Abstract
1. Tight-seal, whole-cell voltage clamp recording techniques were used to characterize monosynaptically evoked GABAB currents in adult rat brain slices maintained at 34-35 degrees C. Responses were recorded from granule cells of the dentate gyrus following the blockade of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX)-, D-2-amino-5-phosphonovaleric acid (D-AP5)- and picrotoxin-sensitive fast synaptic transmission, so that the remaining synaptic currents could be studied in isolation. 2. Under these conditions, stimulation in the molecular layer elicited a slow outward current which was blocked by the selective GABAB antagonist CGP 35348 in a concentration-dependent manner (200-800 microM). This current was absent in recordings made with pipettes containing 10-15 mM of the lidocaine derivative QX-314 or when caesium was substituted for K+. 3. Increasing the [K+]o e-fold (from 2.5 to 6.8 mM) shifted the reversal potential of the GABAB current from -97.9 to -73.2 mV, as predicted by the Nernst equation. Peak conductance was constant, but in 6.8 mM [K+]o at voltages hyperpolarized to EK (equilibrium potential for potassium), a small outward rectification was evident. 4. The time course of the current could be described by fourth-power exponential activation kinetics with double exponential inactivation. At 34-35 degrees C, the average activation time constant (tau m) was 45.2 ms, while the two inactivation time constants (tau h1 and tau h2) were 110.2 and 516.2 ms, with corresponding weighting factors (wh1 and wh2) of 0.84 and 0.16, respectively. The Q10 (temperature coefficient) values for these time constants were between 1.82 and 2.31. Neither tau m, nor tau h1 and tau h2 were voltage dependent in the range from -45 to -95 mV. 5. Paired-pulse depression of the GABAB current was studied by giving identical conditioning and test stimuli over a wide range (50-5000 ms) of interstimulus intervals (ISIs). The maximal depression (48%) occurred at 200 ms ISI, and the depression lasted for over 5 s. The magnitude of paired-pulse depression was not dependent on the postsynaptic membrane potential. 