ABSTRACT
Endoplasmic reticulum (ER) is a crucial organelle associated with cellular homeostasis. Accumulation of improperly folded proteins results in ER stress, accompanied by the reaction involving triggering unfolded protein response (UPR). The UPR is mediated through ER membrane-associated sensors, such as protein kinase-like ER kinase (PERK), inositol-requiring transmembrane kinase/endoribonuclease 1α, and activating transcription factor 6 (ATF6). Prolonged stress triggers cell apoptotic reaction, resulting in cell death. Neuronal cells are especially susceptible to protein misfolding. Notably, ER and UPR malfunctions are linked to many neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), delineated by accumulation of misfolded proteins. Notably, ATF family members play key roles in AD and PD pathogenesis. However, the connection between ER stress, UPR, and neuropathology is not yet fully understood. Here, we discuss our present knowledge of the association between ER stress, the UPR, and neurodegeneration in AD and PD. We also discuss the roles of ATF family members in AD and PD pathogenesis. Moreover, we provide a mechanistic clarification of how disease-related molecules affect ER protein homeostasis and explore recent findings that connect the UPR to neuronal plasticity.
KEYWORDS: Activating transcription factor family, Alzheimer’s disease, Endoplasmic reticulum stress, Parkinson’s disease
INTRODUCTION
Alzheimer’s disease (AD) and Parkinson’s disease (PD) are described by advanced detriment of function in the nervous system, terminating in serious impairment. Although each disease has specific neuropathophysiology, they have a similar pathologic characteristic: misfolded protein aggregates [1,2]. Since the progression of pathology in AD and PD is related to a specific misfolded protein aggregate, these diseases are frequently characterized as protein misfolding disorders [3].
Under the physiological state, chaperones in the cell provide the accurate protein folding and recognize improperly folded proteins and promote protein degradation through lysosome or autophagy pathways [4]. The protein homeostasis [5] is essential for the preservation of cell function since it inhibits improperly folded protein aggregates. In protein misfolding disorders, improperly folded protein aggregates complicate the maintenance of cellular protein homeostasis [6] and leads to endoplasmic reticulum (ER) stress [7]. ER stress triggers a fast and integrated biochemical reaction, termed as the unfolded protein response (UPR). Growing evidence indicates that ER stress and UPR play important pathophysiological role in AD and PD; nevertheless, the molecular mechanism of ER and UPR involved in pathology of AD and PD is still unknown.
ENDOPLASMIC RETICULUM STRESS AND UNFOLDED PROTEIN RESPONSE
The ER controls numerous important cellular processes. Notably, it modulates the protein synthesis and is the major Ca2+ storage organelle that supplies Ca2+ for intracellular signaling. ER homeostasis is primarily regulated by the UPR, a complicated signaling system that modulates translation and transcription in response to demand and enhances the ER’s protein-folding ability [8]. Various conditions – such as reduced calcium in the ER lumen and mutations in proteins that are trafficked through the secretory pathway – can lead to ER dysfunction and ER stress, thereby triggering the UPR [9]. Activation of the UPR in cells can prompt three types of actions: initial adaptation, alarm signaling, and cell apoptosis [10,11]. Under stress, UPR modulates cellular adaption by increasing the ER’s protein-folding ability and concurrently decreasing the synthetic load [12].
UPR is regulated by sensor proteins, such as RNA-activated protein kinase-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring transmembrane kinase/endoribonuclease 1α (IRE1α). In the physiological state, these proteins bind with immunoglobulin heavy-chain binding protein (BiP). Under ER stress, these sensor proteins release BiP, enabling PERK and IRE1α dimerization and autophosphorylation, respectively, and modulated ATF6 proteolysis. These actions lead to the induction of UPR. PERK activation triggers the phosphorylation of eIF2α, and phosphorylated eIF2α decreases global protein synthesis and induces ATF4 translation, which increases the expression of apoptosis-related genes. The endoribonuclease activity of IRE1α enables the splicing of XBP1u protein to the spliced XBP1 (XBP1s). XBP1s increase transcription of numerous genes related to ER-associated degradation and UPR. In unstressed cells, ATF6 was located in ER. Under ER stress, it translocates to the Golgi, where it is cleaved consecutively by the enzymes S1P and S2P. Active ATF6 translocates into nucleus, then it binds promoters of various UPR-related genes, including GADD34, CHOP, BiP, and XBP1 [1,13,14] [Figure 1].
