Abstract
1. Relay neurones were extracellularly recorded from the A-layers of the dorsal lateral geniculate nucleus (dLGN) of the anaesthetized cat. The noradrenergic influence on retinogeniculate transmission was investigated through microiontopheretic techniques in a total of 140 dLGN relay cells using three experimental approaches: (i) the effects of agonists for alpha 1-, alpha 2- and beta-adrenoceptors were separately analysed; (ii) the noradrenergic influence was related to the global state of activity of the relay neurones, which was associated with discrete patterns of the electroencephalogram (EEG); (iii) distinct phases of visual responses evoked from the area of the retinal receptive field, and of binocular and lateral inhibitory responses, were evaluated before, during and after the action of noradrenergic agonists. 2. The spontaneous generation of high-frequency bursts of spikes in dLGN relay neurones, associated with periods of highly synchronized, delta-like patterns of the EEG, was selectively suppressed by the beta-adrenoceptor agonist isoprenaline or the alpha 1- adrenoceptor agonist phenylephrine. Single action potentials, occurring at a low frequency between bursts, were significantly less affected. Depending upon the ejection level of the adrenoceptor agonists, burst activity was suppressed by 23-73%, compared with a reduction in single spike firing in the range 7-24%. The suppression of burst firing occurred in all functional types of dLGN relay neurones (X, Y; on, off), enhanced burst activity was observed in less than 1% of the cells. 3. On-going tonic sequences of action potentials (around 15 Hz), occurring during periods of EEG activity characterized by lower amplitudes and higher frequencies, were separately affected by adrenoceptor agonists. Isoprenaline had no significant effect, phenylephrine induced a global reduction of spike firing with no obvious relation to the ejection level, and the alpha 2-adrenoceptor agonist clonidine inhibited action potential generation in a near dose-dependent manner. 4. Visual response properties were investigated during periods of less synchronized states of EEG activity. Responses to visual stimulation of the retinal receptive field centre were not significantly influenced by isoprenaline, while phenylephrine or clonidine attenuated the phasic and the tonic response component in all functional types of relay neurones and independent of the stimulus contrast being used. At low ejection levels, slight facilitatory effects were observed with isoprenaline (65% of neurones that were tested) or phenylephrine (15%). The inhibitory influence of the antagonistic surround area of the receptive field appeared unaltered during action of isoprenaline or phenylephrine.(ABSTRACT TRUNCATED AT 400 WORDS)
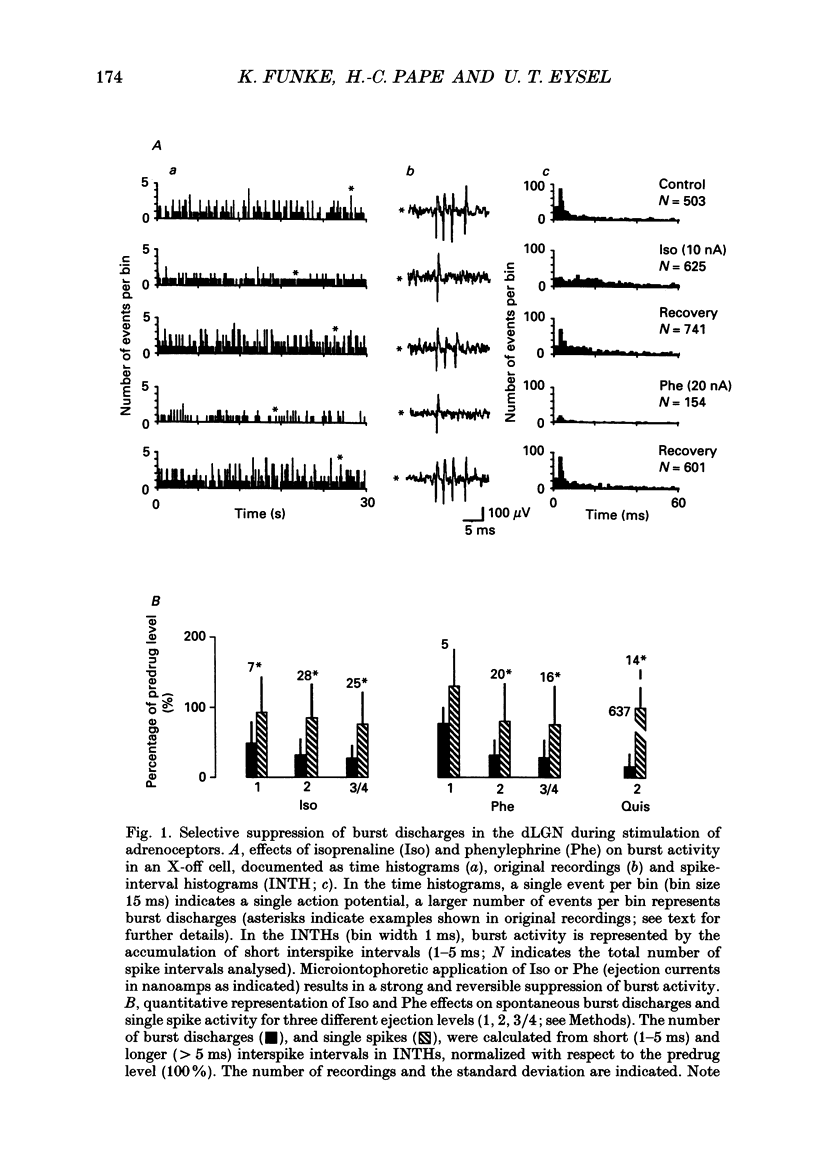
Full text
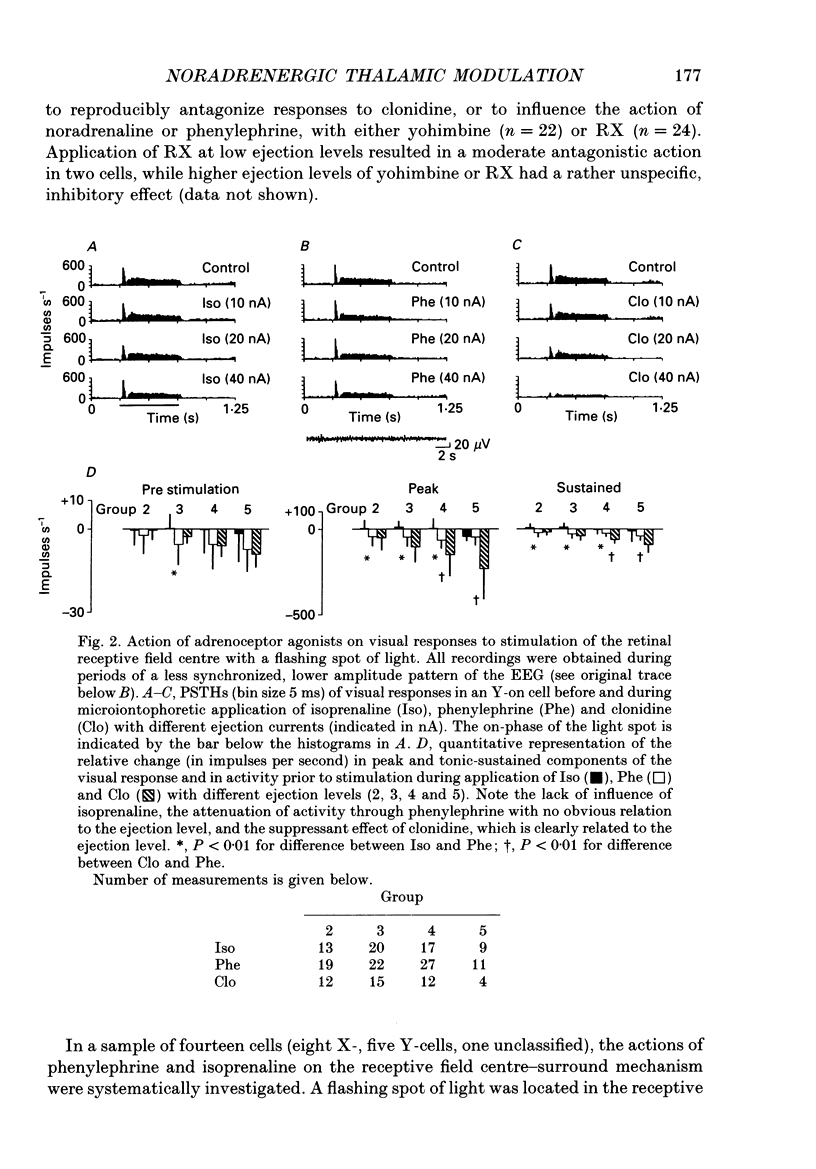
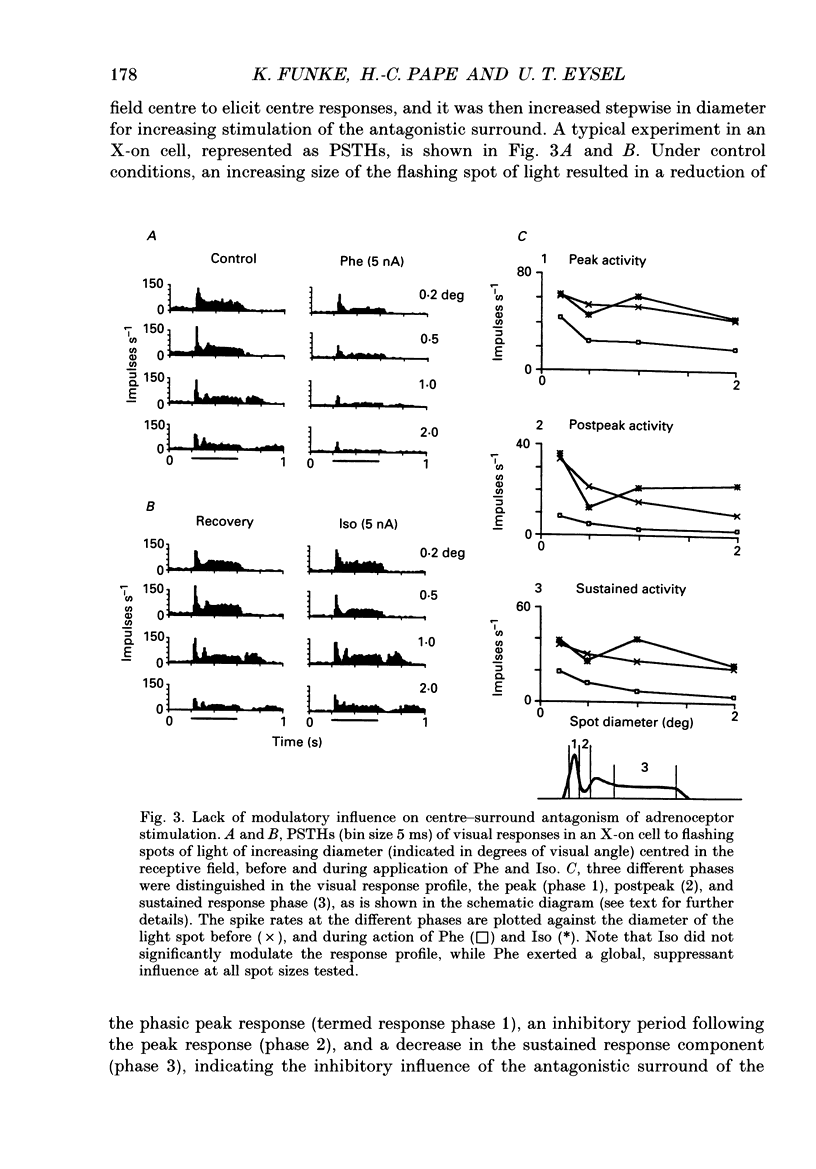
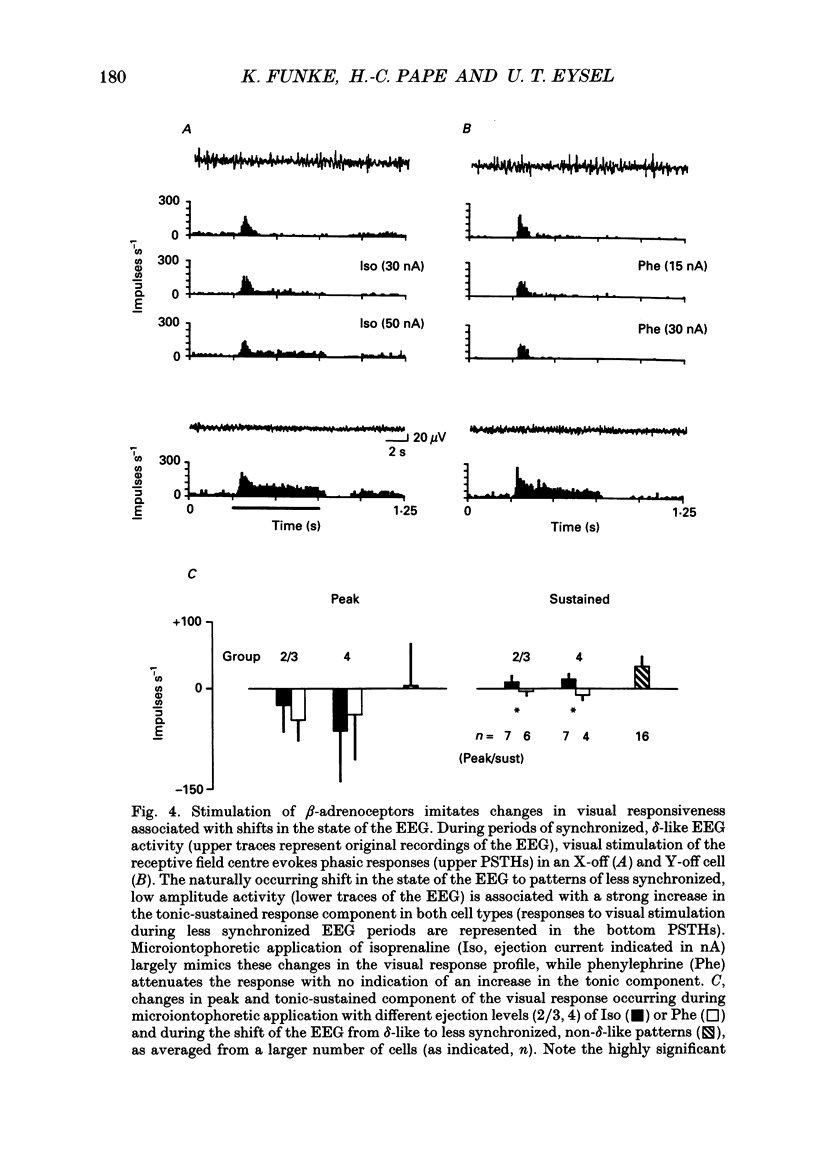
PDF








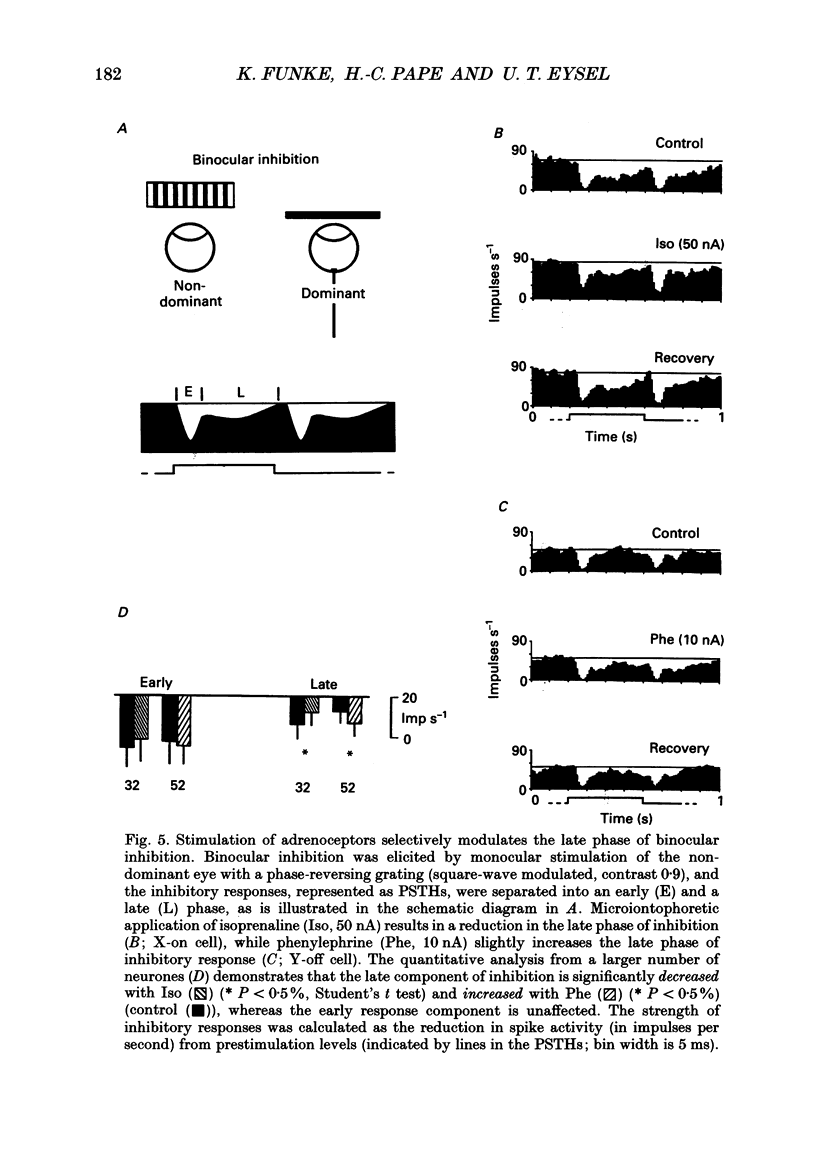
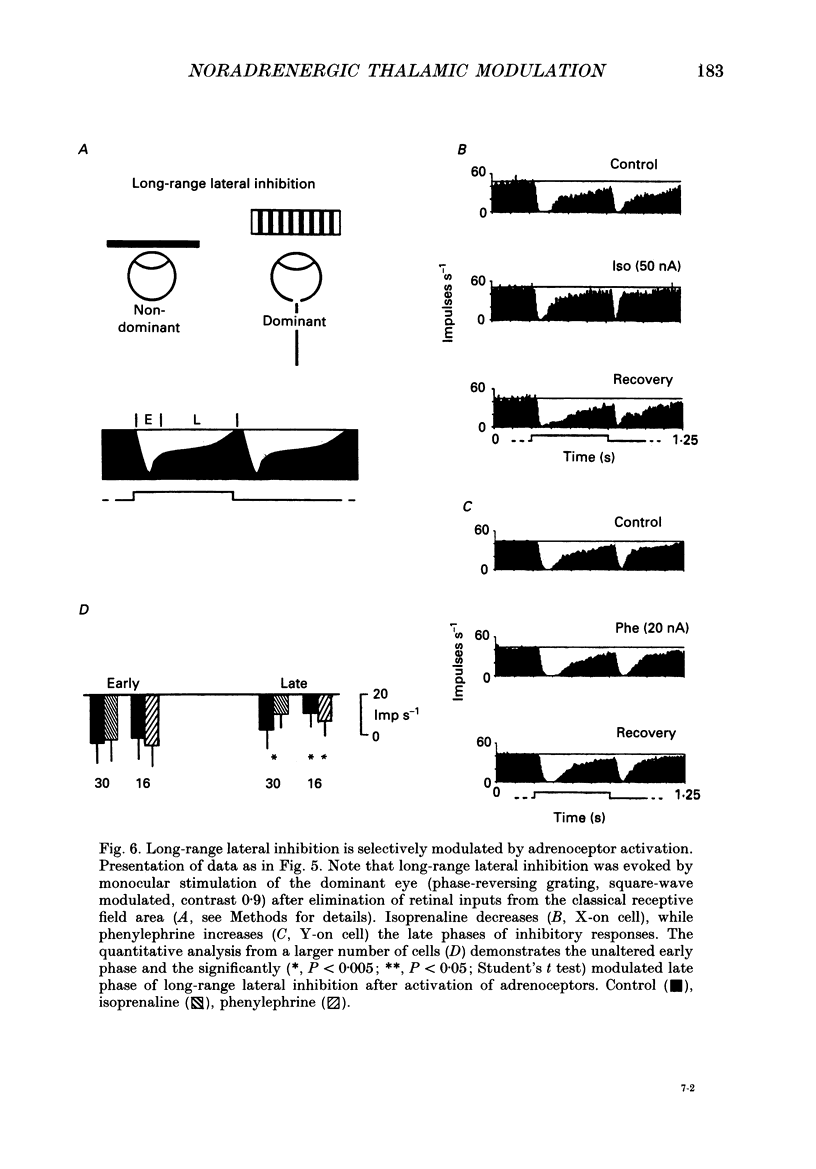
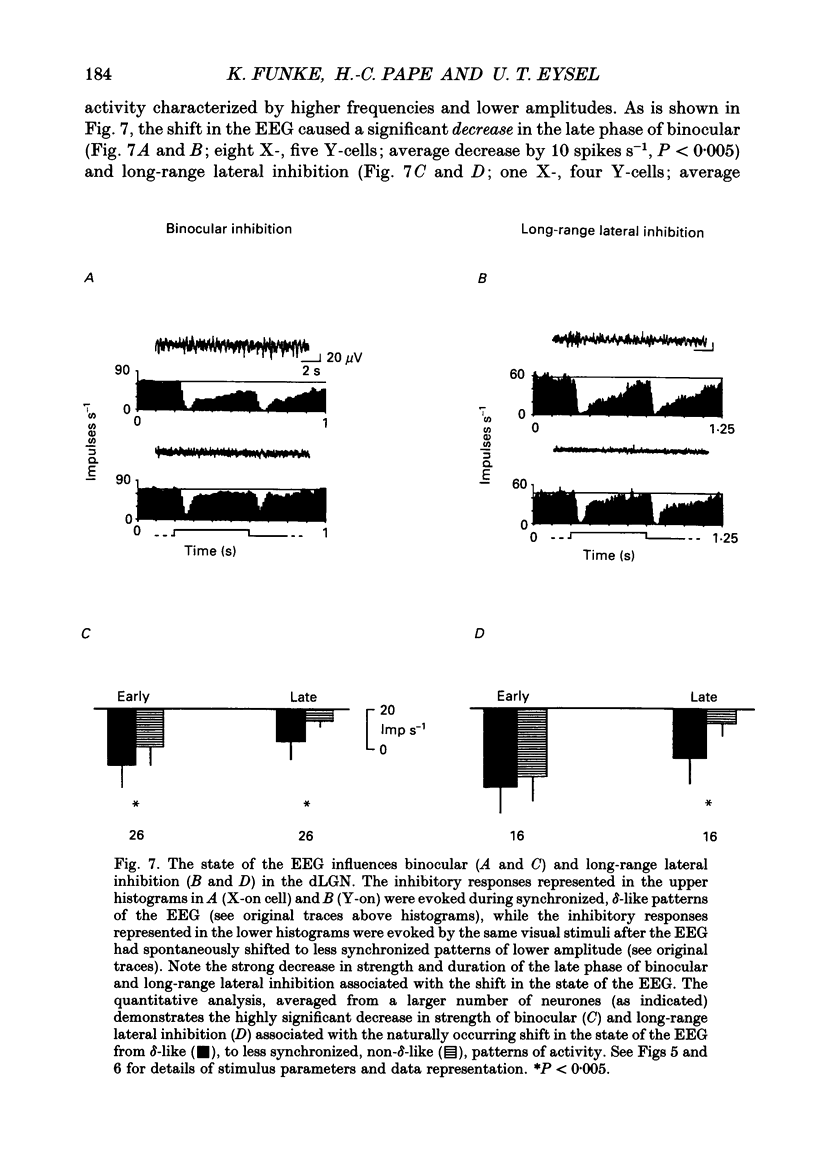







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aston-Jones G., Bloom F. E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981 Aug;1(8):876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund D. B., U'Prichard D. C. Characterization of alpha 1- and alpha 2-adrenergic receptors. Int Rev Neurobiol. 1983;24:343–431. [PubMed] [Google Scholar]
- Bárány E. H. Muscarinic subsensitivity without receptor change in monkey ciliary muscle. Br J Pharmacol. 1985 Jan;84(1):193–198. [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curet O., de Montigny C. Electrophysiological characterization of adrenoceptors in the rat dorsal hippocampus. I. Receptors mediating the effect of microiontophoretically applied norepinephrine. Brain Res. 1988 Dec 13;475(1):35–46. doi: 10.1016/0006-8993(88)90196-5. [DOI] [PubMed] [Google Scholar]