6. Application of the competitive antagonist CGP 35348 such that the peak current was diminished by approximately 50% had no effect on the activation or inactivation kinetics of the current. Similarly, during paired-pulse depression the kinetics of test currents were identical to those of conditioning currents. These findings support the hypothesis that the mechanism responsible for paired-pulse depression involves a reduction in neurotransmitter release without postsynaptic alterations in K+ channel activation/inactivation kinetics.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
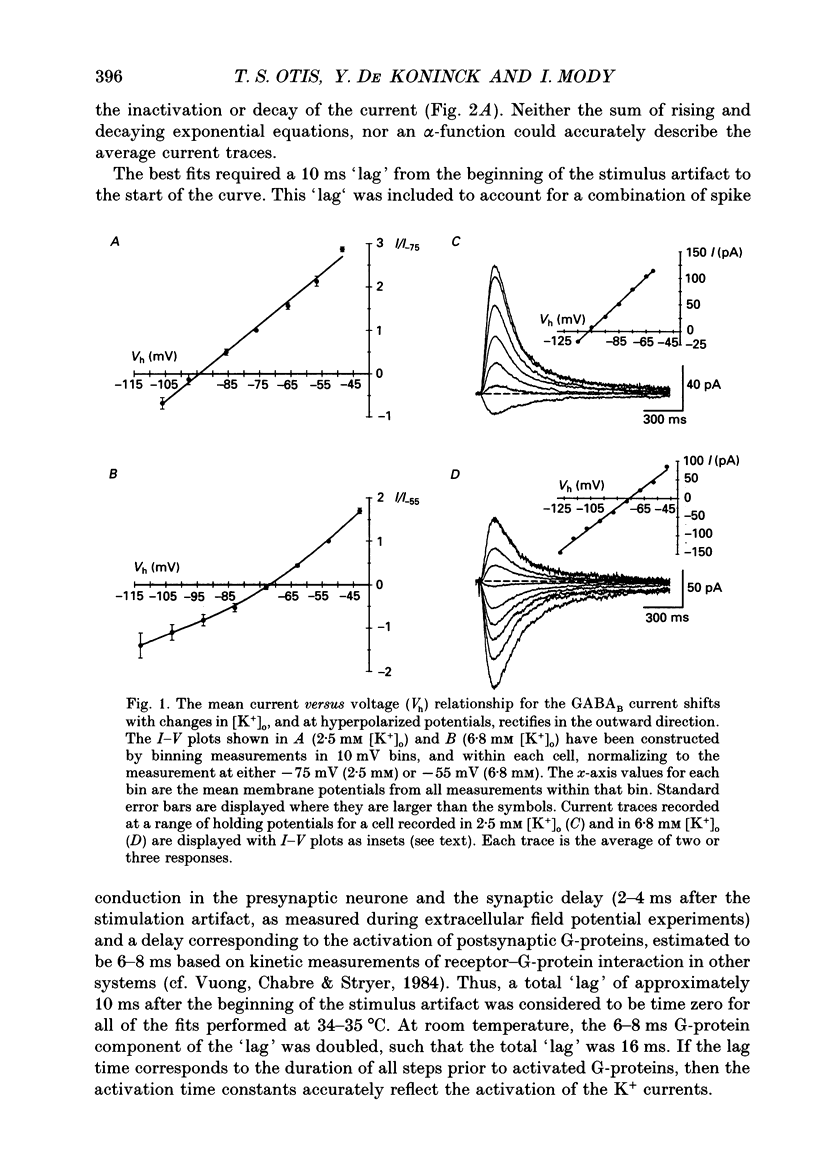
PDF




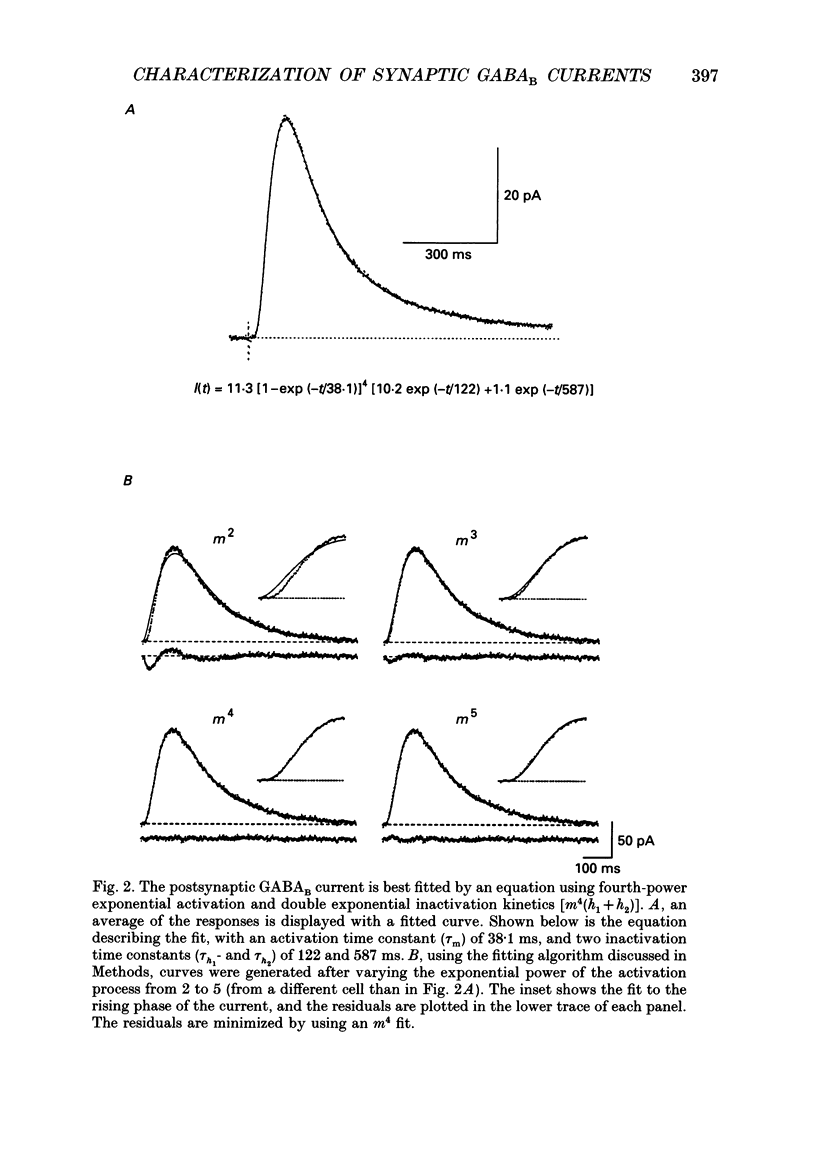
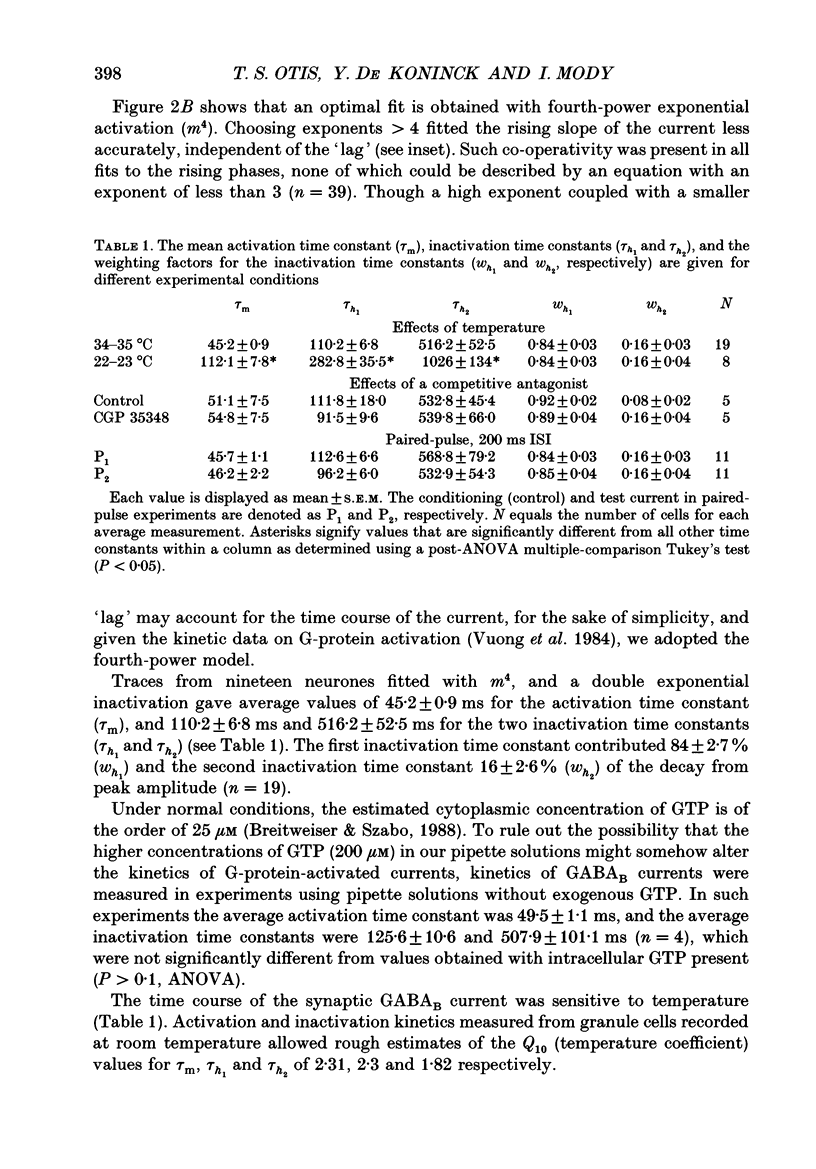
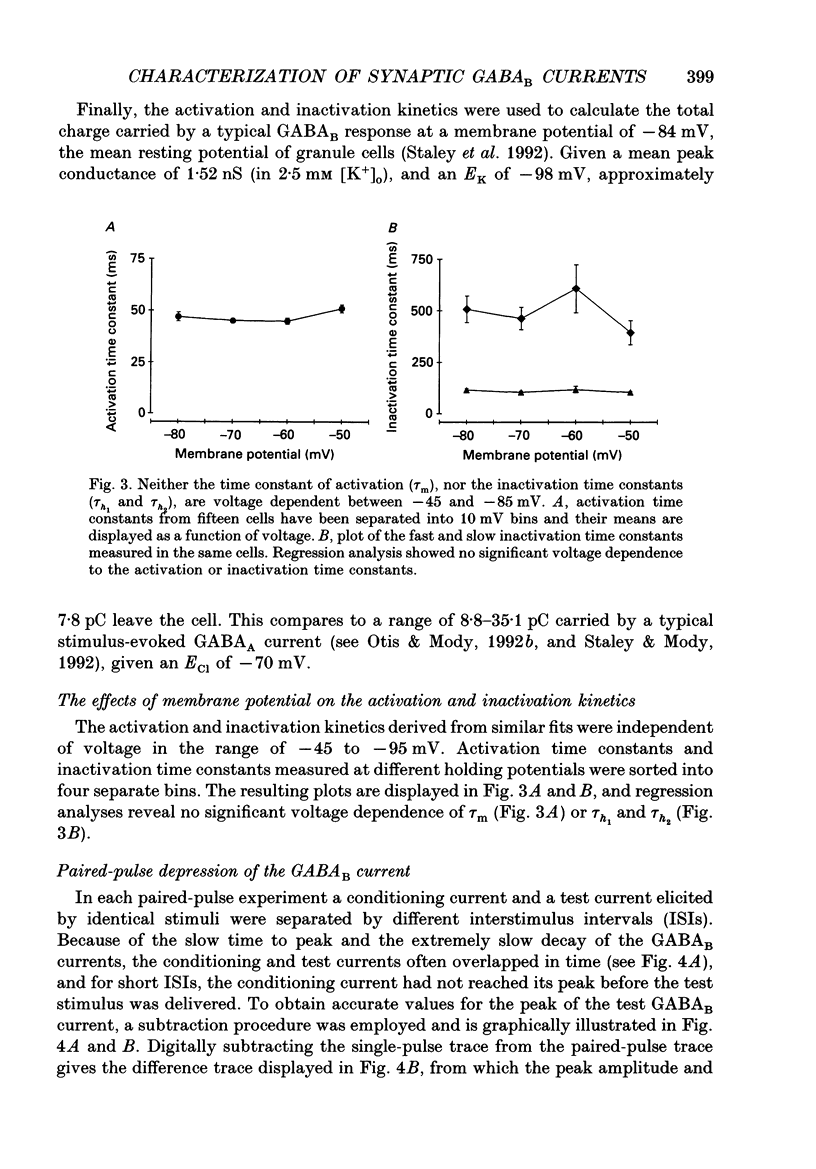





Selected References
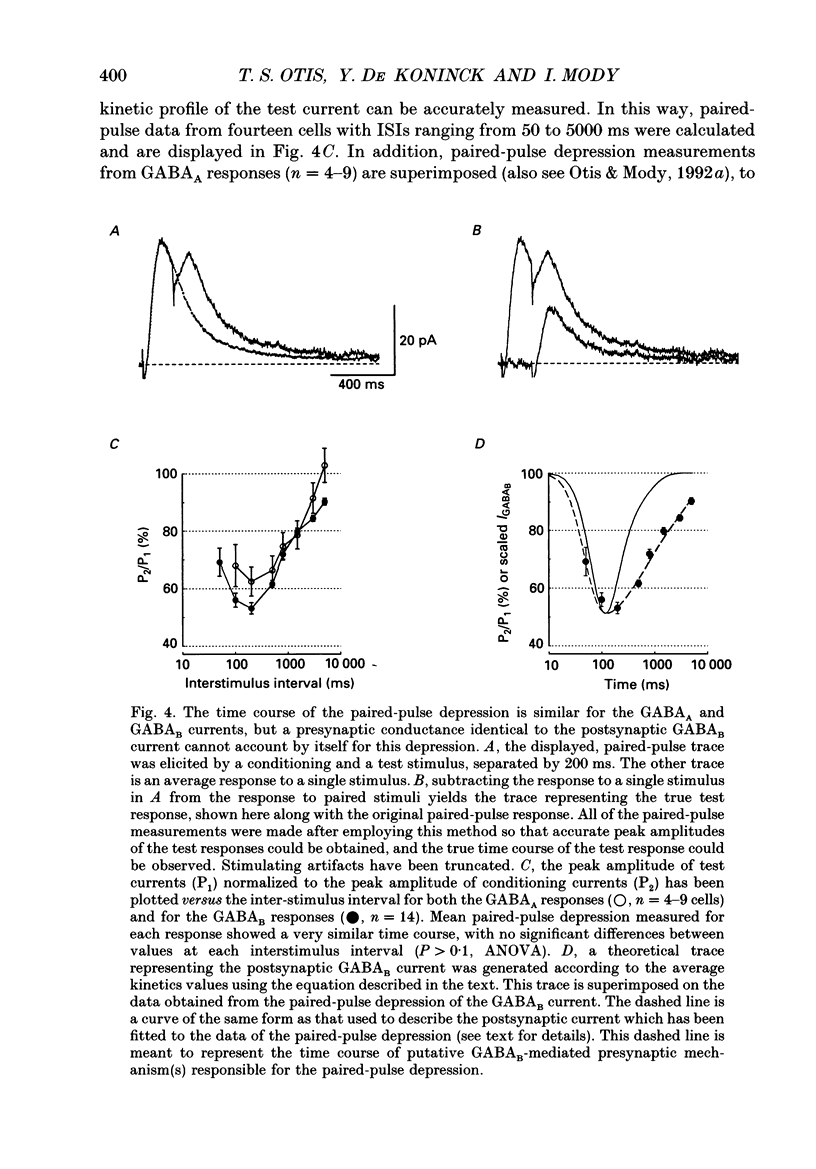
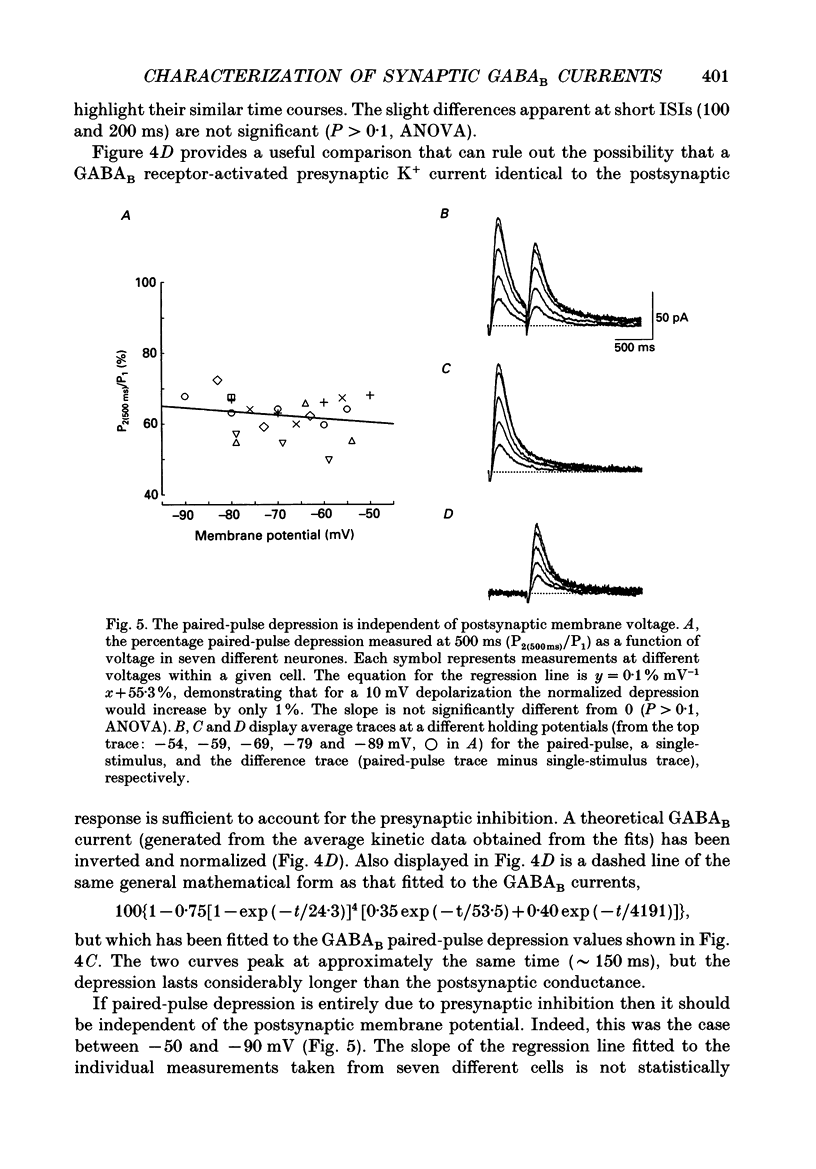
These references are in PubMed. This may not be the complete list of references from this article.
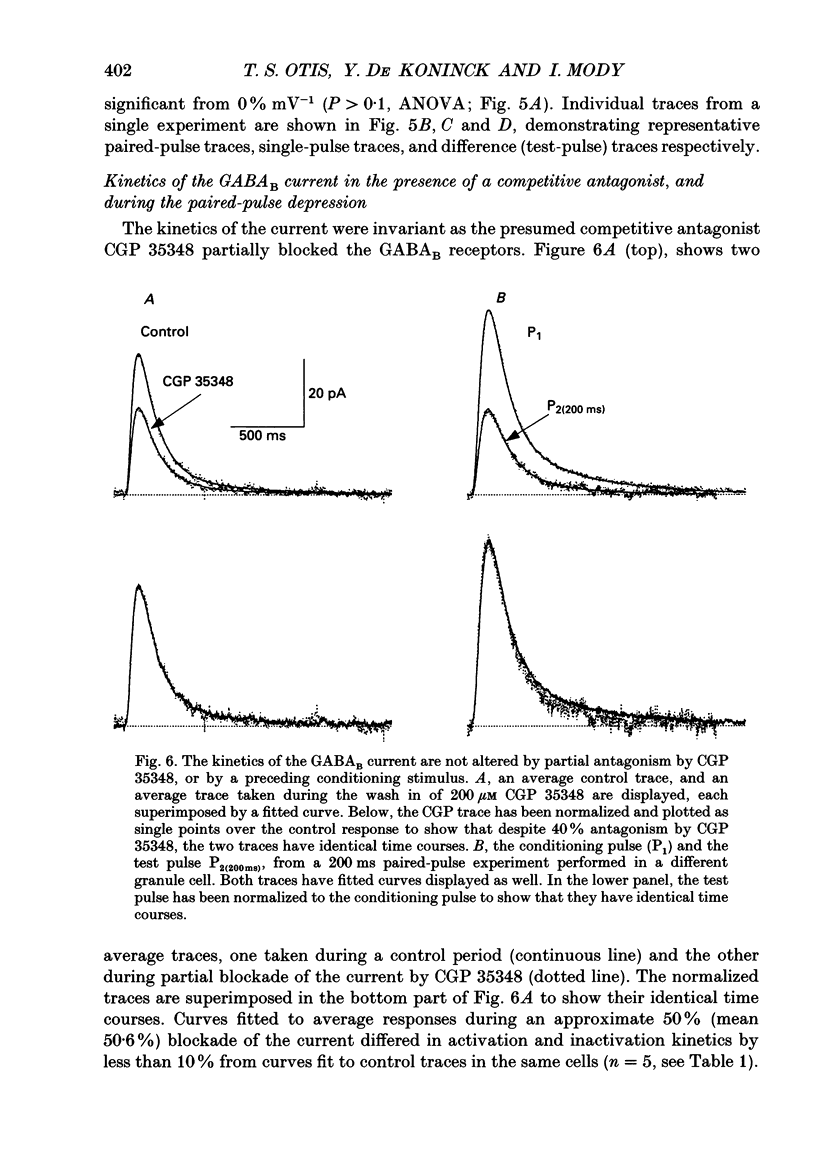
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends Pharmacol Sci. 1989 Oct;10(10):401–407. doi: 10.1016/0165-6147(89)90188-0. [DOI] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Mechanism of muscarinic receptor-induced K+ channel activation as revealed by hydrolysis-resistant GTP analogues. J Gen Physiol. 