Abstract
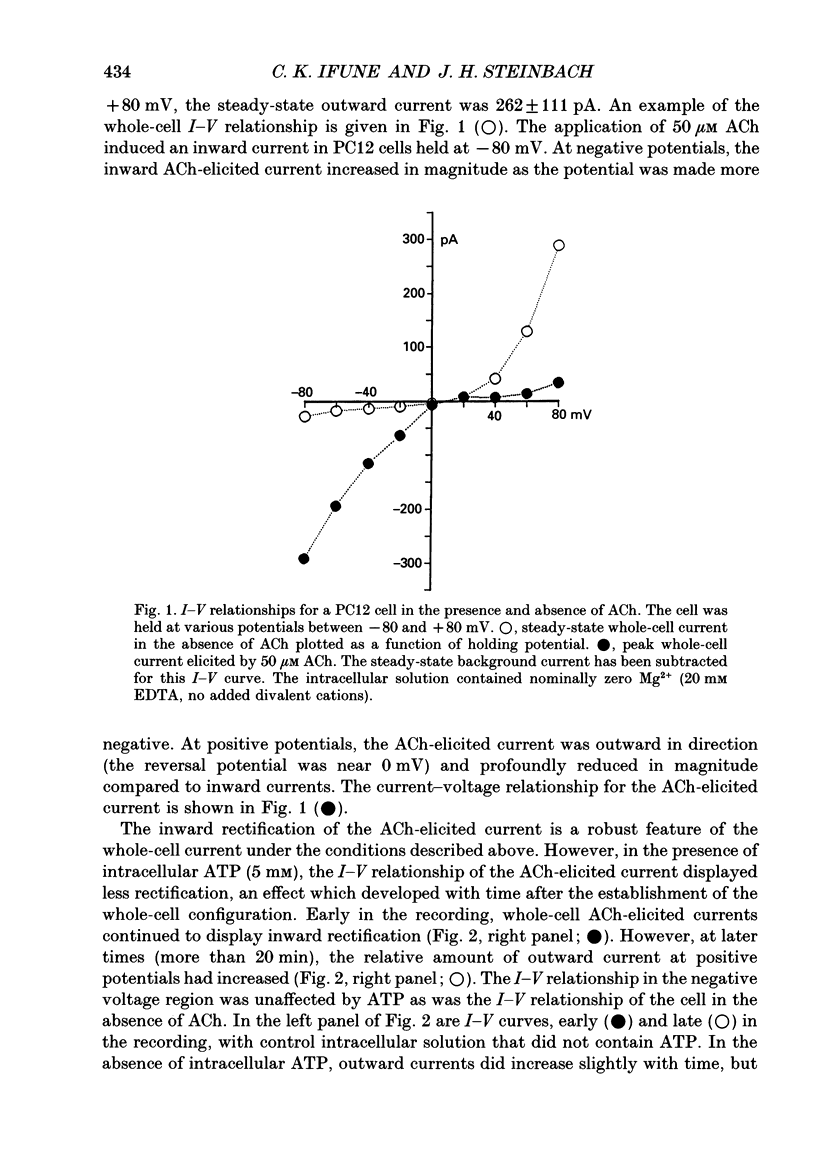
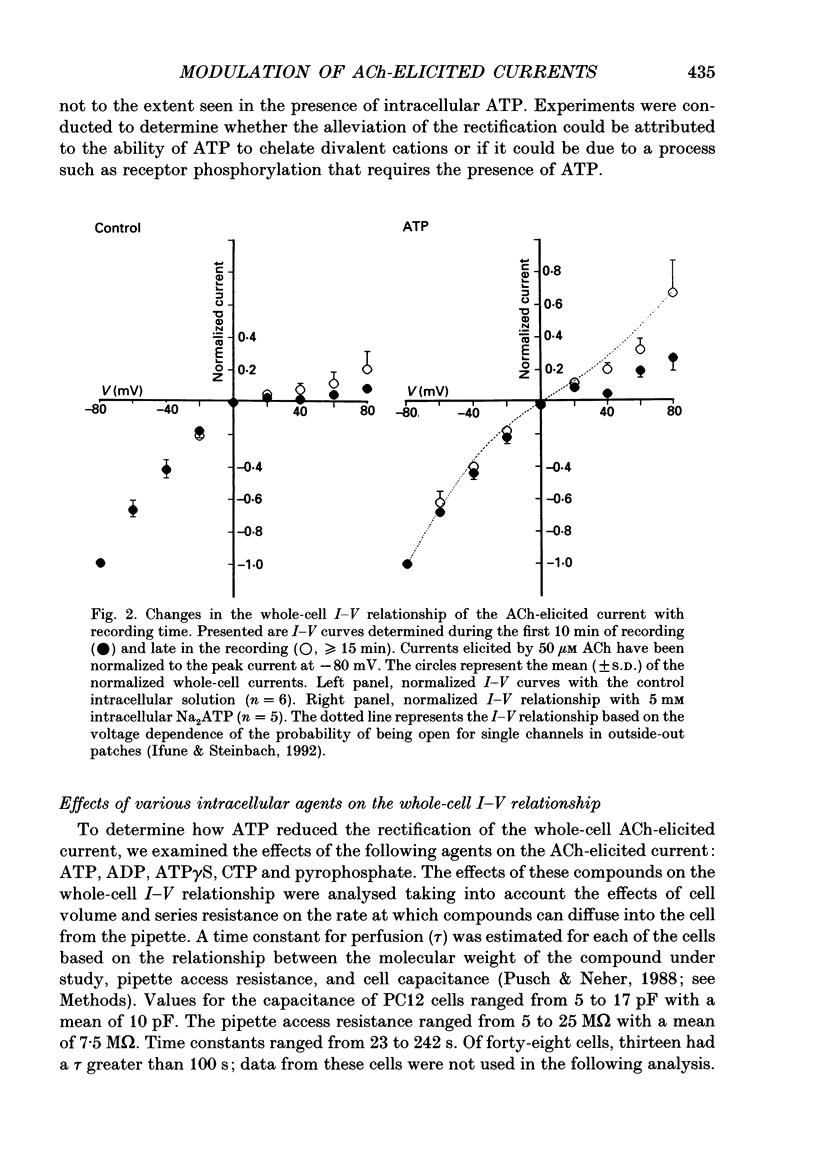
1. Whole-cell voltage clamp techniques were used to examine acetylcholine (ACh)-elicited currents in differentiated cells of the rat phaeochromocytoma cell line, PC12. 2. In the absence of intracellular Mg2+, the whole-cell current-voltage relationship for the ACh-elicited current displayed inward rectification which was reduced in part by the presence of 5 mM internal adenosine 5'-triphosphate (ATP). 3. The reduction in the rectification attributed to ATP developed over the first 15-20 min of whole-cell recording. Similar results were obtained with a non-hydrolysable ATP analogue, adenosine-5'-O-3-thiotriphosphate (ATP gamma S), or cytosine 5'-triphosphate (CTP) in the internal solution, but not with adenosine 5'-diphosphate (ADP) or pyrophosphate. 4. The magnitude of the ACh-elicited current was also dependent on recording time and the composition of the internal pipette solution. The magnitude of the peak ACh-elicited current increased over time when the cell was internally perfused with the control solution or a pipette solution containing pyrophosphate, ATP gamma S, or ADP. The largest sustained increases in ACh-elicited current were observed in the presence of internal pyrophosphate or ATP gamma S. In contrast, with internal ATP or CTP, the whole-cell current initially increased, then steadily decreased with recording time. 5. The desensitization rate of the ACh-elicited current increased with recording time irrespective of the composition of the intracellular solution. 6. The actions of the compounds tested make it likely that the changes in the whole-cell current-voltage relationship, peak current, and desensitization are produced by separate mechanisms. The mechanisms underlying these changes are unknown, but the ability of the compounds to chelate divalent cations is unlikely to be the explanation. Other unlikely explanations include phosphorylation of the ACh receptor or regulation by GTP-binding proteins.
