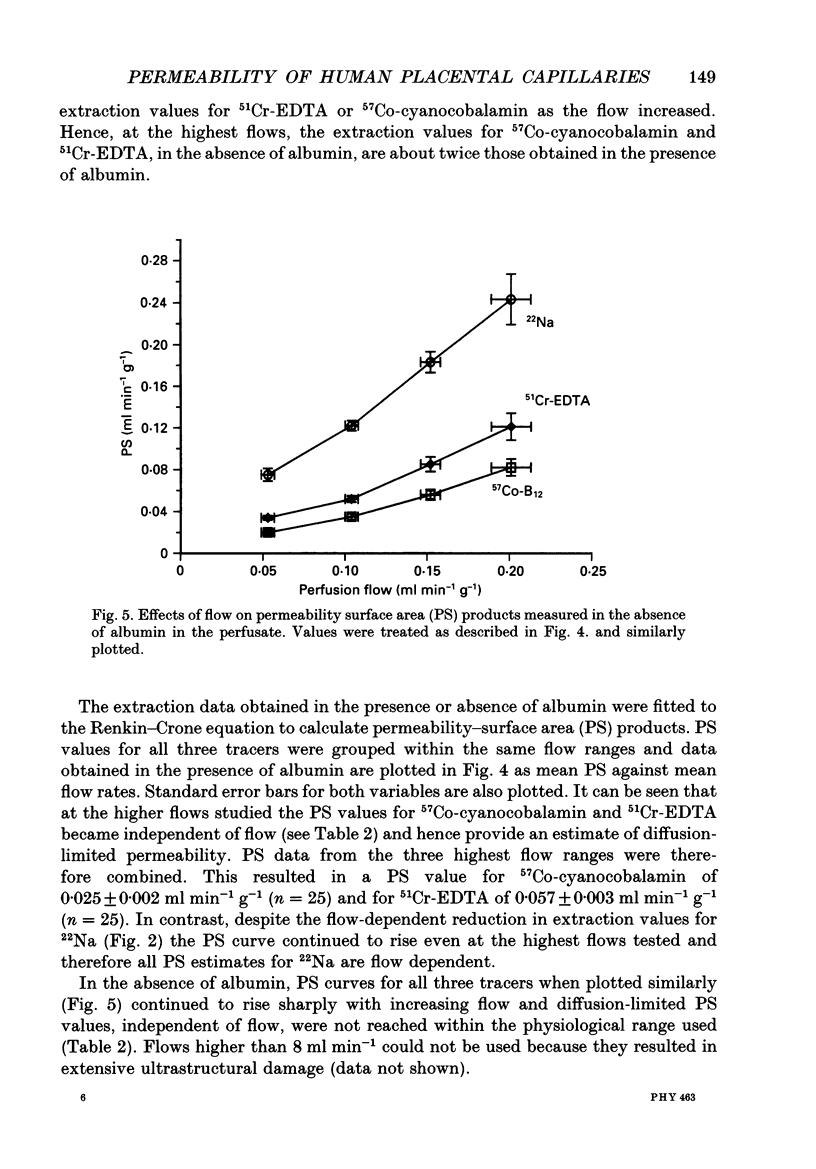




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bain M. D., Copas D. K., Landon M. J., Stacey T. E. In vivo permeability of the human placenta to inulin and mannitol. J Physiol. 1988 May;399:313–319. doi: 10.1113/jphysiol.1988.sp017082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain M. D., Copas D. K., Taylor A., Landon M. J., Stacey T. E. Permeability of the human placenta in vivo to four non-metabolized hydrophilic molecules. J Physiol. 1990 Dec;431:505–513. doi: 10.1113/jphysiol.1990.sp018343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. L., Winlove C. P. Effects of perfusate composition on binding of ruthenium red and gold colloid to glycocalyx of rabbit aortic endothelium. J Histochem Cytochem. 1984 Mar;32(3):259–266. doi: 10.1177/32.3.6198357. [DOI] [PubMed] [Google Scholar]
- Bloxam D. L., Bullen B. E. Condition and performance of the perfused human placental cotyledon. Am J Obstet Gynecol. 1986 Aug;155(2):382–388. doi: 10.1016/0002-9378(86)90835-5. [DOI] [PubMed] [Google Scholar]
- Boyd R. D., Canning J. F., Stacey T. E., Ward R. H., Weedon A. P. Volumes of distribution of sodium and albumin in the sheep placenta. J Physiol. 1983 Mar;336:13–26. doi: 10.1113/jphysiol.1983.sp014562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRONE C. THE PERMEABILITY OF CAPILLARIES IN VARIOUS ORGANS AS DETERMINED BY USE OF THE 'INDICATOR DIFFUSION' METHOD. Acta Physiol Scand. 1963 Aug;58:292–305. doi: 10.1111/j.1748-1716.1963.tb02652.x. [DOI] [PubMed] [Google Scholar]
- Clough G. Relationship between microvascular permeability and ultrastructure. Prog Biophys Mol Biol. 1991;55(1):47–69. doi: 10.1016/0079-6107(91)90011-g. [DOI] [PubMed] [Google Scholar]
- Contractor S. F., Eaton B. M., Firth J. A., Bauman K. F. A comparison of the effects of different perfusion regimes on the structure of the isolated human placental lobule. Cell Tissue Res. 1984;237(3):609–617. doi: 10.1007/BF00228446. [DOI] [PubMed] [Google Scholar]
- Curry F. E. Determinants of capillary permeability: a review of mechanisms based on single capillary studies in the frog. Circ Res. 1986 Oct;59(4):367–380. doi: 10.1161/01.res.59.4.367. [DOI] [PubMed] [Google Scholar]
- Curry F. E., Michel C. C. A fiber matrix model of capillary permeability. Microvasc Res. 1980 Jul;20(1):96–99. doi: 10.1016/0026-2862(80)90024-2. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Haraldsson B., Rippe B. Orosomucoid as one of the serum components contributing to normal capillary permselectivity in rat skeletal muscle. Acta Physiol Scand. 1987 Jan;129(1):127–135. doi: 10.1111/j.1748-1716.1987.tb08047.x. [DOI] [PubMed] [Google Scholar]