Figure 1.
Diagrammatic illustration of the sensor proteins of unfolded protein response (UPR). The principle UPR pathways after binding protein dissociated from sensor proteins when Endoplasmic reticulum (ER) stress happens: (a) protein kinase-like ER kinase (PERK)/eIF2α/ATF4 pathway: trans PERK auto-phosphorylation results in p-eIF2α, then reducing protein translation and upregulates ATF4 translation which increases the expression of gene related to apoptosis, including CHOP, (b) inositol-requiring transmembrane kinase/endoribonuclease 1α (IRE1α)/XBP1 pathway: IRE1α with RNase activity cuts out 26 intronic nucleotides of the XBP1 mRNA, leading to the XBP1s, which is accountable for inducing gene expression related to chaperones and ER-associated degradation, (c) activating transcription factor 6 (ATF6) pathway: Active ATF6 translocates to the nucleus and promotes the expression of genes related to chaperone, ER-associated degradation, and XBP-1
ENDOPLASMIC RETICULUM STRESS IN ALZHEIMER’S DISEASE
AD is a destructive degenerative condition affecting many people. It is described by a significant decrease in memory and cognitive tasks. The mechanisms leading to AD are complex and involve alterations in increased ER stress, calcium imbalance, synaptic transmission, and chronic neuroinflammation [15]. Neuropathological traits of AD include the plaques of amyloid-β (Aβ) peptides and accumulation of hyperphosphorylated tau [15,16]. While tau normally stabilizes neuronal microtubules, its phosphorylated form (p-tau) accumulates into neurofibrillary tangles [17]. Neurotoxic Aβ peptides result in neurodegeneration [18] [Table 1]. During the process of AD development, persistent aggregation of Aβ or p-tau leads to ER calcium dyshomeostasis, ER stress, and aberrant protein folding. Tau reportedly inhibits the ER-associated degradation pathway, resulting in improperly folded protein aggregates in the ER [19]. The neurotoxicity of Aβ peptides is associated with ER stress-modulated apoptosis through JNK activation [20].
Table 1.
Protein misfolding in Alzheimer’s disease and Parkinson’s disease
| Disease | Protein misfolding |
|---|---|
| AD | Deposits of intracellular tau aggregate to form neurofibrillary tangles |
| Extracellular aggregates of amyloid-β form amyloid plaques | |
| PD | Formation of protein inclusion bodies, known as Lewy bodies, that contain aggregated α-synuclein and ubiquitin |
| Accumulation of tau deposits |
AD: Alzheimer’s disease, PD: Parkinson’s disease
Various studies have reported dysregulated ER stress in the brains of AD patients. During moderate ER stress, UPR plays a protective role. However, prolonged ER stress triggers the proapoptotic pathway of the UPR, potentially leading to neurodegeneration. Increased levels of BiP and other chaperones, including Hsp72, Hsp73, and glucose-regulated protein 94 (Grp94), have been found in the cerebrospinal fluids and brains of AD patients [21,22]. In addition, AD brains show substantial upregulation of p-PERK and p-eIF2α, which can be induced by tau aggregates [13,23]. This phosphorylation is induced by tau accumulates [21]. Activation of PERK is linked to increased expression of ATF4 and BACE1 [24]. ATF4 is an important modulator of neuronal plasticity and spatial memory [25].