- Curró Dossi R., Paré D., Steriade M. Various types of inhibitory postsynaptic potentials in anterior thalamic cells are differentially altered by stimulation of laterodorsal tegmental cholinergic nucleus. Neuroscience. 1992;47(2):279–289. doi: 10.1016/0306-4522(92)90244-v. [DOI] [PubMed] [Google Scholar]
- Eysel U. T., Pape H. C., Van Schayck R. Excitatory and differential disinhibitory actions of acetylcholine in the lateral geniculate nucleus of the cat. J Physiol. 1986 Jan;370:233–254. doi: 10.1113/jphysiol.1986.sp015932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi W., Müller C. M., Singer W. Cholinergic mechanisms in the reticular control of transmission in the cat lateral geniculate nucleus. J Neurophysiol. 1988 Jun;59(6):1690–1718. doi: 10.1152/jn.1988.59.6.1690. [DOI] [PubMed] [Google Scholar]
- Funke K., Eysel U. T. EEG-dependent modulation of response dynamics of cat dLGN relay cells and the contribution of corticogeniculate feedback. Brain Res. 1992 Feb 28;573(2):217–227. doi: 10.1016/0006-8993(92)90766-3. [DOI] [PubMed] [Google Scholar]
- Godfraind J. M. Acetylcholine and somatically evoked inhibition on perigeniculate neurones in the cat. Br J Pharmacol. 1978 Jun;63(2):295–302. doi: 10.1111/j.1476-5381.1978.tb09760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W., Tumosa N., Spear P. D. Binocular interactions in the cat's dorsal lateral geniculate nucleus. I. Spatial-frequency analysis of responses of X, Y, and W cells to nondominant-eye stimulation. J Neurophysiol. 1989 Aug;62(2):526–543. doi: 10.1152/jn.1989.62.2.526. [DOI] [PubMed] [Google Scholar]
- Hobson J. A. Sleep and dreaming. J Neurosci. 1990 Feb;10(2):371–382. doi: 10.1523/JNEUROSCI.10-02-00371.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Sensitivity of neurones in visual cortex (area 17) under different levels of anaesthesia. Exp Brain Res. 1974;20(5):471–484. doi: 10.1007/BF00238014. [DOI] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984 Apr;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayama Y., Negi T., Sugitani M., Iwama K. Effects of locus coeruleus stimulation on neuronal activities of dorsal lateral geniculate nucleus and perigeniculate reticular nucleus of the rat. Neuroscience. 1982 Mar;7(3):655–666. doi: 10.1016/0306-4522(82)90071-9. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981 Jun 18;291(5816):554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- McCarley R. W., Benoit O., Barrionuevo G. Lateral geniculate nucleus unitary discharge in sleep and waking: state- and rate-specific aspects. J Neurophysiol. 1983 Oct;50(4):798–818. doi: 10.1152/jn.1983.50.4.798. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992 Oct;39(4):337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Acetylcholine inhibits identified interneurons in the cat lateral geniculate nucleus. Nature. 1988 Jul 21;334(6179):246–248. doi: 10.1038/334246a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol. 1987 Nov;392:147–165. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J Neurophysiol. 1988 Mar;59(3):978–996. doi: 10.1152/jn.1988.59.3.978. [DOI] [PubMed] [Google Scholar]
- Nakai Y., Takaori S. Influence of norepinephrine-containing neurons derived from the locus coeruleus on lateral geniculate neuronal activities of cats. Brain Res. 1974 May 10;71(1):47–60. doi: 10.1016/0006-8993(74)90190-5. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Tsujimura R., Nomura J. Interaction between alpha 2- and beta-adrenergic receptors in rat cerebral cortical membranes: clonidine-induced reduction in agonist and antagonist affinity for beta-adrenergic receptors. Brain Res. 1991 Mar 1;542(2):181–186. doi: 10.1016/0006-8993(91)91564-h. [DOI] [PubMed] [Google Scholar]
