Abstract
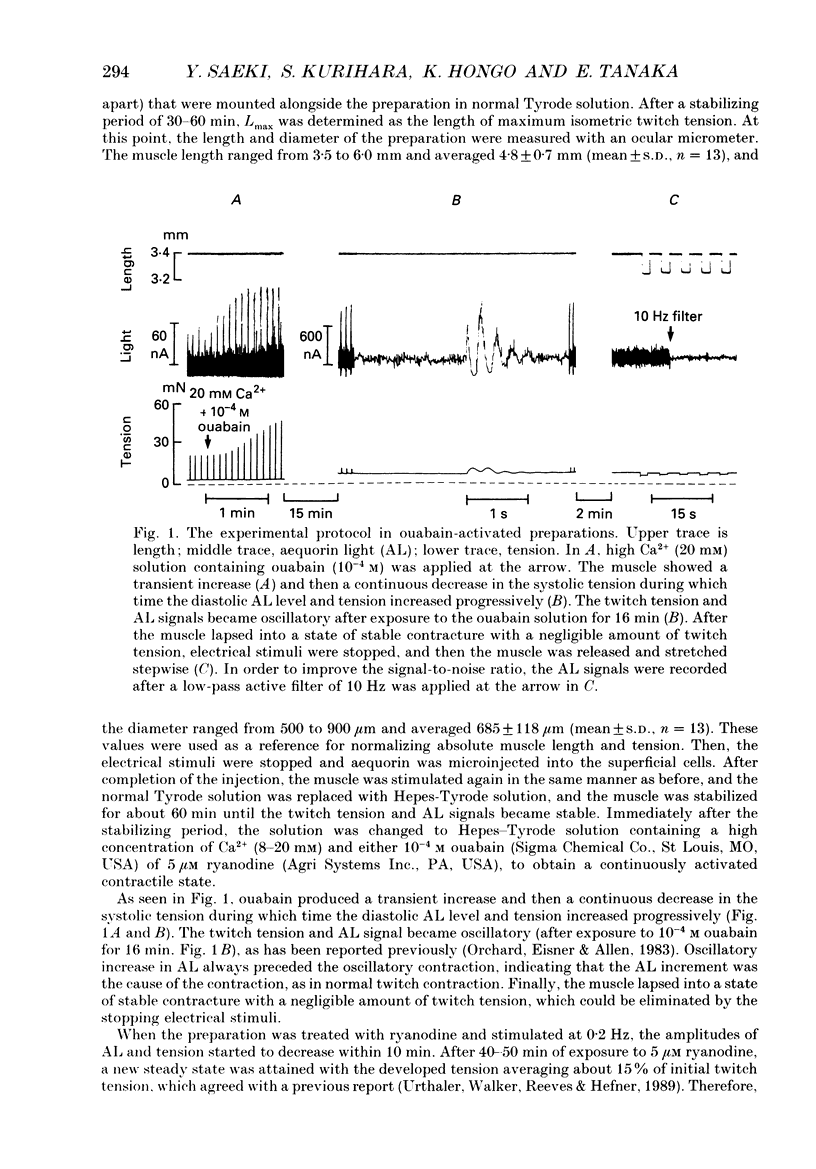
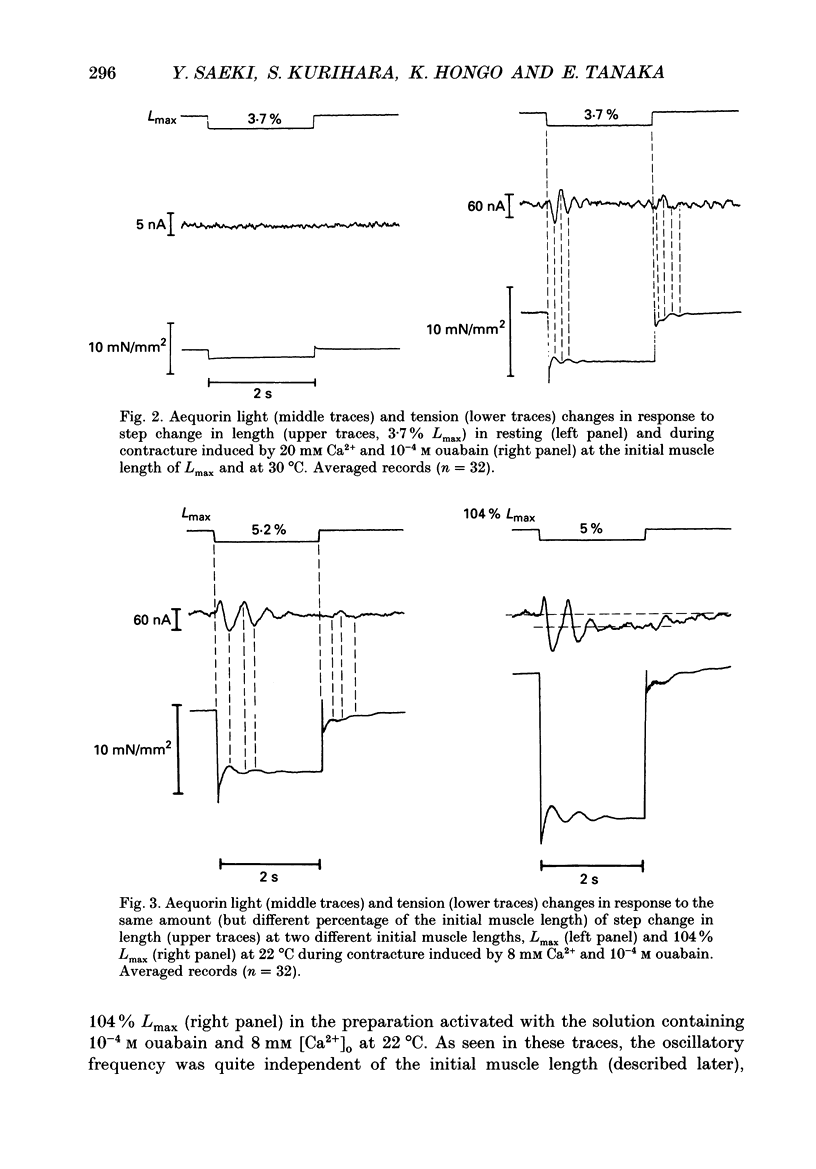
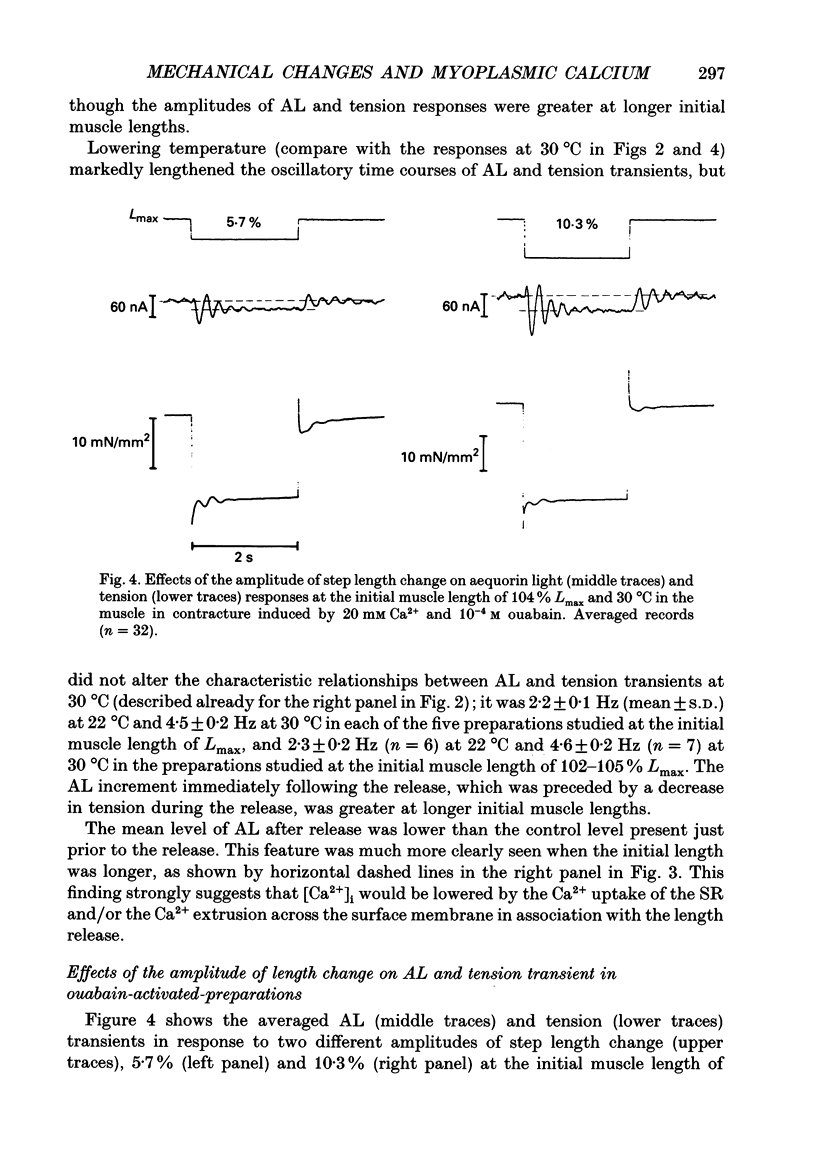
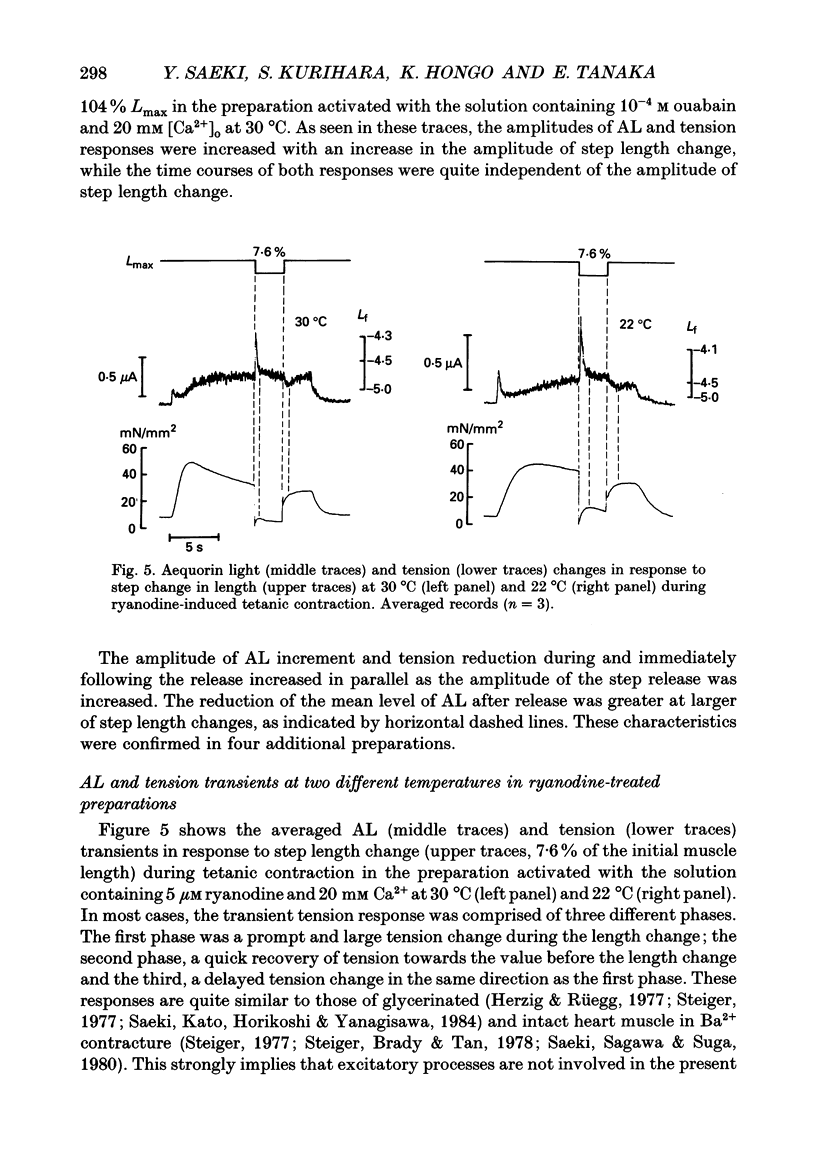
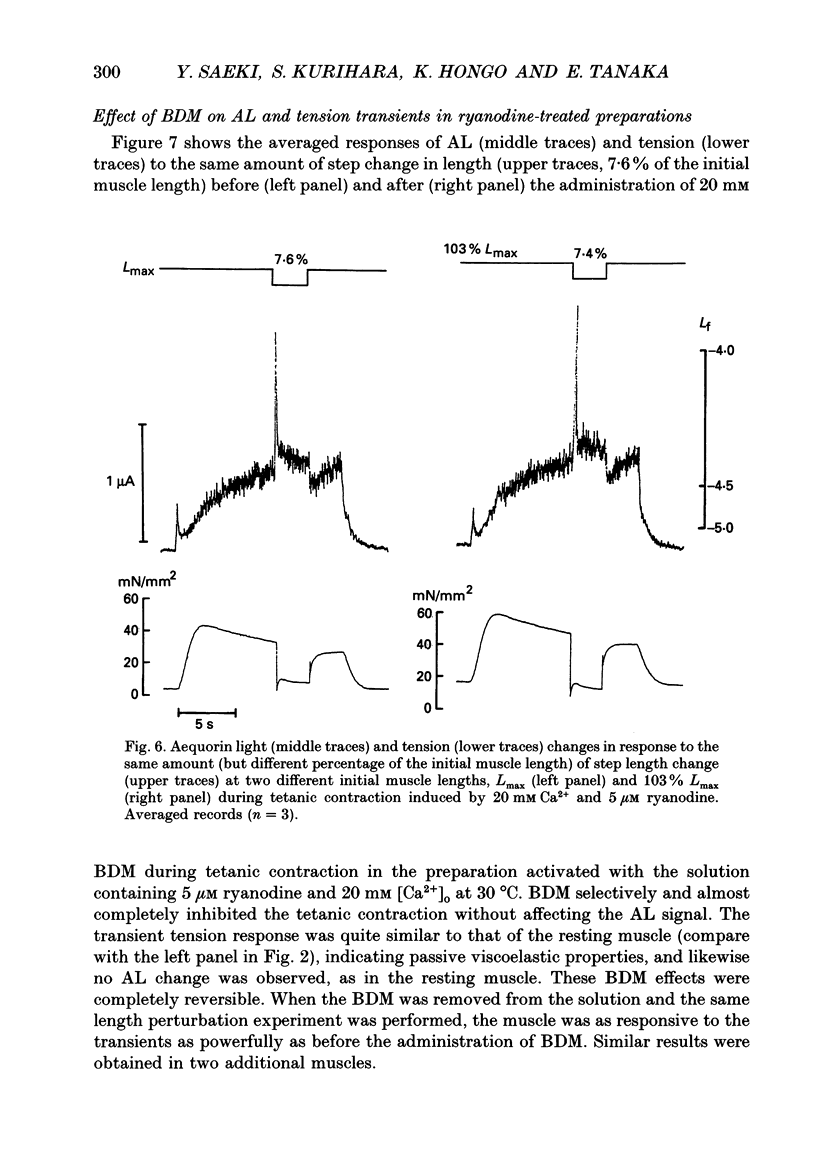
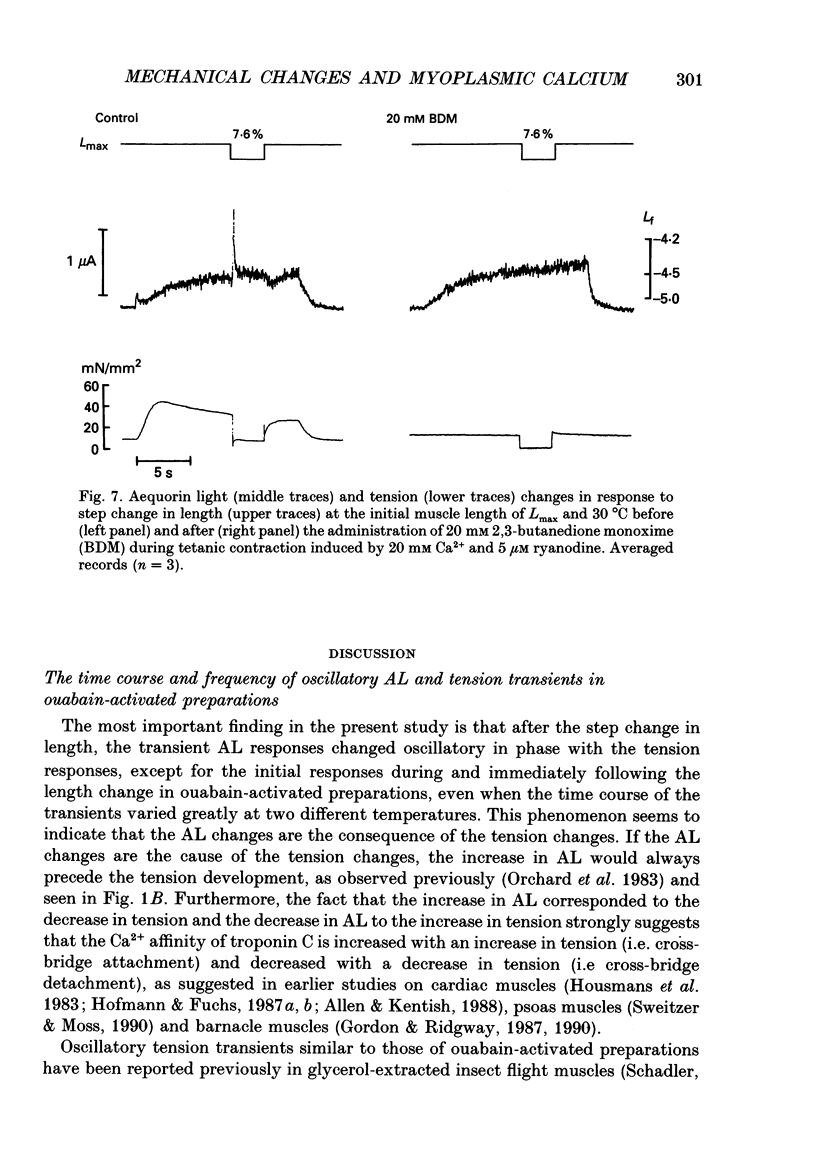
1. To study the effects of mechanical constraints on the calcium (Ca2+) affinity of cardiac troponin C, we analysed the tension and aequorin light (AL, intracellular Ca2+) transients in response to a step length change in aequorin-injected ferret right ventricular papillary muscles. The muscle preparations were continuously activated with ouabain (10(-4) M) (ouabain contracture) or with high frequency stimuli in the presence of ryanodine (5 microM) (tetanic contraction). 2. The tension transient in response to either the release or stretch was oscillatory: tension decreased rapidly during the release and then increased, after which it lapsed into a new steady level in a series of damped oscillations. The opposite was true for the stretch. The oscillatory responses were conspicuous and less damped in ouabain-activated preparations (oscillation frequency of 2.2-2.3 Hz at 22 degrees and 4.5-4.6 Hz at 30 degrees C) and much more damped in ryanodine-treated preparations. 3. The transient AL response was also oscillatory, the time course of which corresponded to that of the transient tension response. Regardless of the difference in the time course of the transients in two different preparations and at two different temperatures, the increase in AL corresponded to the decrease in tension, likewise the decrease in AL to the increase in tension. 