IRE1 activation in human brain tissue is positively associated with the progression of AD. IRE1 deleted the RNase domain in the nervous system, decreased Aβ oligomer content, and led to recovery of memory capacity and learning in mouse AD model [26]. In addition, XBP1 promoter polymorphism increases a risk factor for it [27]. XBP1 can decrease BACE1 expression through HMG-CoA reductase degradation 1, resulting in a reduction of Aβ plaques [28]. ATF6 reduces APP expression level, thereby inhibiting Aβ levels, decreasing the expression of BACE1 and promoter activity, and facilitating the spatial memory retention in mouse AD model [29].
In the brains of AD patients, CHOP, caspase-12 and GADD34, linking ER stress to apoptosis, is increased [30]. An increase in CHOP results in the production of reactive oxygen species (ROS), elevated levels of Aβ oligomers, and ultimately, cell death [31].
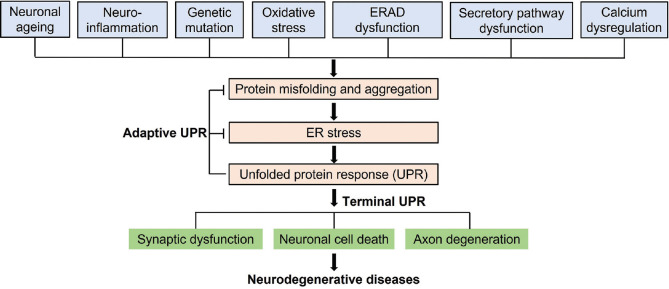
Sadleir et al. found that 5XFAD transgenic mice (with familial AD) showed increased expressions of APP and presenilin 1 (PS1; the most common cause of familial AD). These mice did not show UPR activation and did not exhibit increased expressions of sensor proteins, suggesting that the role of ER stress in AD is still controversial [32]. In summary, ER and UPR appear to be important factors in the development of AD [Figure 2].
Figure 2.
Endoplasmic reticulum (ER) stress and unfolded protein response (UPR) in neurodegeneration. Neuronal ageing, neuro-inflammation, genetic mutations, and other stimuli may trigger improperly folded protein accumulation and aggregation resulting in ER stress. In order to rescue ER stress, the response via UPR is triggered. However, long-lasting ER stress triggers apoptosis influencing neurons and synaptic function, thereby resulting in neurodegenerative
ENDOPLASMIC RETICULUM STRESS IN PARKINSON’S DISEASE
PD is a serious neurodegenerative disease portrayed by both motor and nonmotor symptoms, eventually resulting in immobility [33,34]. The pathogenic factors involved in PD remain largely unclear. Most cases of PD are sporadic with an unknown etiology, while only 10%–15% result from mutations in several genes, such as PRKN, SNCA/PARK1, and PINK1 [35].
PD is typically identified by two major features: the impairment of dopaminergic neurons in the substantia nigra and the aggregation of improperly folded alpha-synuclein (α-SYN) in neuronal somas (Lewy bodies) or within axons and dendrites (Lewy neurites) [36] [Table 1]. High α-SYN expression is detected in the presynaptic terminals of neurons but can also be found in blood and tissues [37,38]. SNCA gene mutations, including A53T, A30P, and E46K, are recognized as inherited causes of PD [39,40,41]. α-SYN is prone to accumulate, and their β-sheet form extends into insoluble fibrils [42].
Numerous studies indicate that ER stress acts a key role in α-SYN toxicity and modulates the death of dopaminergic neurons. α-SYN accumulation interacting with BiP results in UPR signaling pathway [14,43]. α-SYN is reportedly more plentiful in the ER/microsome fractions of brain tissue from mice and humans with PD, compared with non-PD controls [44]. In addition, ER stress markers, including p-PERK and p-eIF2α, have been detected in the dopaminergic neurons of PD patients [45].
α-synucleinopathy was positively correlated with the activation of ER chaperones and aberrant UPR in pathological neurons of A53T transgenic mouse model. This correlation is validated by the upregulated accumulation of polyubiquitin chains and caspase-12 triggering [44]. Notably, lack of glucose caused α-SYN-induced cell apoptosis in dopaminergic differentiated SH-SY5Y cells. α-SYN plays a role in stress detection; lack of glucose results in α-SYN overexpression, leading to interaction with BiP, and subsequent triggering of the PERK/ATF4/cAMP response element binding protein-2 (CREB-2) pathways [14].
