Abstract
The synthesis of unprecedented triskelion‐shaped hexa‐peri‐hexabenzocoronenes with C3 , C3h or D3h symmertry is reported. We present a new, five step synthetic access to tris‐iodinated HBC derivatives carrying different solubilizing moieties (tert‐butyl and mesityl), which serve as suitable building blocks for further functionalization. These molecules can undergo sonogashira cross coupling reactions to obtain a series of seven ethynyl tris‐substituted HBCs. The coupling partners range from carbon‐based aromatic scaffolds (tBu‐benzene, naphthalene, phenanthrene) to ligands for metal complexes (bipyridine, phenanthroline, terpyridine, acetylacetone). The optoelectronic properties were investigated by UV/vis absorption spectroscopy resulting in a significant bathochromic shift of the absorption maximum compared to the iodinated starting material. Semiempirical calculations were used to determine the conformation and symmetry of the compounds.
Keywords: tris-substitution, Sonogashira coupling, Hexa-peri-hexabenzocoronene, C3 symmetry, π-extension
We report a new five step synthetic approach for tris‐iodinated hexa‐peri‐hexabenzocoronenes (HBCs). These molecules serve as precursors for the synthesis of a library of seven triskelion‐shaped HBCs via sonogashira cross coupling reactions. Furthermore, the optoelectronic properties of the compounds are presented and discussed.
Introduction
The preparation of graphene related compounds with non‐zero band gaps, such as polycyclic aromatic hydrocarbons (PAHs), became of major interest in recent years. [1] These molecules are potential candidates for a wide range of applications, for example, in organic field transistors or photovoltaic devices.[ 2 , 3 , 4 ] One of the most intensively studied PAH is hexa‐peri‐hexabenzocoronene (HBC) due to its straightforward synthetic availability and promising properties like high stability or facile self‐assembly. [1] The optoelectronic properties of these molecules can be modulated by varying the shape and number of substituents.[ 1 , 5 ]
The first synthetic approaches towards HBCs were reported by clar and halleux, which used harsh conditions and obtained the targeted molecule only in poor yields.[ 6 , 7 ] In 1995, müllen and coworkers developed a highly efficient synthetic route towards HBCs, which has been established as common procedure today. [8] Here, the HBC core is generated by oxidative cyclodehydrogenation of the corresponding hexaarylbenzene (HAB) in a scholl‐oxidation.[ 8 , 9 ] Since then, a large variety of synthetic procedures towards HABs with specific substitution patterns has been developed.[ 1 , 10 , 11 ] One of the most prominent synthetic routes includes a diels‐alder reaction of a tolane and tetraphenylcyclopentadienone derivative to generate precursors for mainly mono‐ and ortho‐disubstituted HBCs. [10] For the synthesis of hexa‐substituted HBC derivatives the cyclotrimerization of symmetric tolanes has been used predominantly. [12] The synthetic access of other more elaborate substitution patterns, however, is more challenging. Especially, tris‐substituted HBCs are still rare although interesting structural and optical properties are expected.[ 5 , 13 ] A potential reason for this lack of examples is the challenging synthetic access of suitable D3h ‐symmetric HBC building blocks carrying functionalizable iodine or bromine substituents.[ 5 , 11 ] Several unsuccessful attempts to generate symmetric tris‐substituted HAB derivatives substituted with solubilizing groups and halogens were described by müllen et al., for example, the cyclotrimerization of asymmetric tolanes. [5] Due to the failure of these approaches müllen and coworkers developed a new strategy to synthesize a tris‐iodinated HBC via 1,3,5‐tris‐(2’‐biphenyl)benzene derivatives, but the missing solubilizing groups could lead to problems regarding further wet chemical functionalization.[ 5 , 14 ] In 2016, jux et al. developed a new approach for the synthesis of HABs with different substitution patterns via functionalization of para‐nitroaniline. [11] Within this method it was also possible to synthesize a symmetric tris‐substituted HAB precursor carrying three iodine and three tert‐butyl substituents. However, ten synthetic steps are required to access this building block. [11] Here, we present a new synthetic strategy for the generation of tris‐iodinated HBC building blocks carrying different solubilizing groups (tert‐butyl and mesityl). These molecules serve as ideal precursors for subsequent sonogashira cross coupling reactions, which leads to the first synthesis of ethynyl tris‐substituted HBCs (triskelion‐shaped HBCs). Within this scope we were able to provide access to a series of these π‐extended HBCs. The substituents range from carbon‐only based aromatics (tBu‐benzene, naphthalene and phenanthrene) to ligands as heterocycles (bipyridine (bpy), phenanthroline (phen), terpyridine (terpy)) or acetylacetone (acac).