4. The mean level of AL after release was lower than the control level present just prior to the release in ouabain-activated preparations, but the AL after release finally returned to the nearly control level in ryanodine-treated preparations. 5. When the ryanodine-treated muscle was further treated with 2,3-butanedione monoxime (BDM) (20 mM), the tetanic tension decreased remarkably without affecting the AL signal. The tension transient of this preparation was quite similar to that of the resting muscle, which changed in a nearly stepwise fashion; AL was hardly affected by step length changes, as in the resting muscle, in spite of the higher AL level. 6. These results suggest that the Ca2+ affinity of cardiac troponin C is increased with an increase in tension (i.e. the cross-bridge attachment) and decreased with a decrease in tension i.e. the cross-bridge detachment), and that the mean [Ca2+]i is lowered by release, at least in a Ca(2+)-overloaded condition, mainly through the sarcoplasmic reticulum.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Kentish J. C. Calcium concentration in the myoplasm of skinned ferret ventricular muscle following changes in muscle length. J Physiol. 1988 Dec;407:489–503. doi: 10.1113/jphysiol.1988.sp017427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Kentish J. C. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985 Sep;17(9):821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1982 Jun;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M. R., Peterson J. N., Yue D. T., Hunter W. C. Effect of isoproterenol on force transient time course and on stiffness spectra in rabbit papillary muscle in barium contracture. J Mol Cell Cardiol. 1988 May;20(5):415–426. doi: 10.1016/s0022-2828(88)80133-0. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Bridge J. H., MacLeod K. T. The mechanism of ryanodine action in rabbit ventricular muscle evaluated with Ca-selective microelectrodes and rapid cooling contractures. Can J Physiol Pharmacol. 1987 Apr;65(4):610–618. doi: 10.1139/y87-103. [DOI] [PubMed] [Google Scholar]
- Blanchard E. M., Smith G. L., Allen D. G., Alpert N. R. The effects of 2,3-butanedione monoxime on initial heat, tension, and aequorin light output of ferret papillary muscles. Pflugers Arch. 1990 Apr;416(1-2):219–221. doi: 10.1007/BF00370248. [DOI] [PubMed] [Google Scholar]
- Cooper G., 4th Load and length regulation of cardiac energetics. Annu Rev Physiol. 1990;52:505–522. doi: 10.1146/annurev.ph.52.030190.002445. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Effects of ryanodine in skinned cardiac cells. Fed Proc. 1985 Dec;44(15):2970–2976. [PubMed] [Google Scholar]