1988 Apr;91(4):469–493. doi: 10.1085/jgp.91.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezina V. Guanosine 5'-triphosphate analogue activates potassium current modulated by neurotransmitters in Aplysia neurones. J Physiol. 1988 Dec;407:15–40. doi: 10.1113/jphysiol.1988.sp017401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Leresche N. A role for GABAB receptors in excitation and inhibition of thalamocortical cells. Trends Neurosci. 1991 Jan;14(1):16–21. doi: 10.1016/0166-2236(91)90178-w. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz R. A., Prince D. A. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989 May;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988 Sep;1(7):585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Gage P. W. Activation and modulation of neuronal K+ channels by GABA. Trends Neurosci. 1992 Feb;15(2):46–51. doi: 10.1016/0166-2236(92)90025-4. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N. L., Lange G. D., Barker J. L. (-)-Baclofen activates presynaptic GABAB receptors on GABAergic inhibitory neurons from embryonic rat hippocampus. Neurosci Lett. 1988 Feb 15;85(1):105–109. doi: 10.1016/0304-3940(88)90437-5. [DOI] [PubMed] [Google Scholar]
- Harrison N. L. On the presynaptic action of baclofen at inhibitory synapses between cultured rat hippocampal neurones. J Physiol. 1990 Mar;422:433–446. doi: 10.1113/jphysiol.1990.sp017993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolimek W., Misgeld U. On the inhibitory actions of baclofen and gamma-aminobutyric acid in rat ventral midbrain culture. J Physiol. 1992;451:419–443. doi: 10.1113/jphysiol.1992.sp019171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille J. C. Postsynaptic potentials mediated by excitatory and inhibitory amino acids in interneurons of stratum pyramidale of the CA1 region of rat hippocampal slices in vitro. J Neurophysiol. 1991 Nov;66(5):1441–1454. doi: 10.1152/jn.1991.66.5.1441. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991 Mar 21;350(6315):232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Müller W., Brunner H. Effects of (-)baclofen on inhibitory neurons in the guinea pig hippocampal slice. Pflugers Arch. 1989 Jun;414(2):139–144. doi: 10.1007/BF00580955. [DOI] [PubMed] [Google Scholar]