Full text
PDF


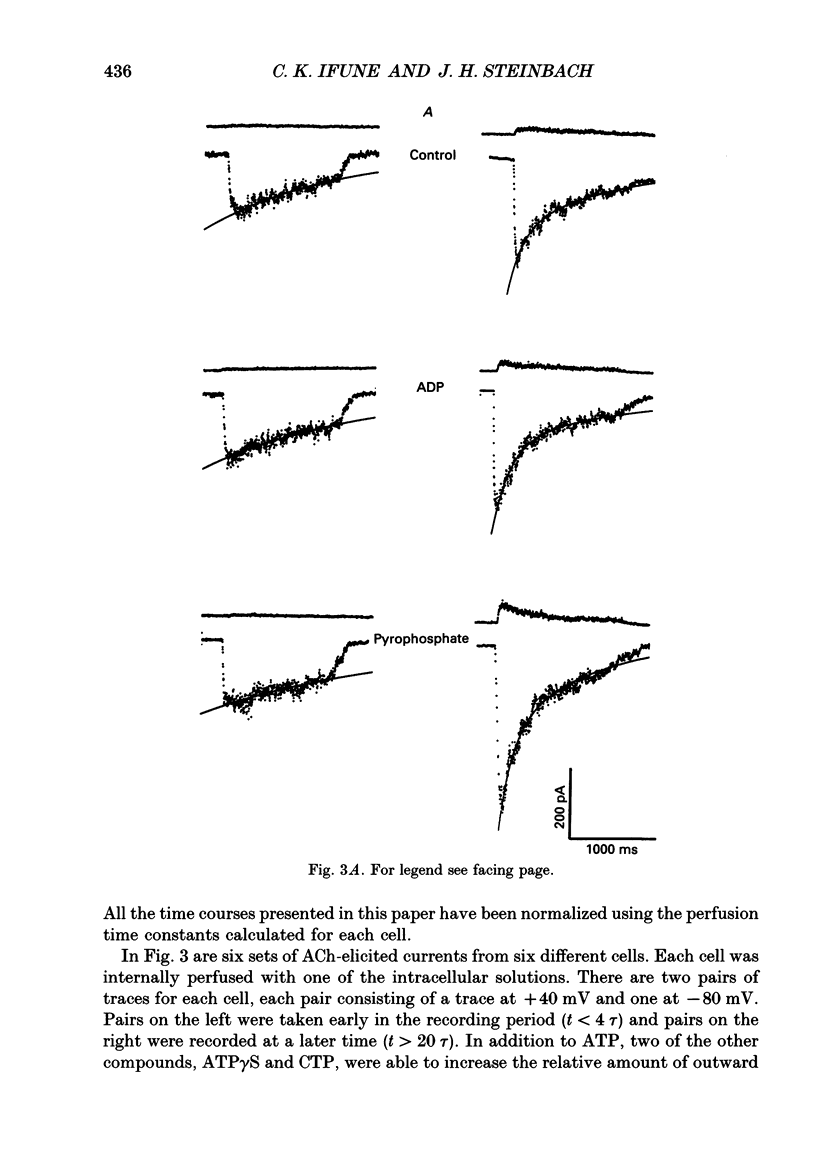
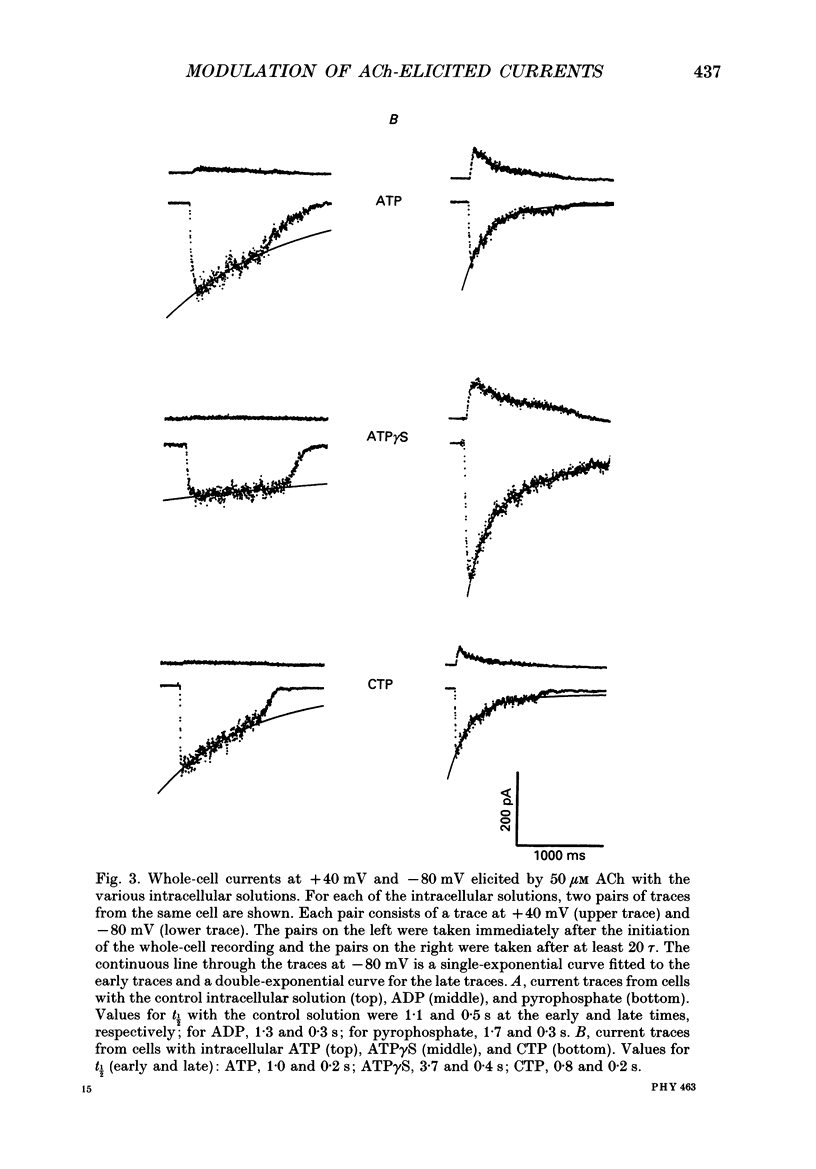
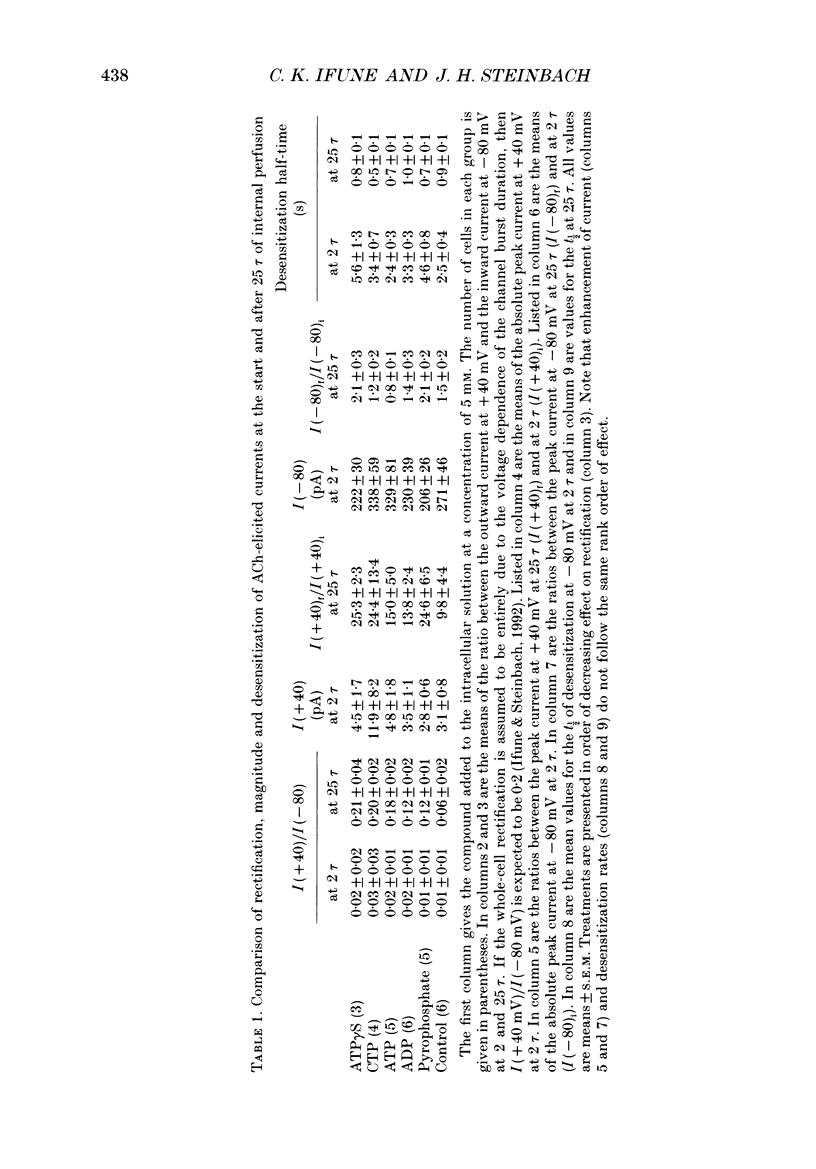
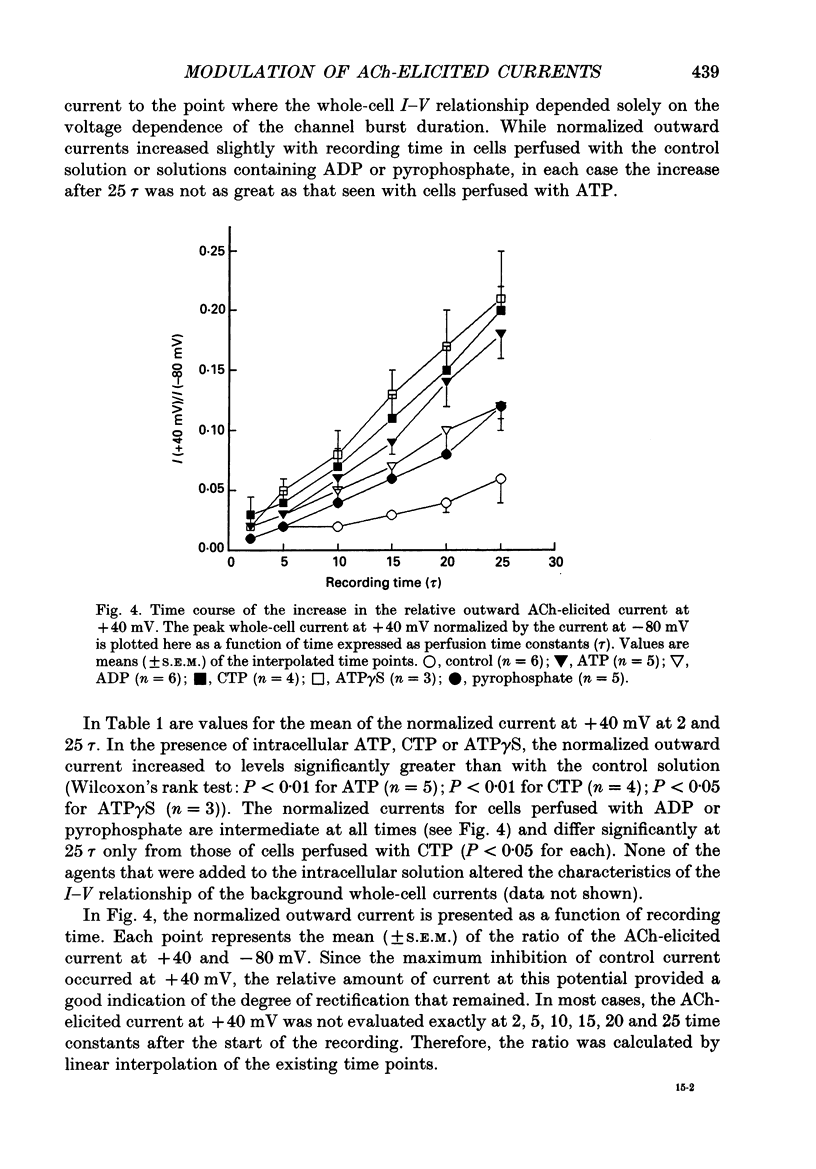
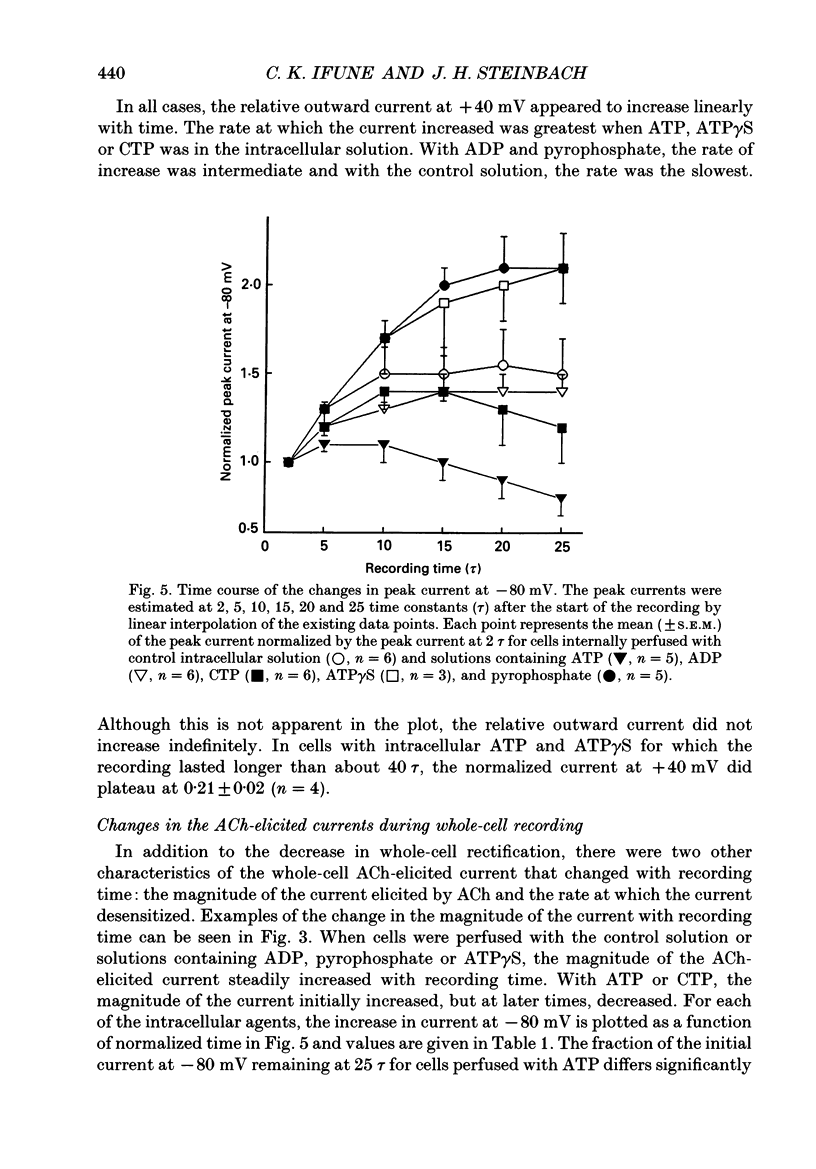






Selected References
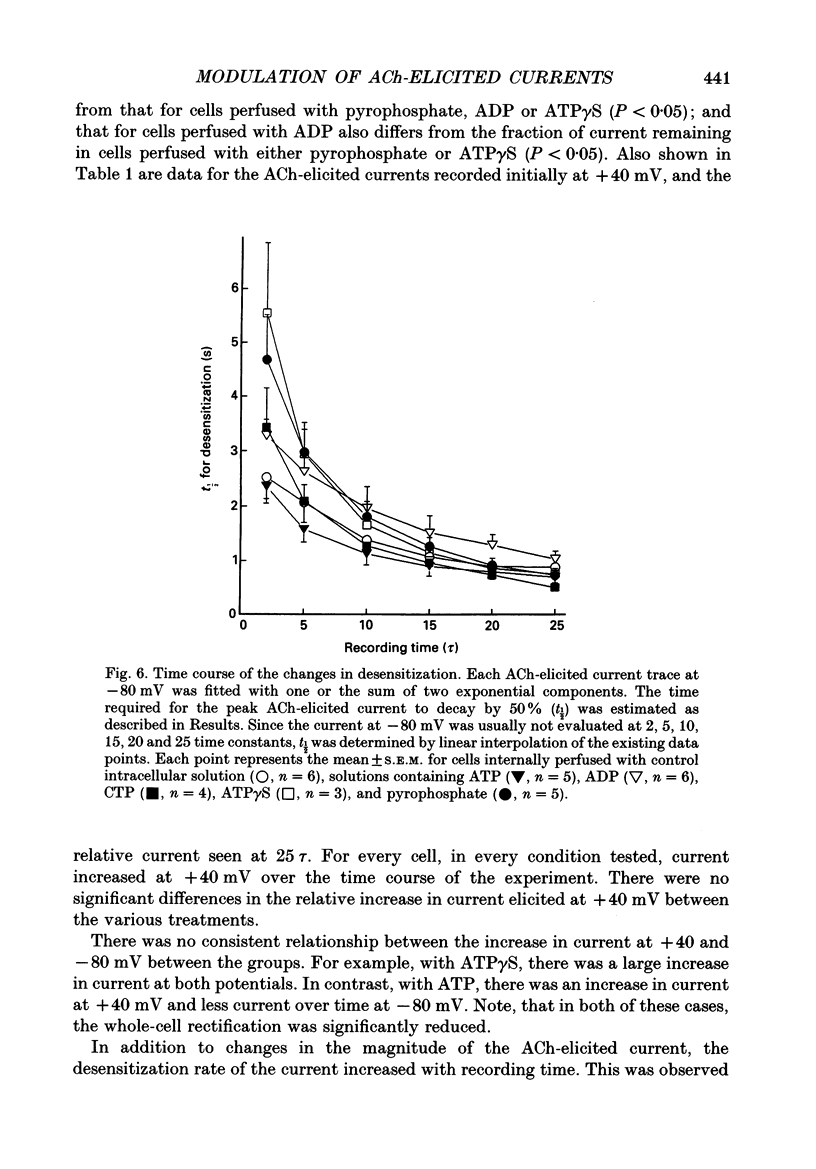
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Pedersen S. E., Scott C. W., Ross E. M. Reconstitution of catecholamine-stimulated binding of guanosine 5'-O-(3-thiotriphosphate) to the stimulatory GTP-binding protein of adenylate cyclase. Biochemistry. 1984 Nov 6;23(23):5460–5467. doi: 10.1021/bi00318a013. [DOI] [PubMed] [Google Scholar]
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V. A., Selyanko A. A., Skok V. I. Acetylcholine-induced current fluctuations and fast excitatory post-synaptic currents in rabbit sympathetic neurones. J Physiol. 