- Gordon A. M., Ridgway E. B. Extra calcium on shortening in barnacle muscle. Is the decrease in calcium binding related to decreased cross-bridge attachment, force, or length? J Gen Physiol. 1987 Sep;90(3):321–340. doi: 10.1085/jgp.90.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Ridgway E. B. Stretch of active muscle during the declining phase of the calcium transient produces biphasic changes in calcium binding to the activating sites. J Gen Physiol. 1990 Nov;96(5):1013–1035. doi: 10.1085/jgp.96.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G., Lakatta E. G. Ryanodine releases calcium from sarcoplasmic reticulum in calcium-tolerant rat cardiac myocytes. J Physiol. 1987 Sep;390:453–467. doi: 10.1113/jphysiol.1987.sp016711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann P. A., Fuchs F. Effect of length and cross-bridge attachment on Ca2+ binding to cardiac troponin C. Am J Physiol. 1987 Jul;253(1 Pt 1):C90–C96. doi: 10.1152/ajpcell.1987.253.1.C90. [DOI] [PubMed] [Google Scholar]
- Hofmann P. A., Fuchs F. Evidence for a force-dependent component of calcium binding to cardiac troponin C. Am J Physiol. 1987 Oct;253(4 Pt 1):C541–C546. doi: 10.1152/ajpcell.1987.253.4.C541. [DOI] [PubMed] [Google Scholar]
- Housmans P. R., Lee N. K., Blinks J. R. Active shortening retards the decline of the intracellular calcium transient in mammalian heart muscle. Science. 1983 Jul 8;221(4606):159–161. doi: 10.1126/science.6857274. [DOI] [PubMed] [Google Scholar]