- Heinrich D., Metz J., Raviola E., Forssmann W. G. Ultrastructure of perfusion-fixed fetal capillaries in the human placenta. Cell Tissue Res. 1976 Sep 14;172(2):157–169. doi: 10.1007/BF00226024. [DOI] [PubMed] [Google Scholar]
- Leach L., Firth J. A. Fine structure of the paracellular junctions of terminal villous capillaries in the perfused human placenta. Cell Tissue Res. 1992 Jun;268(3):447–452. doi: 10.1007/BF00319151. [DOI] [PubMed] [Google Scholar]
- Levick J. R., Michel C. C. The effect of bovine albumin on the permeability of frog mesenteric capillaries. Q J Exp Physiol Cogn Med Sci. 1973 Jan;58(1):87–97. doi: 10.1113/expphysiol.1973.sp002194. [DOI] [PubMed] [Google Scholar]
- MARTIN P., YUDILEVICH D. A THEORY FOR THE QUANTIFICATION OF TRANSCAPILLARY EXCHANGE BY TRACER-DILUTION CURVES. Am J Physiol. 1964 Jul;207:162–168. doi: 10.1152/ajplegacy.1964.207.1.162. [DOI] [PubMed] [Google Scholar]
- Mann G. E. Alterations of myocardial capillary permeability by albumin in the isolated, perfused rabbit heart. J Physiol. 1981;319:311–323. doi: 10.1113/jphysiol.1981.sp013910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann G. E., Smaje L. H., Yudilevich D. L. Permeability of the fenestrated capillaries in the cat submandibular gland to lipid-insoluble molecules. J Physiol. 1979 Dec;297(0):335–354. doi: 10.1113/jphysiol.1979.sp013043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. C., Curry F. E., Michel C. C. The effects of proteins upon the filtration coefficient of individually perfused frog mesenteric capillaries. Microvasc Res. 1977 Mar;13(2):185–202. doi: 10.1016/0026-2862(77)90084-x. [DOI] [PubMed] [Google Scholar]
- Michel C. C. Capillary permeability and how it may change. J Physiol. 1988 Oct;404:1–29. doi: 10.1113/jphysiol.1988.sp017275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Mossaz A., Ryser J. E., Orci L., Vassalli P. Leukocyte interleukins induce cultured endothelial cells to produce a highly organized, glycosaminoglycan-rich pericellular matrix. J Cell Biol. 1984 Nov;99(5):1706–1715. doi: 10.1083/jcb.99.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., RENKIN E. M., BORRERO L. M. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Physiol. 1951 Oct;167(1):13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- Paaske W. P. Capillary permeability in skeletal muscle. Acta Physiol Scand. 1977 Sep;101(1):1–14. doi: 10.1111/j.1748-1716.1977.tb05977.x. [DOI] [PubMed] [Google Scholar]