- Pape H. C., McCormick D. A. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989 Aug 31;340(6236):715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Tebècis A. K., York D. H. The inhibitory action of monoamines on lateral geniculate neurones. J Physiol. 1967 Jun;190(3):563–581. doi: 10.1113/jphysiol.1967.sp008228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski M. A., Aghajanian G. K. Modulation of lateral geniculate neurone excitability by noradrenaline microiontophoresis or locus coeruleus stimulation. Nature. 1980 Oct 23;287(5784):731–734. doi: 10.1038/287731a0. [DOI] [PubMed] [Google Scholar]
- Ruffolo R. R., Jr, Nichols A. J., Stadel J. M., Hieble J. P. Structure and function of alpha-adrenoceptors. Pharmacol Rev. 1991 Dec;43(4):475–505. [PubMed] [Google Scholar]
- Shapley R., Hochstein S. Visual spatial summation in two classes of geniculate cells. Nature. 1975 Jul 31;256(5516):411–413. doi: 10.1038/256411a0. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A., Berardi N. The cholinergic influence on the function of the cat dorsal lateral geniculate nucleus (dLGN). Brain Res. 1983 Dec 5;280(2):299–307. doi: 10.1016/0006-8993(83)90059-8. [DOI] [PubMed] [Google Scholar]