- Kurihara S., Saeki Y., Hongo K., Tanaka E., Sudo N. Effects of length change on intracellular Ca2+ transients in ferret ventricular muscle treated with 2,3-butanedione monoxime (BDM). Jpn J Physiol. 1990;40(6):915–920. doi: 10.2170/jjphysiol.40.915. [DOI] [PubMed] [Google Scholar]
- Le Guennec J. Y., White E., Gannier F., Argibay J. A., Garnier D. Stretch-induced increase of resting intracellular calcium concentration in single guinea-pig ventricular myocytes. Exp Physiol. 1991 Nov;76(6):975–978. doi: 10.1113/expphysiol.1991.sp003560. [DOI] [PubMed] [Google Scholar]
- Marban E., Kusuoka H., Yue D. T., Weisfeldt M. L., Wier W. G. Maximal Ca2+-activated force elicited by tetanization of ferret papillary muscle and whole heart: mechanism and characteristics of steady contractile activation in intact myocardium. Circ Res. 1986 Sep;59(3):262–269. doi: 10.1161/01.res.59.3.262. [DOI] [PubMed] [Google Scholar]
- Marban E., Wier W. G. Ryanodine as a tool to determine the contributions of calcium entry and calcium release to the calcium transient and contraction of cardiac Purkinje fibers. Circ Res. 1985 Jan;56(1):133–138. doi: 10.1161/01.res.56.1.133. [DOI] [PubMed] [Google Scholar]
- Mörner S. E., Wohlfart B. The action of 2,3-butane-dionemonoxime on the inotropic state in guinea-pig myocardium. Acta Physiol Scand. 1991 Jun;142(2):211–219. doi: 10.1111/j.1748-1716.1991.tb09149.x. [DOI] [PubMed] [Google Scholar]