- Miyake M., Christie M. J., North R. A. Single potassium channels opened by opioids in rat locus ceruleus neurons. Proc Natl Acad Sci U S A. 1989 May;86(9):3419–3422. doi: 10.1073/pnas.86.9.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan T., Jensen M. S., Lambert J. D. The slow inhibitory postsynaptic potential in rat hippocampal CA1 neurones is blocked by intracellular injection of QX-314. Neurosci Lett. 1990 Mar 14;110(3):309–313. doi: 10.1016/0304-3940(90)90865-7. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Olpe H. R., Karlsson G., Pozza M. F., Brugger F., Steinmann M., Van Riezen H., Fagg G., Hall R. G., Froestl W., Bittiger H. CGP 35348: a centrally active blocker of GABAB receptors. Eur J Pharmacol. 1990 Oct 2;187(1):27–38. doi: 10.1016/0014-2999(90)90337-6. [DOI] [PubMed] [Google Scholar]
- Otis T. S., Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992 Jul;49(1):13–32. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- Otis T. S., Staley K. J., Mody I. Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 1991 Apr 5;545(1-2):142–150. doi: 10.1016/0006-8993(91)91280-e. [DOI] [PubMed] [Google Scholar]
- Premkumar L. S., Chung S. H., Gage P. W. GABA-induced potassium channels in cultured neurons. Proc Biol Sci. 1990 Aug 22;241(1301):153–158. doi: 10.1098/rspb.1990.0079. [DOI] [PubMed] [Google Scholar]
- Scholz K. P., Miller R. J. GABAB receptor-mediated inhibition of Ca2+ currents and synaptic transmission in cultured rat hippocampal neurones. J Physiol. 1991 Dec;444:669–686. doi: 10.1113/jphysiol.1991.sp018900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. A subset of local interneurons generate slow inhibitory postsynaptic potentials in hippocampal neurons. Brain Res. 1990 Mar 12;511(1):163–164. doi: 10.1016/0006-8993(90)90236-5. [DOI] [PubMed] [Google Scholar]
- Staley K. J., Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol. 1992 Jul;68(1):197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- Staley K. J., Otis T. S., Mody I. Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole-cell recordings. J Neurophysiol. 1992 May;67(5):1346–1358. doi: 10.1152/jn.1992.67.5.1346. [DOI] [PubMed] [Google Scholar]
