Abstract
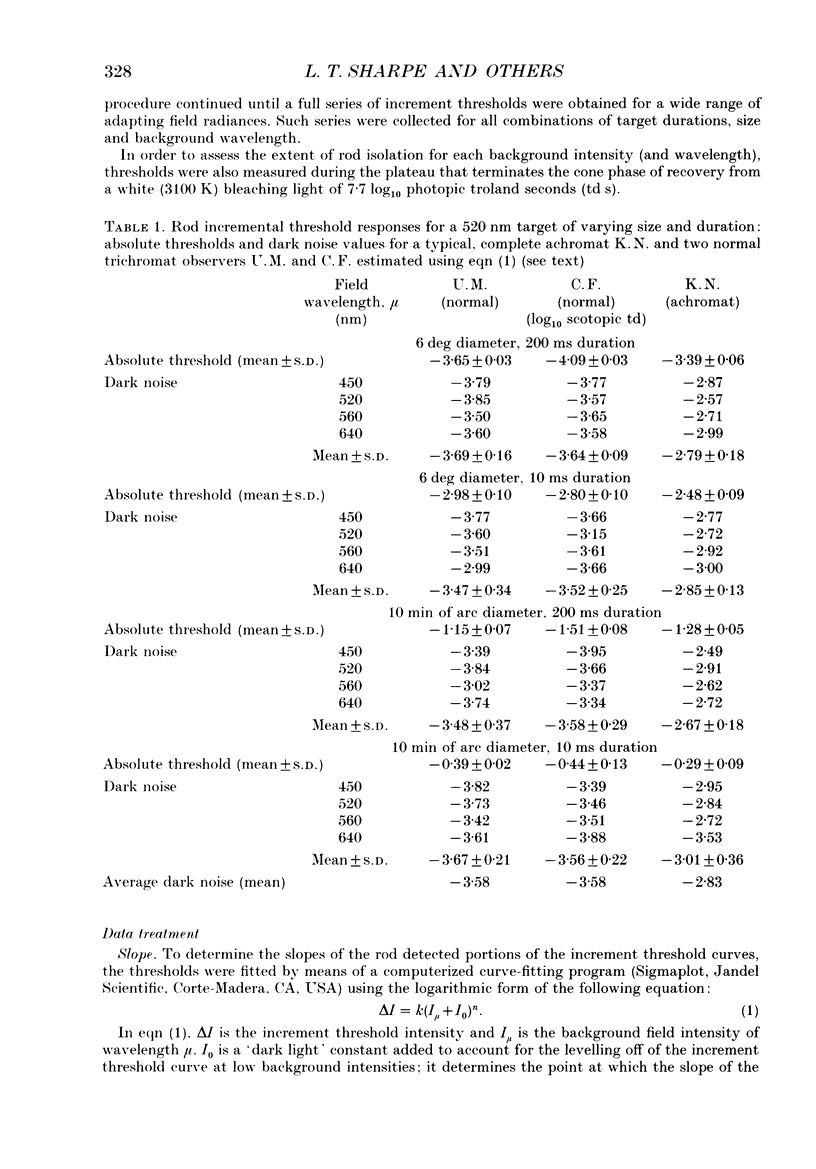
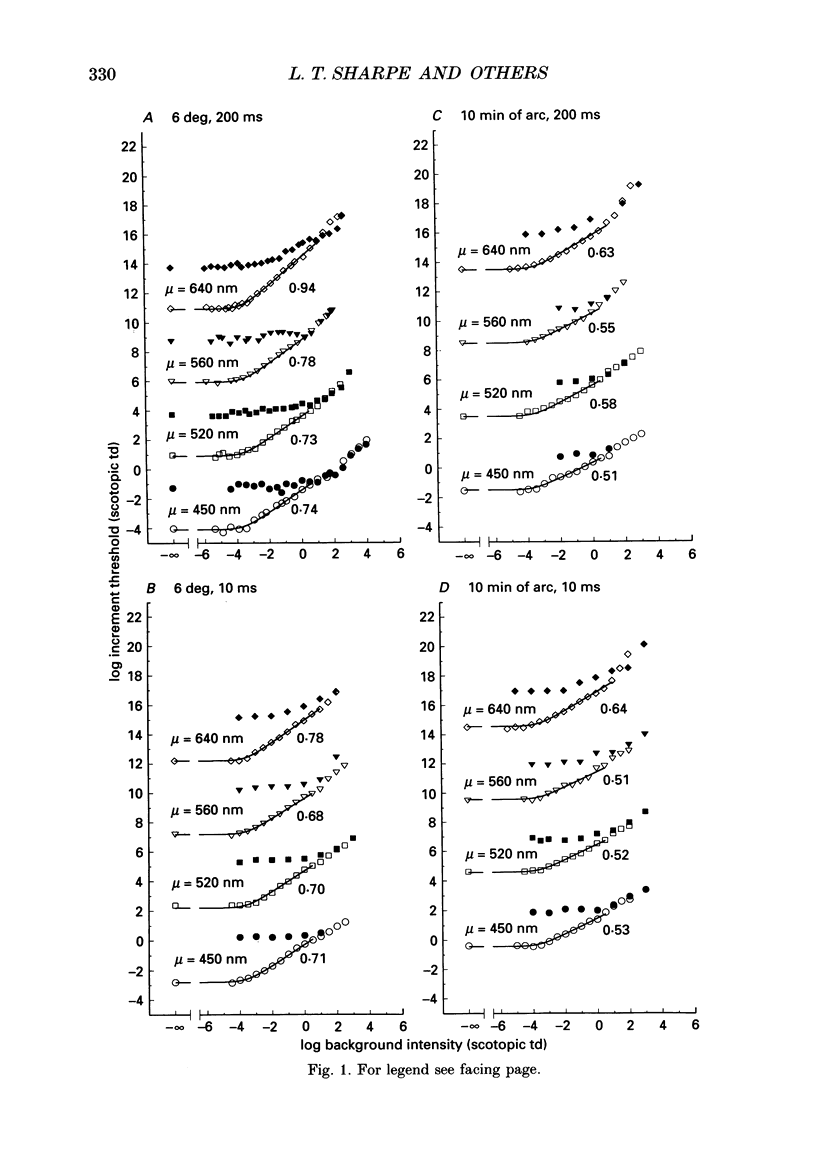
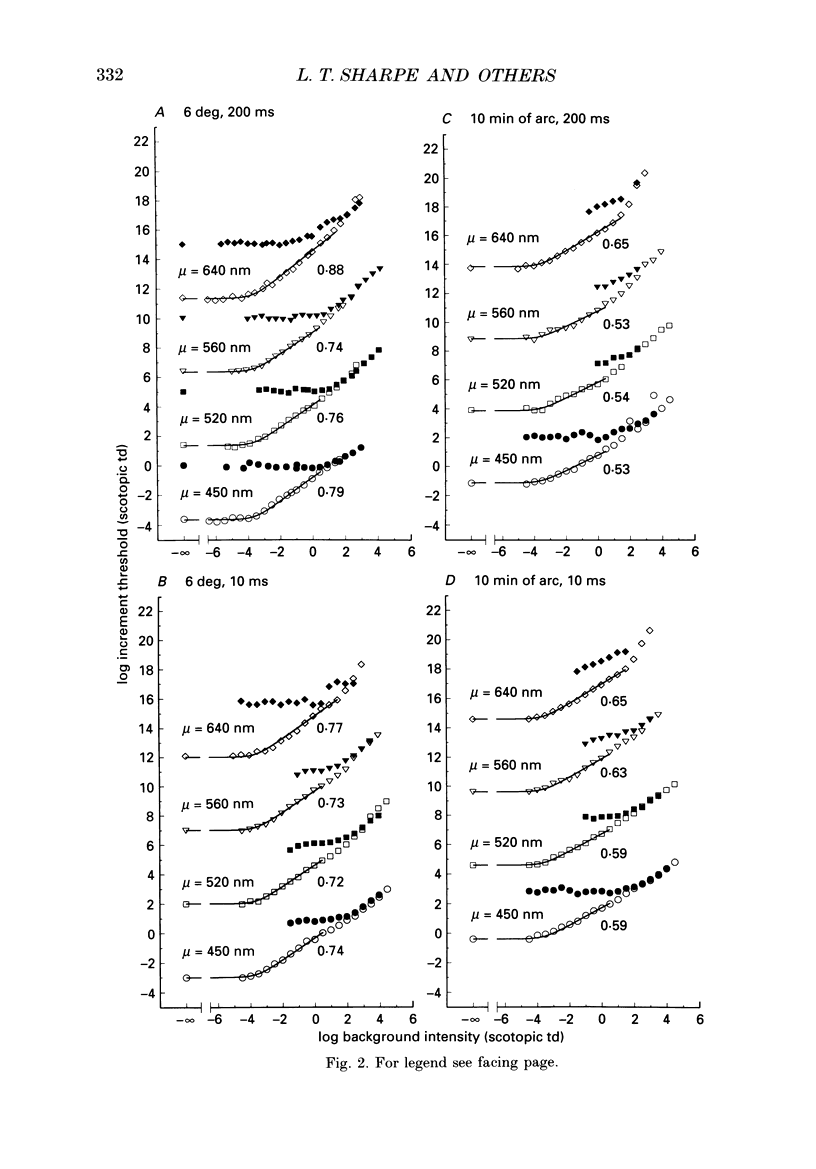
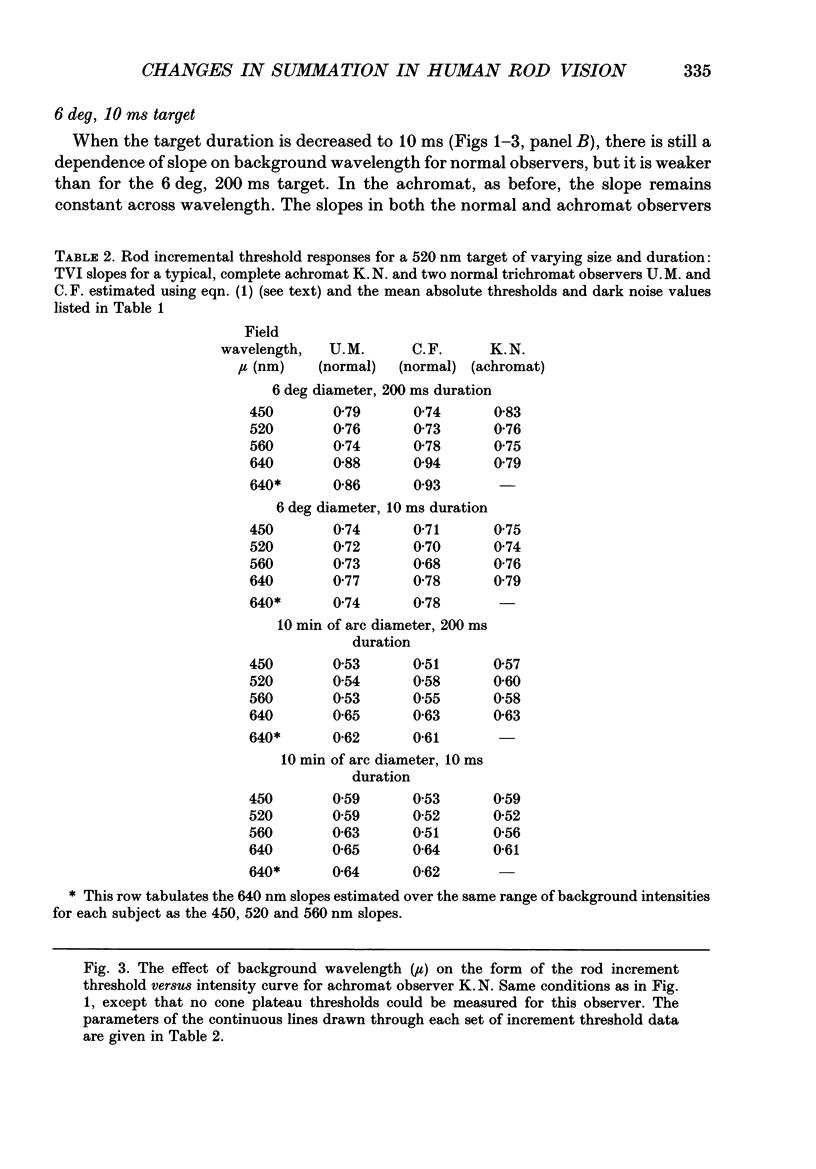
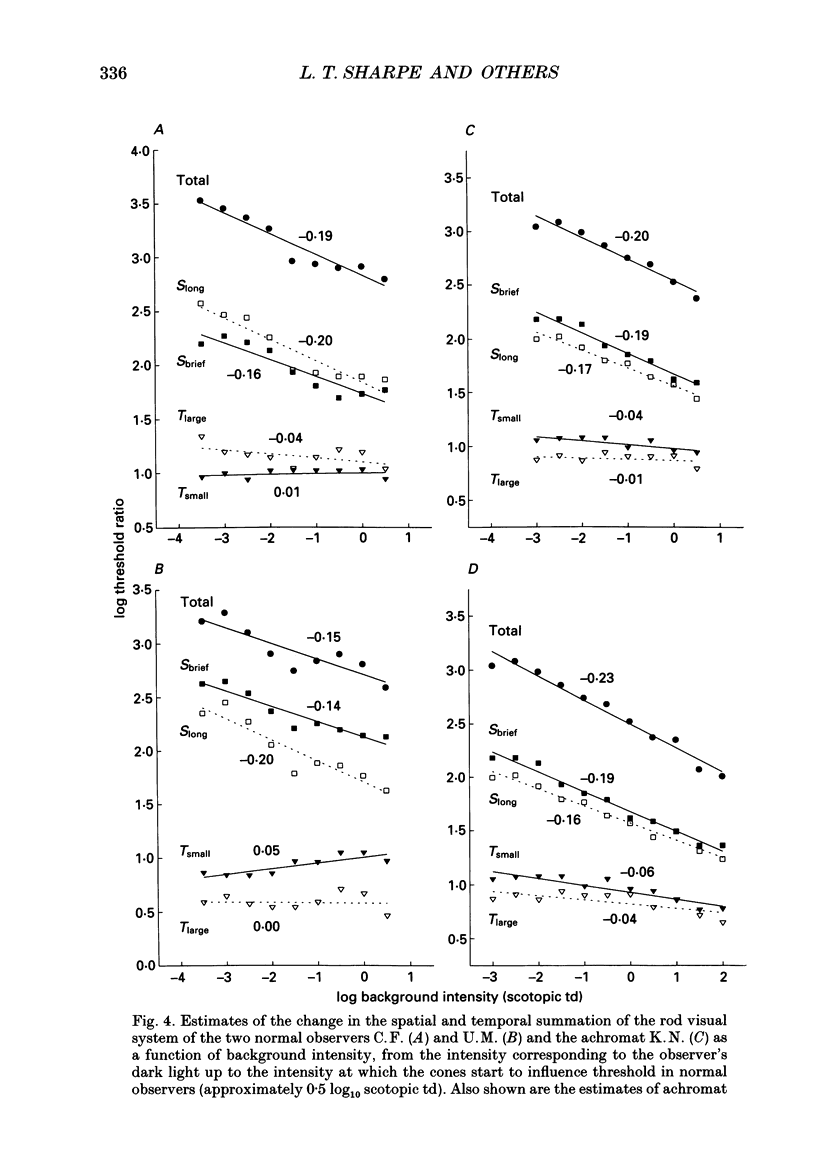
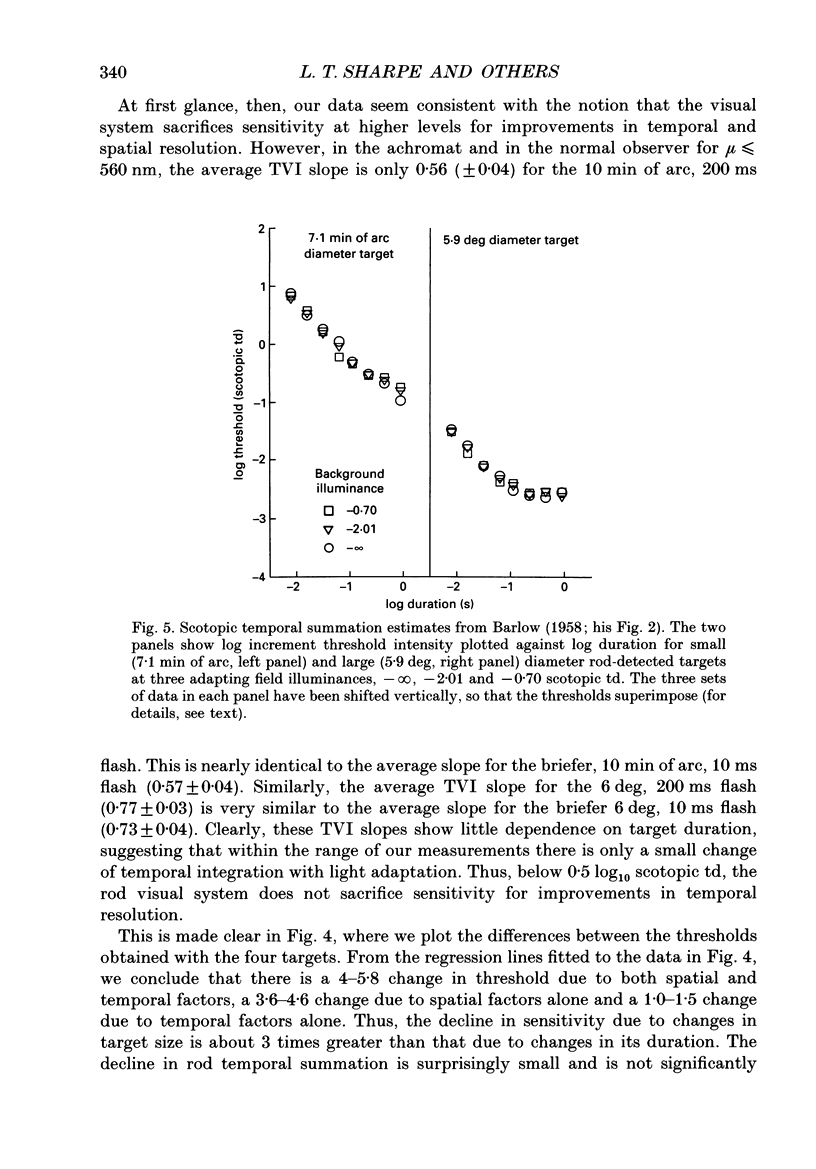
1. Absolute and increment thresholds were measured in a retinal region 12 deg temporal from the fovea with 520 nm targets of varying size and duration. Measurements were made under rod-isolation conditions in two normal observers and in a typical, complete achromat observer who has no cone-mediated vision. The purpose of these experiments was to determine how the temporal and spatial summation of rod-mediated vision changes with light adaptation. 2. The absolute threshold and the rise in increment threshold with background intensity depend upon target size and duration, but the psychophysically estimated dark light of the eye (the hypothetical light assumed to be equivalent to photoreceptor noise) does not. 3. The rise in increment threshold for tiny (10 min of arc), brief (10 ms) targets approaches the de Vries-Rose square-root law, varying according to the quantal fluctuations of the background light. The slope of the rod increment threshold versus background intensity (TVI) curves in logarithmic co-ordinates is about 0.56 +/- 0.04 (when cones are not influencing rod field adaptation). For large (6 deg) and long (200 ms) targets, a maximum slope of about 0.77 +/- 0.03 is attained. 