1983 Mar;336:511–526. doi: 10.1113/jphysiol.1983.sp014595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieber L. A., Adams D. J. Acetylcholine-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991 Mar;434:215–237. doi: 10.1113/jphysiol.1991.sp018466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hirano T., Kidokoro Y., Ohmori H. Acetylcholine dose-response relation and the effect of cesium ions in the rat adrenal chromaffin cell under voltage clamp. Pflugers Arch. 1987 Apr;408(4):401–407. doi: 10.1007/BF00581136. [DOI] [PubMed] [Google Scholar]
- Ifune C. K., Steinbach J. H. Inward rectification of acetylcholine-elicited currents in rat phaeochromocytoma cells. J Physiol. 1992 Nov;457:143–165. doi: 10.1113/jphysiol.1992.sp019369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifune C. K., Steinbach J. H. Rectification of acetylcholine-elicited currents in PC12 pheochromocytoma cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4794–4798. doi: 10.1073/pnas.87.12.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifune C. K., Steinbach J. H. Regulation of sodium currents and acetylcholine responses in PC12 cells. Brain Res. 1990 Jan 8;506(2):243–248. doi: 10.1016/0006-8993(90)91257-h. [DOI] [PubMed] [Google Scholar]
- Ifune C. K., Steinbach J. H. Voltage-dependent block by magnesium of neuronal nicotinic acetylcholine receptor channels in rat phaeochromocytoma cells. J Physiol. 1991 Nov;443:683–701. doi: 10.1113/jphysiol.1991.sp018858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Kuriyama H. Properties of the nicotinic-receptor-activated current in adrenal chromaffin cells of the guinea-pig. Pflugers Arch. 1991 Aug;419(1):13–20. doi: 10.1007/BF00373741. [DOI] [PubMed] [Google Scholar]
- Margiotta J. F., Berg D. K., Dionne V. E. Cyclic AMP regulates the proportion of functional acetylcholine receptors on chicken ciliary ganglion neurons. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8155–8159. doi: 10.1073/pnas.84.22.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A., Colquhoun D., Cull-Candy S. G. Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. J Physiol. 1990 Aug;427:625–655. doi: 10.1113/jphysiol.1990.sp018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A., Cull-Candy S. G., Colquhoun D. Conductance and kinetic properties of single nicotinic acetylcholine receptor channels in rat sympathetic neurones. J Physiol. 1991 Aug;439:717–750. doi: 10.1113/jphysiol.1991.sp018690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A., Cull-Candy S. G., Colquhoun D. Single-channel and whole-cell currents evoked by acetylcholine in dissociated sympathetic neurons of the rat. Proc R Soc Lond B Biol Sci. 1987 Nov 23;232(1267):239–248. doi: 10.1098/rspb.1987.0072. [DOI] [PubMed] [Google Scholar]
- Mulle C., Changeux J. P. A novel type of nicotinic receptor in the rat central nervous system characterized by patch-clamp techniques. J Neurosci. 1990 Jan;10(1):169–175. doi: 10.1523/JNEUROSCI.10-01-00169.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus R., Cachelin A. B. Changes in the conductance of the neuronal nicotinic acetylcholine receptor channel induced by magnesium. Proc Biol Sci. 1990 Aug 22;241(1301):78–84. doi: 10.1098/rspb.1990.0069. [DOI] [PubMed] [Google Scholar]
- Otero A. S., Breitwieser G. E., Szabo G. Activation of muscarinic potassium currents by ATP gamma S in atrial cells. Science. 1988 Oct 21;242(4877):443–445. doi: 10.1126/science.3051383. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Sands S. B., Barish M. E. Neuronal nicotinic acetylcholine receptor currents in phaeochromocytoma (PC12) cells: dual mechanisms of rectification. J Physiol. 1992 Feb;447:467–487. doi: 10.1113/jphysiol.1992.sp019012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann A., Marty A. Activation of Ca-dependent K channels by carbamoylcholine in rat lacrimal glands. Proc Natl Acad Sci U S A. 1984 Jan;81(2):611–615. doi: 10.1073/pnas.81.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel J. L., Shao X. M., Jackson M. B. Activation and desensitization of the 5-HT3 receptor in a rat glioma x mouse neuroblastoma hybrid cell. J Physiol. 1991 May;436:293–308. doi: 10.1113/jphysiol.1991.sp018551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel J. L., Trussell L. O., Jackson M. B. Three serotonin responses in cultured mouse hippocampal and striatal neurons. J Neurosci. 1988 Apr;8(4):1273–1285. doi: 10.1523/JNEUROSCI.08-04-01273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H. Rectification of synaptic and acetylcholine currents in the mouse submandibular ganglion cells. J Physiol. 1989 Oct;417:307–322. doi: 10.1113/jphysiol.1989.sp017803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. W., Feltz P. Nicotinic acetylcholine receptors in porcine hypophyseal intermediate lobe cells. J Physiol. 1990 Mar;422:83–101. doi: 10.1113/jphysiol.1990.sp017974. [DOI] [PMC free article] [PubMed] [Google Scholar]