Controversy remains regarding the IRE1-XBP1 pathway involved in ER stress in PD. In neurotoxin 6-hydroxydopamine-induced PD model animals, the active form of XBP1 is reportedly neuroprotective [46]. However, in a PD model fruit fly, IRE1 induce cell loss in photoreceptor neurons, in an XBP1-unrelated way [47]. Moreover, in yeast, α-SYN aggregate induces ER stress through suppressing ER-to-Golgi transport [48,49]. This trafficking damage is reportedly induced by RAB1 GTPase, or by ATF6 [48,50]. Notably, the coexpression of RAB1 with α-SYN has recovered the damage of dopaminergic neurons in animal models [48].
In addition, α-SYN suppresses ATF6 activation through coat COPII-modulated ER-Golgi trafficking, triggered on ER stress, resulting in induction of apoptosis [51]. In addition, ER stress triggered by α-SYN accumulation is through destabilized ER Ca2+ homeostasis. α-SYN accumulation stimulates ER calcium pump SERCA protein in neurons, resulting in changes of calcium metabolism and apoptosis [52]. Knockout mice of the CaBP-9k gene showed upregulation of α-SYN and activation of apoptosis in neurons. CaBP-9k knockout mice treated with ER stress inhibitor tauroursodeoxycholic acid restored ER stress markers and cleaved caspase-12 to regular levels [53]. In conclusion, UPR and ER stress-related pathways appear to be a novel target for PD treatment [Figure 2].
ACTIVATING TRANSCRIPTION FACTOR FAMILY IN ALZHEIMER’S DISEASE AND PARKINSON’S DISEASE
ATF family acts significant roles in the neuropathogenesis of AD and PD. ATFs contain a basic leucine zipper-like domain that enables the execution of critical transcriptional modulatory functions [54,55]. The ATF family includes ATF1-7 [56,57], and these transcription factors exhibit differential expression in human tissues [58]. Notably, ATF-2, -4, and -6 are highly expressed in the brain compared to other tissues, while ATF-1, ATF-3, ATF-5, and ATF-7 are expressed at lower levels in the brain relative to other tissues.
ATF-1 binds with CREB to exert beneficial effects on neurons [59] and modulate several stress responses [60]. ATF-2 is involved in DNA damage and apoptosis [61,62] and can regulate the inflammation in microglia cells, which is related to AD [63]. ATF-2 exhibits cytoplasmic localization in brain tissue from AD patients, suggesting that the pathogenesis of AD may involve altered subcellular localization of ATF-2 [64]. Kang et al. found that metformin activates ATF-2/CREB/PGC-1α pathway, resulting in neuroprotection. PGC-1α, CREB, and ATF-2 appear important for cell viability against mitochondrial stress, as SH-SY5Y cells with knockdown of these genes are susceptible to MPP+ toxicity [65]. In addition, in dopaminergic neuron-specific conditional ATF-2 mutant mice, MPTP-triggered neurodegeneration was significantly mitigated, suggesting that ATF-2 activation acts a harmful function in PD neuropathogenesis [66].
ATF-3 expression is low under normal situations but is rapidly induced by multiple stresses [67]. ATF-3 binds to the cyclic AMP response element, typically decreasing the expressions of various target genes, and ATF-3-mediated responses can be adaptive or maladaptive [67,68]. ATF-3 is increased in damaged neurons to help neuronal regeneration [69]. Upregulation of ATF3 is linked to neuroprotection and regeneration [70]. ATF3 protects neurons from death and rebuilds synaptic links after neurotoxic injury [71]. In the peripheral nervous system, ATF3 is also involved in axonal regeneration [70].