4. The steeper slopes of the rod-detected TVI curves for large, long targets implies some reduction in temporal or spatial summation. In fact, the change in summation area is much more critical: under conditions where only the rod system is active the TVI curve slope is independent of target duration, suggesting that temporal summation is practically independent of background intensity. 5. The rise in threshold also depends on the wavelength of the background field in the normal observer but not in the achromat, confirming reports that the field adaptation of the rods is not independent of the quantal absorptions in the cones. The cone influence is most conspicuous on long-wavelength backgrounds and is found for all target sizes and durations, but is greater for large and long targets than for the other conditions.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARDEN G. B., WEALE R. A. Nervous mechanisms and dark-adaptation. J Physiol. 1954 Sep 28;125(3):417–426. doi: 10.1113/jphysiol.1954.sp005169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho A. C., Donner K., Hydén C., Reuter T., Orlov OYu Retinal noise, the performance of retinal ganglion cells, and visual sensitivity in the dark-adapted frog. J Opt Soc Am A. 1987 Dec;4(12):2321–2329. doi: 10.1364/josaa.4.002321. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B. Increment thresholds at low intensities considered as signal/noise discriminations. J Physiol. 1957 May 23;136(3):469–488. doi: 10.1113/jphysiol.1957.sp005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Retinal noise and absolute threshold. J Opt Soc Am. 1956 Aug;46(8):634–639. doi: 10.1364/josa.46.000634. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B. Temporal and spatial summation in human vision at different background intensities. J Physiol. 1958 Apr 30;141(2):337–350. doi: 10.1113/jphysiol.1958.sp005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Threshold setting by the surround of cat retinal ganglion cells. J Physiol. 1976 Aug;259(3):737–757. doi: 10.1113/jphysiol.1976.sp011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C. B., Rushton W. A. Dark adaptation and increment threshold in a rod monochromat. J Physiol. 1965 Dec;181(3):612–628. doi: 10.1113/jphysiol.1965.sp007786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Robson J. G. Application of Fourier analysis to the visibility of gratings. J Physiol. 