Results and Discussion
Synthesis of Carbon‐Only Based Triskelion‐Shaped HBCs
The synthesis of a D3h ‐symmetric HBC carrying three iodo and three tert‐butyl substituents (tBu3‐I3‐HBC) was achieved in five steps (Scheme 1). Starting with the iodination of 1,3,5‐tribromobenzene 1, compound 2 was synthesized using a literature procedure.[ 15 , 16 ] In the second step it was possible to selectively substitute the iodine atoms via suzuki cross coupling reactions to obtain 3 in a yield of 42 %. Followed by a subsequent threefold suzuki cross coupling, 4 was obtained in good yields. The iodination of 4 was performed using PIFA and iodine as reported in literature to afford tBu‐I3‐HAB 5. [11] It is noticeable that only four steps were necessary to produce 5 in good yields compared to the previously known synthetic route, which required twice as many synthetic steps. [11] In the last step the HAB 5 could be easily converted to the corresponding HBC derivative 6 in a scholl‐oxidation using DDQ/TfOH conditions. The desired HBC 6 can now serve as an ideal precursor for further cross coupling reactions to expand the scope of tris‐substituted HBC derivatives.
Scheme 1.
Synthesis route towards the tBu3‐I3‐HBC 6. a) H5IO6, KI, H2SO4, 0 °C→r.t., 3 d; b) Ar, Pd(dppf)Cl2, K3PO4, toluene/H2O, 100 °C, 16 h; c) Ar, Pd(dppf)Cl2, K3PO4, toluene/H2O, 100 °C, 16 h; d) Ar, PIFA, I2, CH2Cl2, r.t., 16 h; e) Ar, TfOH, DDQ, CH2Cl2, 0 °C→r.t., 2 h.
For a basic understanding of the properties of tris‐substituted π‐extended HBCs, first some carbon based scaffolds (tBu‐benzene, naphthalene and phenanthrene) were chosen for coupling via acetylene bridges. The acetylene linkers allow a linear extension of the aromatic π‐system and the formation of highly symmetrical planar structures. This results in a triskelion‐shaped geometry of the HBCs with D3h (compund 10) or C3h symmetry (compounds 11 and 12).
The ethynyl coupled derivatives of naphthalene (8 b) and phenanthrene (9 b) were synthesized starting from their corresponding bromo derivatives via sonogashira cross coupling (see supporting information). Compound 7 is commercially available and was used without further purification. Finally, 7, 8 b and 9 b were coupled to the HBC precursor 6 in a sonogashira cross coupling reaction to generate 10, 11 and 12 (Scheme 2). HBC 10 was purified via column chromatography and obtained in a good yield of 79 %. Due to their poor solubility in common organic solvents, the isolation of 11 and 12 was rather challenging and the compounds were isolated in low yields. After some failed attempts, the purification was achieved using a short plug filtration (silica, CH2Cl2), collection of the yellow baseline and extraction of the baseline with hot CH2Cl2.
Scheme 2.
Synthesis of 10, 11 and 12. a) Ar, Pd(PPh3)2Cl2, CuI, Et3N, rt, 16 h.
Synthesis of Ligand‐Substituted Triskelion‐Shaped HBCs
In the next step the ligands bpy, phen, terpy and acac were coupled to the HBC moiety.
The tris‐bipyridine HBC 14 was synthesized in good yields via sonogashira cross coupling of 6 to the ethynyl‐bipyridine 13 b (Scheme 3), which was previously obtained from the bromo derivative (see supporting information). After successful synthesis, the aim was to expand the scope to phenanthroline and terpyridine HBC derivatives via the same route. The formation of both target molecules was verified by MALDI mass spectrometry. However, the isolation of the compounds could not be achieved due to solubility issues.