- Okazaki O., Suda N., Hongo K., Konishi M., Kurihara S. Modulation of Ca2+ transients and contractile properties by beta-adrenoceptor stimulation in ferret ventricular muscles. J Physiol. 1990 Apr;423:221–240. doi: 10.1113/jphysiol.1990.sp018019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard C. H., Eisner D. A., Allen D. G. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983 Aug 25;304(5928):735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Exchange of calcium ions in the mammalian myocardium. Mechanisms and physiological significance. Circ Res. 1974 May;34(5):599–605. doi: 10.1161/01.res.34.5.599. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Kato C., Horikoshi T., Yanagisawa K. Effects of Ba2+ on the mechanical properties of glycerinated heart muscle. Pflugers Arch. 1984 Mar;400(3):235–240. doi: 10.1007/BF00581553. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Sagawa K., Suga H. Transient tension responses of heart muscle in Ba2+ contracture to step length changes. Am J Physiol. 1980 Mar;238(3):H340–H347. doi: 10.1152/ajpheart.1980.238.3.H340. [DOI] [PubMed] [Google Scholar]
- Schädler M., Steiger G. J., Rüegg J. C. Mechanical activation and isometric oscillation in insect fibrillar muscle. Pflugers Arch. 1971;330(3):217–229. doi: 10.1007/BF00588613. [DOI] [PubMed] [Google Scholar]