1968 Aug;197(3):551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., MacLeod D. I., Stockman A. Improvement in human vision under bright light: grain or gain? J Physiol. 1987 Dec;394:41–66. doi: 10.1113/jphysiol.1987.sp016859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Jensen R. J., Brunken W. J. Rod pathways in mammalian retinae. Trends Neurosci. 1990 Mar;13(3):110–115. doi: 10.1016/0166-2236(90)90187-f. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Lennie P. The influence of temporal frequency and adaptation level on receptive field organization of retinal ganglion cells in cat. J Physiol. 1982 Dec;333:343–366. doi: 10.1113/jphysiol.1982.sp014457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Flux, not retinal illumination, is what cat retinal ganglion cells really care about. J Physiol. 1973 Sep;233(2):311–326. doi: 10.1113/jphysiol.1973.sp010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P. E. Quantum efficiency and false positive rate. J Physiol. 1969 Jun;202(2):421–436. doi: 10.1113/jphysiol.1969.sp008819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P. E. Rapid changes and hysteresis in spatial integration for human rod vision. J Physiol. 1971 Jun;215(2):433–447. doi: 10.1113/jphysiol.1971.sp009478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY D. H. Visual response to time-dependent stimuli. I. Amplitude sensitivity measurements. J Opt Soc Am. 1961 Apr;51:422–429. doi: 10.1364/josa.51.000422. [DOI] [PubMed] [Google Scholar]
- Lennie P. Scotopic increment thresholds in retinal ganglion cells. Vision Res. 1979;19(4):425–430. doi: 10.1016/0042-6989(79)90108-1. [DOI] [PubMed] [Google Scholar]
- Makous W., Boothe R. Cones block signals from rods. Vision Res. 1974 Apr;14(4):285–294. doi: 10.1016/0042-6989(74)90078-9. [DOI] [PubMed] [Google Scholar]
- Makous W., Peeples D. Rod-cone interaction: reconciliation with Flamant and Stiles. Vision Res. 1979;19(6):695–698. doi: 10.1016/0042-6989(79)90246-3. [DOI] [PubMed] [Google Scholar]
- Sakitt B. Counting every quantum. J Physiol. 1972 May;223(1):131–150. doi: 10.1113/jphysiol.1972.sp009838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapf J. L., Nunn B. J., Meister M., Baylor D. A. Visual transduction in cones of the monkey Macaca fascicularis. J Physiol. 1990 Aug;427:681–713. doi: 10.1113/jphysiol.1990.sp018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe L. T., Fach C. C., Stockman A. The field adaptation of the human rod visual system. J Physiol. 