Scheme 3.
Synthesis of 14. a) Ar, Pd(PPh3)2Cl2, CuI, Et3N, DMF, 80 °C, 16 h.
In order to improve the solubility of the triskelion‐shaped HBCs, we decided to replace the tert‐butyl groups by mesityl groups to increase the solubility of the compounds. For this purpose, the synthetic route for the synthesis of the appropriate triiodo‐HBC precursor was adapted (Scheme 4). Starting again with the synthesis of 2 via iodination of 1,3,5‐tribromobenzene, the subsequent selective suzuki cross coupling was performed with TMS‐phenylboronic acid to obtain 15 in a good yield of 76 %. It was also possible to obtain a crystal structure of 15 (Figure 1). After a second suzuki cross coupling using mesitylphenyl boronic pinacol ester 16, the synthesis of the HAB derivative 17 could be achieved. The TMS‐groups were subsequently substituted with iodine via iodo‐desylation using iodine monochloride. Finally, the scholl oxidation of 18 allowed for the formation of the desired HBC derivative 19.
Scheme 4.
Synthesis route towards the Mes3‐I3‐HBC 19. a) H5IO6, KI, H2SO4, 0 °C→ r.t., 3 d; b) Ar, Pd(dppf)Cl2, K3PO4, toluene/H2O, 100 °C, 16 h; c) Ar, Pd(dppf)Cl2, K3PO4, toluene/H2O, 100 °C, 4 d.; d) Ar, ICl, CH2Cl2, −78 °C→r.t., 16 h; e) Ar, TfOH, DDQ, CH2Cl2, 0 °C→r.t., 3 h.
Figure 1.

Crystal structure of 15.
With 19 in hand, the sonogashira cross coupling with ethynyl‐phenanthroline 20 b and ‐terpyridine 21 b was carried out to successfully synthesize the HBCs 22 and 23 in good to moderate yields, as expected (Scheme 5) . Due to the improved solubility, it was possible to purify 22 and 23 via column chromatography.
Scheme 5.
Synthesis of 22 and 24. a) Ar, Pd(PPh3)2Cl2, CuI, Et3N, DMF, 80 °C, 16 h.
To complete our scope, the acetylacetone (acac) ligand was coupled to the HBC moiety (Scheme 6). In contrast to the neutral ligands bpy, phen and terpy, the negatively charged acac in its deprotonated form provides additional opportunities for metal complexation. Therefore, an asymmetric acac derivative carrying a tert‐butyl group to improve the solubility of the compound was designed. For this purpose, pinacolone and 4‐bromobenzoyl chloride were reacted under basic conditions in a condensation reaction to obtain the acac derivative 24. Compound 24 can undergo a sonogashira cross coupling with TMS‐acetylene to generate 25 in quantitative yields. After deprotection of the triple bond, 26 was formed as an ideal precursor for the sonogashira cross coupling with 19. This led to the successful synthesis of the desired target molecule 27 in good yields.
Scheme 6.
Synthesis route towards 26. a) Ar, LiHMDS in THF, THF, 0 °C, 7 h; b) Ar, TMSA, Pd(PPh3)2Cl2, CuI, Et3N, THF, 70 °C, 16 h; c) Ar, TBAF in THF, THF, rt, 45 min; d) Ar, 19, Pd(PPh3)2Cl2, CuI, Et3N, THF, 70 °C, 16 h.