- Thalmann R. H. Evidence that guanosine triphosphate (GTP)-binding proteins control a synaptic response in brain: effect of pertussis toxin and GTP gamma S on the late inhibitory postsynaptic potential of hippocampal CA3 neurons. J Neurosci. 1988 Dec;8(12):4589–4602. doi: 10.1523/JNEUROSCI.08-12-04589.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Comparison of the actions of baclofen at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDongen A. M., Codina J., Olate J., Mattera R., Joho R., Birnbaumer L., Brown A. M. Newly identified brain potassium channels gated by the guanine nucleotide binding protein Go. Science. 1988 Dec 9;242(4884):1433–1437. doi: 10.1126/science.3144040. [DOI] [PubMed] [Google Scholar]
- Vuong T. M., Chabre M., Stryer L. Millisecond activation of transducin in the cyclic nucleotide cascade of vision. Nature. 1984 Oct 18;311(5987):659–661. doi: 10.1038/311659a0. [DOI] [PubMed] [Google Scholar]
- Wilden U., Hall S. W., Kühn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Mattera R., Codina J., Graf R., Okabe K., Padrell E., Iyengar R., Brown A. M., Birnbaumer L. The G protein-gated atrial K+ channel is stimulated by three distinct Gi alpha-subunits. Nature. 1988 Dec 15;336(6200):680–682. doi: 10.1038/336680a0. [DOI] [PubMed] [Google Scholar]