1992 Jan;445:319–343. doi: 10.1113/jphysiol.1992.sp018926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe L. T., Fach C., Nordby K., Stockman A. The incremental threshold of the rod visual system and Weber's law. Science. 1989 Apr 21;244(4902):354–356. doi: 10.1126/science.2711186. [DOI] [PubMed] [Google Scholar]
- Sharpe L. T., Stockman A., MacLeod D. I. Rod flicker perception: scotopic duality, phase lags and destructive interference. Vision Res. 1989;29(11):1539–1559. doi: 10.1016/0042-6989(89)90137-5. [DOI] [PubMed] [Google Scholar]
- Sharpe L. T., Whittle P., Nordby K. Spatial integration and sensitivity changes in the human rod visual system. J Physiol. 1993 Feb;461:235–246. doi: 10.1113/jphysiol.1993.sp019511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman A., Sharpe L. T., Zrenner E., Nordby K. Slow and fast pathways in the human rod visual system: electrophysiology and psychophysics. J Opt Soc Am A. 1991 Oct;8(10):1657–1665. doi: 10.1364/josaa.8.001657. [DOI] [PubMed] [Google Scholar]
- VAN DEN BRINK G., BOUMAN M. A. Variation of integrative actions in the retinal system; an adaptational phenomenon. J Opt Soc Am. 1954 Aug;44(8):616–620. doi: 10.1364/josa.44.000616. [DOI] [PubMed] [Google Scholar]