NMR Spectroscopy
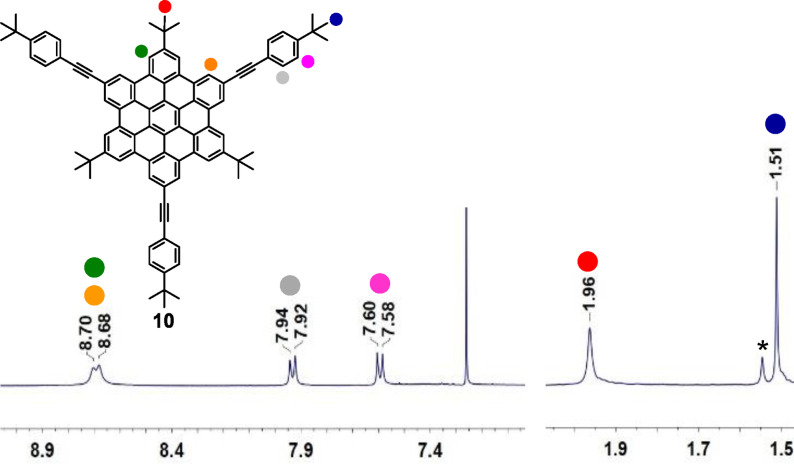
All presented compounds were characterized by high resolution mass spectrometry and NMR spectroscopy. Due to the symmetry of the synthesized HBCs, the NMR investigations show one set of signals. The 1H NMR spectrum of 10 is shown as an example in Figure 2. In the aliphatic region two singlets at 1.96 ppm and 1.51 ppm were found. The more downfield shifted signal can be assigned to directly to the HBC core connected the tert‐butyl substituents (red), whereas the more upfield shifted singlet was assigned to the tert‐butyl groups located at the phenyl substituents (blue). The signals of the two different HBC protons (green/orange) can be found as the most downfield shifted signals in the spectrum giving two broad singlets. The remaining two signals for the phenyl protons (grey/pink) appear in the region from 8.00–7.50 ppm showing the typical splitting for 1,4‐substituted phenyl derivatives. In analogy, the NMR spectra of the other tert‐butyl substituted HBCs 6, 11, 12 and 14 were obtained with the appropriate signals of the respective substituents.
Figure 2.
1H NMR of 10 in CDCl3 at rt (zoom in) *H2O.
As example for the mesityl substituted HBCs, the 1H NMR spectrum of compound 23 is depicted in Figure 3. The singlets in the aliphatic region were assigned to the methyl groups of the mesityl substituents (red/blue). Furthermore, the signals of the aromatic HBC protons (green) refer to the most deshielded signals at 9.44 ppm and 9.18 ppm, which is significantly downfield shifted compared to the HBC protons of the tert‐butyl substituted HBCs. The most upfield shifted singlet of the aromatic protons at 7.18 ppm can be assigned to the mesityl groups (grey). The additional signals in the aromatic region from 8.71–7.30 ppm correspond to the protons of terpy substituent. The NMR investigation of 19, 22 and 27 show analogous spectra with the remaining signals of the substituents.
Figure 3.
1H NMR of 23 in C2D2Cl4 at 100 °C (zoom in).
UV/vis and Fluorescence Spectroscopy
All these new HBC derivatives reveal a bright yellow to brownish color and green‐yellow fluorescence emission. They were characterized by UV/Vis and fluorescence spectroscopy in deoxygenated dichloromethane (c=10−6 mol/L). For fluorescence spectroscopy the corresponding wavelength at the absorption maximum was used for excitation. Comparing the absorption maxima of 6, 10, 11 and 12 a significant red‐shift up to 21 nm was detected (Figure 4). The absorption maximum of 6 is located at 361 nm whereas the absorption maxima of the HBCs 10 (λmax=375 nm), 11 (λmax=378 nm) and 12 (λmax=382 nm) are shifted to longer wavelengths. This bathochromic shift can be explained by the addition of phenyl rings which leads to an extended π‐system of the substituents. It is noticeable that the shape of the absorption spectra slightly differs compared to the characteristic three band maxima of HBCs. The lower wavelength maximum seems to overlap with the absorption maximum giving a broad band. In wavelength region above 390 nm two shoulders appear in the spectra of each compound, which is again redshifted in similar fashion as described for the absorption maxima. In contrast to the non–fluorescent compound 6, the HBCs 10, 11 and 12 show green‐yellow fluorescence emission (Figure 5). The shape of the emission spectra of the compounds is quite similar, while the respective emission maxima are slightly red shifted from 10 (λmax, em=503 nm) to 11 (λmax, em=504 nm) and 12 (λmax, em=505 nm) with the extended π‐system of the substituents.
Figure 4.