López-Cerdán et al. highlighted sex-based different mechanisms in PD hallmarks, including inflammatory reaction, mitochondrial malfunction, and oxidative stress. In female PD patients, specific transcription factors were activated with normalized enrichment scores of >0 including ATF-3, B-cell lymphoma 6, and Polycomb Group Ring Finger 2, which have been previously linked to neurodegenerative diseases or cognitive disabilities [72]. In addition, a PD model exhibited alterations of ATF-3 in response to ROS production and neurological damage [73]. Moreover, in a mouse model of PD, suppression of the ROS/ATF-3/CHOP pathway mitigates cell apoptosis in neurons, as ATF-3 activation induces CHOP expression, ultimately leading to cell apoptosis [73]. Francis et al. reported that ATF3 overexpression protects rat neurons from kainic acid-triggered neurotoxicity, having antiapoptotic effects on cells [74]. Although these results are contradictory, they suggest that ATF-3 is implicated in apoptosis. Accordingly, it is possible that ATF-3 activation or overexpression might modulate AD or PD through regulation of apoptosis.
ATF-4 is generally expressed at low levels but is increased on stimulation. It can be as both a transcriptional repressor and activator [75]. ATF-4 is involved in cell apoptosis [76], redox homeostasis, mitochondrial function, and amino acid metabolism in neurons [77,78]. ATF-4 is also involved in cell death [79,80]. The AD brain shows a significantly increased protein level of ATF-4 [81]. This may be related to the finding that ATF-4 may function as the downstream effector of Aβ and an upstream initiator for the neuropathological features in AD [82]. It is possible that increased ATF-4 is related to upregulated phosphorylation of tau, through protein phosphatase 1 kinases and glycogen synthase kinase 3, which could result in neuronal damage. Sun et al. found that human PD brain samples showed intense ATF-4 immunostaining [83]. Aimé et al. found that the drug adaptaquin blocks ATF-4/CHOP-dependent pro-death Tribbles pseudokinase 3 induction and protects in cellular and mouse models of PD [84]. Wang et al. found that verbascoside suppresses the progress of AD through downregulating PERK-eIF2α-ATF-4-CHOP axis triggered by ER stress in U251 glioma cells and in APP/PS1 transgenic mice [85].
ATF-5 is an opposing modulator of differentiation in neurons [86]. A decrease of ATF-5 is necessary to enhance neural cell cycle exit and neuronal differentiation [86,87]. ATF-5 can suppress apoptosis [88]. ATF-6 activates the UPR in response to ER stress [89]. The first line of defense against ER stress, the UPR can preserve ER homeostasis, although its activation may also lead to cell death [90]. ATF-6 is involved in the UPR pathway and can decrease ER stress [91,92]. When lasting presence of stimuli damages ER function, the ATF-6 pathway triggers the ER stress-regulated apoptosis, stimulating the expression of caspase-12 and CHOP to provoke apoptotic pathway [93,94]. In summary, ATFs act an important role in modulating cell repair, injury, and regeneration in neurons.
CONCLUSION
ER stress acts a critical role in AD and PD characterized by improperly folded protein aggregates. However, it is not yet completely clear how the UPR is involved, and related mechanisms through which ER stress leads to neuropathogenesis are unknown, and may have opposite effects. This may explain the interaction between ER stress, UPR, and neuroinflammation. As previously reviewed, studies using both in vitro and in vivo neurodegenerative disease models have demonstrated that the disease-related aggregates of improperly folded protein result in synaptic and neuronal dysfunction. Targeting ER- and UPR-related pathways appears to be a promising approach to treat neurodegenerative diseases. A comprehensive understanding of the cellular signaling pathways and physiological roles of ER- and UPR-related proteins will assist to guide the development of new therapeutic strategies. It will be important to discover novel drugs that can regulate ER stress and UPR signaling in various cell and animal models, which will provide substantial information regarding how the ER and UPR are involved in AD and PD progression.