- Paaske W. P., Sejrsen P. Permeability of continuous capillaries. Dan Med Bull. 1989 Dec;36(6):570–590. [PubMed] [Google Scholar]
- RENKIN E. M. Transport of potassium-42 from blood to tissue in isolated mammalian skeletal muscles. Am J Physiol. 1959 Dec;197:1205–1210. doi: 10.1152/ajplegacy.1959.197.6.1205. [DOI] [PubMed] [Google Scholar]
- Reviriego J., Alonso M. J., Ibañez C., Marín J. Action of adenosine and characterization of adenosine receptors in human placental vasculature. Gen Pharmacol. 1990;21(2):227–233. doi: 10.1016/0306-3623(90)90906-3. [DOI] [PubMed] [Google Scholar]
- Schneeberger E. E., Hamelin M. Interaction of serum proteins with lung endothelial glycocalyx: its effect on endothelial permeability. Am J Physiol. 1984 Aug;247(2 Pt 2):H206–H217. doi: 10.1152/ajpheart.1984.247.2.H206. [DOI] [PubMed] [Google Scholar]
- Sibley C. P., Bauman K. F., Firth J. A. Permeability of the foetal capillary endothelium of the guinea-pig placenta to haem proteins of various molecular sizes. Cell Tissue Res. 1982;223(1):165–178. doi: 10.1007/BF00221507. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Permeability of muscle capillaries to exogenous myoglobin. J Cell Biol. 1973 May;57(2):424–452. doi: 10.1083/jcb.57.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweiry J. H., Mann G. E. Pancreatic microvascular permeability in caerulein-induced acute pancreatitis. Am J Physiol. 1991 Oct;261(4 Pt 1):G685–G692. doi: 10.1152/ajpgi.1991.261.4.G685. [DOI] [PubMed] [Google Scholar]
- Thornburg K. L., Faber J. J. The steady state concentration gradients of an electron-dense marker (ferritin in the three-layered hemochorial placenta of the rabbit. J Clin Invest. 1976 Oct;58(4):912–925. doi: 10.1172/JCI108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. J., Bauman K. F., Firth J. A. Interendothelial junctions of cardiac capillaries in rats: their structure and permeability properties. Cell Tissue Res. 1988 Apr;252(1):57–66. doi: 10.1007/BF00213826. [DOI] [PubMed] [Google Scholar]
- Wheeler C. P., Yudilevich D. L. Transport and metabolism of adenosine in the perfused guinea-pig placenta. J Physiol. 1988 Nov;405:511–526. doi: 10.1113/jphysiol.1988.sp017345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudilevich D. L., De Rose N. Blood-brain transfer of glucose and other molecules measured by rapid indicator dilution. Am J Physiol. 1971 Mar;220(3):841–846. doi: 10.1152/ajplegacy.1971.220.3.841. [DOI] [PubMed] [Google Scholar]