UV/Vis absorption spectra of 6, 10, 11 and 12 in dichloromethane.
Figure 5.
Normalized fluorescence emission spectra of 10, 11 and 12 in dichloromethane.
The UV/Vis absorption spectra of the ligand‐substituted HBCs 14, 22, 23 and 27 as well as the Mes3I3‐HBC precursor 19 are depicted in Figure 6. The absorption spectrum of the Mes3I3‐HBC 19 shows the characteristic three band maxima shape with an absorption maximum at 365 nm similar to the related precursor 6. The threefold substitution of the HBCs lead to broadened absorption spectra of 14, 22, 23 and 27. Again, the lower wavelength maximum seems to overlap with the absorption maximum resulting in a broad band with a shoulder. In comparison to the precursors 6 or respectively 19, the absorption maximum of the substituted compounds is shifted to longer wavelengths (14: λmax=378 nm, 22: λmax=388 nm, 23: λmax=382 nm and 27: λmax=388 nm). In wavelength areas larger than 400 nm, two shoulders appear in the spectrum of compound 14. For compounds 22, 23 and 27 a second maximum was observed. Compared to precursor 19, the intensity of this second absorption maximum is significantly increased in relation to the absorption maximum of each compound. Again, in contrast to the non‐fluorescent compound 19, the ligand‐substituted HBCs 14 (λmax, em=503 nm), 22 (λmax, em=507 nm), 23 (λmax, em=506 nm) and 27 (λmax, em=507 nm) show green‐yellow fluorescence emission (Figure 7). The respective shape of the emission spectrum of 22, 23 and 27 is quite similar, whereas the shape of the emission spectrum of 14 differs. The difference can be explained by the different solubilizing substituents, since compound 14 is carrying tert‐butyl substituents whereas 22, 23 and 27 are substituted with mesityl groups.
Figure 6.
UV/Vis absorption spectra of 19, 14, 22, 23 and 27 in dichloromethane.
Figure 7.
Normalized fluorescence emission spectra of 14, 22, 23 and 27 in dichloromethane.
Semi‐Empirical Calculations
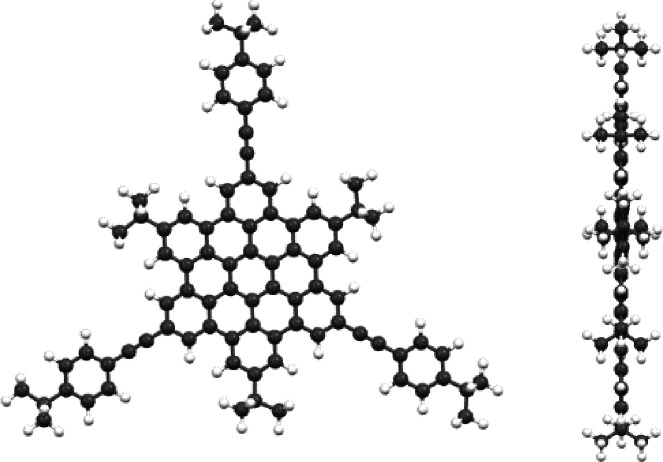
To investigate the structure and preferred conformation of the new HBC compounds, theoretical calculations with the software HyperChemTM were conducted. The results of the semi‐empirical calculations (PM3) confirmed the expectation of a planar conformation of all presented HBCs. Depending on the substituents connected to the HBC the symmetries vary from D3h , C3h to C3 , as presumed. For the tert‐butyl substituted compounds HBCs 6 and 10 the calculations indicate a D3h symmetry (Figure 8). The symmetry of 11, 12 and 14 was determined as C3h (Figure 9) due to the not existing C2 rotation axes perpendicular to the C3 axis.
Figure 8.
Structure of 10 (example D3h symmetry) top and side view calculated by semi‐empirical methods.
Figure 9.
Structure of 11 (example C3h symmetry) top and side view calculated by semi‐empirical methods.