- Singer W. Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiol Rev. 1977 Jul;57(3):386–420. doi: 10.1152/physrev.1977.57.3.386. [DOI] [PubMed] [Google Scholar]
- Singer W. Inhibitory binocular interaction in the lateral geniculate body of the cat. Brain Res. 1970 Feb 17;18(1):165–170. doi: 10.1016/0006-8993(70)90463-4. [DOI] [PubMed] [Google Scholar]
- Starke K., Göthert M., Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989 Jul;69(3):864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschenes M. The thalamus as a neuronal oscillator. Brain Res. 1984 Nov;320(1):1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Steriade M., Dossi R. C., Nuñez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. J Neurosci. 1991 Oct;11(10):3200–3217. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlrich D. J., Cucchiaro J. B., Humphrey A. L., Sherman S. M. Morphology and axonal projection patterns of individual neurons in the cat perigeniculate nucleus. J Neurophysiol. 1991 Jun;65(6):1528–1541. doi: 10.1152/jn.1991.65.6.1528. [DOI] [PubMed] [Google Scholar]
- Unnerstall J. R., Kopajtic T. A., Kuhar M. J. Distribution of alpha 2 agonist binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agents. Brain Res. 1984 Mar;319(1):69–101. doi: 10.1016/0165-0173(84)90030-4. [DOI] [PubMed] [Google Scholar]