- Steiger G. J., Brady A. J., Tan S. T. Intrinsic regulatory properties of contractility in the myocardium. Circ Res. 1978 Mar;42(3):339–350. doi: 10.1161/01.res.42.3.339. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Wendt I. R. Length dependence of changes in sarcoplasmic calcium concentration and myofibrillar calcium sensitivity in striated muscle fibres. J Muscle Res Cell Motil. 1984 Jun;5(3):243–272. doi: 10.1007/BF00713107. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Ito K., Kenyon J. L. Ryanodine: a modifier of sarcoplasmic reticulum calcium release in striated muscle. Fed Proc. 1985 Dec;44(15):2984–2988. [PubMed] [Google Scholar]
- Sweitzer N. K., Moss R. L. The effect of altered temperature on Ca2(+)-sensitive force in permeabilized myocardium and skeletal muscle. Evidence for force dependence of thin filament activation. J Gen Physiol. 1990 Dec;96(6):1221–1245. doi: 10.1085/jgp.96.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urthaler F., Walker A. A., Reeves R. C., Hefner L. L. Effects of ryanodine on contractile performance of intact length-clamped papillary muscle. Circ Res. 1989 Nov;65(5):1270–1282. doi: 10.1161/01.res.65.5.1270. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Marban E., Wier W. G. Relationship between force and intracellular [Ca2+] in tetanized mammalian heart muscle. J Gen Physiol. 1986 Feb;87(2):223–242. doi: 10.1085/jgp.87.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]