For the mesityl‐substituted compounds 19, 22, 23 and 27 the calculations indicate also a planar structure with the difference that the mesityl substituents are tilted about 45 ° out of the plane. This was expected since the mesityl groups were introduced to hinder the π–π‐stacking of the HBCs, which led to a better solubility of the compounds. Due to that fact, no additional mirror planes or rotation axes were determined, indicating a C3 symmetry for all presented mesityl substituted HBCs (Figure 10). The plots of the calculated structures of the further HBC compounds can be found in the supporting information.
Figure 10.
Structure of 22 (example C3 symmetry) top and side view calculated by semi‐empirical methods.
Conclusions
We report the first synthesis of a series of ethynyl‐substituted triskelion‐shaped HBC derivatives. The new synthetic strategy for tris‐iodinated HBC precursors provides the basis and opened up the opportunity to introduce different substituents. Key step in this synthetic route is a selective suzuki coupling, which allows the C3 symmetric pre‐functionalization. The exchange of the solubilizing groups from tert‐butyl to mesityl substituents facilitated the purification of the compounds due to the increased solubility. Therefore, it was possible to successfully obtain in sum seven different triskelion‐shaped HBCs. Spectroscopic investigations show, that the tris‐substitution of HBCs led to a bathochromic shift of the absorption maximum up to 23 nm compared to the iodinated precursor molecules 6 and 19 (λmax=365 nm). Furthermore, all tris‐substituted compounds (10, 11, 12, 14, 22, 23 and 27) show in contrast to the iodinated precursor molecules (6 and 19) green‐yellow fluorescence emission. Using semi‐empirical calculations, the three‐dimensional structure of the HBC compounds was determined indicating a D3h , C3h or C3 symmetry of the compounds depending on the substituents. Especially the ligand‐based compounds 14, 22, 23 and 27 might be potential candidates for catching transition metal cations, which is currently studied. Due to the tris‐substitution they might be also potential candidates for the formation of new metal organic frameworks.
Experimental Section
The synthetic procedures for precursors 2–9, 13, 15–21 and 24–26, the complementing data, as well as information about the used materials and instrumentation are given in the Supporting Information.
General procedure Sonogashira coupling: In a pressure vial, CuI (0.15–0.3 eq), Pd(PPh3)2Cl2 (0.15–0.3 eq) and the ethynyl coupling partner (6–10 eq) were dissolved in deoxygenated triethylamine (10, 11, 12), triethylamine/THF mixture (27) or triethylamine/DMF mixture (14, 22, 23). The tris‐iodiated HBC precursor (1 eq) was added and the reaction mixture was stirred at rt, 70 °C or 80 °C for 16 h. After aqueous workup, the crude product was purified by column chromatography. For purification of compounds 11 and 12 a short plug filtration, collection of the yellow baseline and extraction of the baseline with hot CH2Cl2 was used.
Supporting Information
The authors have cited additional references within the Supporting Information.11, 15 [11, 15‐ 26]
Conflict of Interests
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgments
The authors acknowledge financial support by the Deutsche Forschungsgemeinschaft (DFG) (Project number 522357978). Open Access funding enabled and organized by Projekt DEAL.
Buck J., Hampel F., Hirsch A., Chem. Eur. J. 2025, 31, e202404000. 10.1002/chem.202404000
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The original data supporting this study are open available in Zenodo (10.5281/zenodo.13990188). Deposition Number 2393679 contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service (http://www.ccdc.cam.ac.uk/structures).
References
- 1. Narita A., Wang X., Feng X., Müllen K., Chem. Soc. Rev. 2015, 44, 6616–6643. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt-Mende L., Fechtenkötter A., Müllen K., Moons E., Friend R. H., Mackenzie J. D., Science 2001, 293, 1119–1122. [DOI] [PubMed] [Google Scholar]
- 3. van de Craats A. M., Stutzmann N., Bunk O., Nielsen M. M., Watson M., Müllen K., Chanzy H. D., Sirringhaus H., Friend R. H., Adv. Mater. 2003, 15, 495–499. [Google Scholar]
- 4. van de Craats A. M., Warman J. M., Fechtenkötter A., Brand J. D., Harbison M. A., Müllen K., Adv. Mater. 1999, 11, 1469–1472. [Google Scholar]
- 5. Wu J., Baumgarten M., Debije M. G., Warman J. M., Müllen K., Angew. Chem. Int. Ed. 2004, 43, 5331–5335. [DOI] [PubMed] [Google Scholar]
- 6. Clar E., Ironside C. T., Proc. Chem. Soc. 1958, 150–150. [Google Scholar]
- 7. Halleux A., Martin R., King G., Helv. Chim. Acta 1958, 41, 1177–1183. [Google Scholar]
- 8. Stabel A., Herwig P., Müllen K., Rabe J. P., Angew. Chem. Int. Ed. 1995, 34, 1609–1611. [Google Scholar]
- 9. Scholl R., Mansfeld J., Ber. Dtsch. Chem. Ges. 1910, 43, 1734–1746. [Google Scholar]
- 10. Wu J., Pisula W., Müllen K., Chem. Rev. 2007, 107, 718–747. [DOI] [PubMed] [Google Scholar]
- 11. Lungerich D., Reger D., Hölzel H., Riedel R., Martin M. M. J. C., Hampel F., Jux N., Angew. Chem. Int. Ed. 2016, 55, 5602–5605. [DOI] [PubMed] [Google Scholar]
- 12. Hyatt J. A., Org. Prep. Proced. Int. 1991, 23, 460–463. [Google Scholar]
- 13. Martin M. M., Lungerich D., Hampel F., Langer J., Ronson T. K., Jux N., Chem. Eur. J. 2019, 25, 15083–15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng X., Wu J., Enkelmann V., Müllen K., Org. Lett. 2006, 8, 1145–1148. [DOI] [PubMed] [Google Scholar]
- 15. Barluenga J., González J. M., García-Martín M. A., Campos P. J., Tetrahedron Lett. 1993, 34, 3893–3896. [Google Scholar]
- 16. Kobayashi K., Kobayashi N., J. Org. Chem. 2004, 69, 2487–2497. [DOI] [PubMed] [Google Scholar]
- 17. Rajkiewicz A. A., Wojciechowska N., Kalek M., ACS Catal. 2020, 10 (1), 831–841. [Google Scholar]
- 18. Bharath D., Chithiravel S., Sasikumar M., Chereddy N. R., Shanigaram B., Bhanuprakash K., Krishnamoorthy K., Rao V. J., RSC Adv. 2015, 5, 94859–94865. [Google Scholar]
- 19. Hakoda Y., Aoyagi M., Irisawa K., Kato S., Nakamura Y., Yamaji M., Photochem Photobiol Sci 2016, 15, 1586–1593. [DOI] [PubMed] [Google Scholar]
- 20. Sarobe M., Jenneskens L. W., Steggink R. G. B., Visser T., J. Org. Chem. 1999, 64 (11), 3861–3866. [Google Scholar]
- 21. Scattergood P. A., Roberts J., Omar S. A. E., Elliott P. I. P., Inorg. Chem. 2019, 58 (13), 8607–8621. [DOI] [PubMed] [Google Scholar]
- 22. Bingöl B., Durrell A. C., Keller G. E., Palmer J. H., Grubbs R. H., Gray H. B., J. Phys. Chem. B 2013, 117 (16), 4177–4182. [DOI] [PubMed] [Google Scholar]
- 23. Wipf P., Jung J., J. Org. Chem. 2000, 65 (20), 6319–6337. [DOI] [PubMed] [Google Scholar]
- 24. Calimsiz S., Sayah M., Mallik D., Organ M. G., Angew. Chem. Int. Ed. 2010, 49, 2014–2017. [DOI] [PubMed] [Google Scholar]
- 25. Vrábel M., Hocek M., Havran L., Fojta M., Votruba I., Klepetarova B., Pohl R., Rulisek L., Zendlova L., Hobza P., Shih I., Mabery E., Mackman R., Eur. J. Inorg. Chem. 2007, 1752–1769. [Google Scholar]
- 26. Grosshenny V., Romero F. M., Ziessel R., J. Org. Chem. 1997, 62 (5), 1491–1500. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The original data supporting this study are open available in Zenodo (10.5281/zenodo.13990188). Deposition Number 2393679 contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service (http://www.ccdc.cam.ac.uk/structures).