Here, we also summarized the roles of ATFs in the neuropathogenesis of AD and PD. Since ATF expressions are significantly changed during AD or PD, it can be suggested that ATFs family may be the causative genes for AD or PD. ATFs play diverse roles during AD and PD and may be involved in these diseases through several pathways, including modulation of ER stress and apoptosis. Thus, ATFs may act as a potential target for the therapy of AD and PD. However, there are presently few drugs that target ATFs to treat AD or PD, and there remains a need for further research to investigate the particular mechanism of ATFs in AD and PD.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Conflicts of interest
Dr. Ching-Feng Cheng, an editorial board member at Tzu Chi Medical Journal, had no role in the peer review process of or decision to publish this article. The other authors declared no conflicts of interest in writing this paper.
Funding Statement
This work was supported by the Ministry of Science and Technology (MOST 110-2314-B-303-011-MY3 to C-FC), Buddhist Tzu Chi Medical Foundation (TCMMP111-01-02, TCRD-TPE-111-RT-4, TCRD-TPE-NCU-113-01 to C-FC), and Tzu Chi and Academia Sinica cooperation (TCAS-112-02 translational research grants to C-FC).
REFERENCES
- 1.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 2.Aguzzi A, O’Connor T. Protein aggregation diseases: Pathogenicity and therapeutic perspectives. Nat Rev Drug Discov. 2010;9:237–48. doi: 10.1038/nrd3050. [DOI] [PubMed] [Google Scholar]
- 3.Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–5. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- 4.Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med. 2015;21:1406–15. doi: 10.1038/nm.4001. [DOI] [PubMed] [Google Scholar]
- 5.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–9. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 6.Frake RA, Ricketts T, Menzies FM, Rubinsztein DC. Autophagy and neurodegeneration. J Clin Invest. 2015;125:65–74. doi: 10.1172/JCI73944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–35. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 8.Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: A link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167:35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercado G, Valdés P, Hetz C. An ERcentric view of Parkinson's disease. Trends Mol Med. 2013;19:165–75. doi: 10.1016/j.molmed.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–30. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 11.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: Cell life and death decisions. J Clin Invest. 2005;115:2656–64. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu M, Lawrence DA, Marsters S, Acosta-Alvear D, Kimmig P, Mendez AS, et al. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345:98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stutzbach LD, Xie SX, Naj AC, Albin R, Gilman S, PSP Genetics Study Group et al. The unfolded protein response is activated in disease-affected brain regions in progressive supranuclear palsy and Alzheimer's disease. Acta Neuropathol Commun. 2013;1:31. doi: 10.1186/2051-5960-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellucci A, Navarria L, Zaltieri M, Falarti E, Bodei S, Sigala S, et al. Induction of the unfolded protein response by α-synuclein in experimental models of Parkinson's disease. J Neurochem. 2011;116:588–605. doi: 10.1111/j.1471-4159.2010.07143.x. [DOI] [PubMed] [Google Scholar]
- 15.Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, et al. Plasma tau in Alzheimer disease. Neurology. 2016;87:1827–35. doi: 10.1212/WNL.0000000000003246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh DM, Selkoe DJ. A beta oligomers –A decade of discovery. J Neurochem. 2007;101:1172–84. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 17.Gendron TF, Petrucelli L. The role of tau in neurodegeneration. Mol Neurodegener. 2009;4:13. doi: 10.1186/1750-1326-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Ma Q, Zhang YW, Xu H. Proteolytic processing of Alzheimer's β-amyloid precursor protein. J Neurochem. 2012;120(Suppl 1):9–21. doi: 10.1111/j.1471-4159.2011.07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abisambra JF, Jinwal UK, Blair LJ, O’Leary JC, 3rd, Li Q, Brady S, et al. Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation. J Neurosci. 2013;33:9498–507. doi: 10.1523/JNEUROSCI.5397-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song J, Park KA, Lee WT, Lee JE. Apoptosis signal regulating kinase 1 (ASK1): Potential as a therapeutic target for Alzheimer's disease. Int J Mol Sci. 2014;15:2119–29. doi: 10.3390/ijms15022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García-González P, Cabral-Miranda F, Hetz C, Osorio F. Interplay between the unfolded protein response and immune function in the development of neurodegenerative diseases. Front Immunol. 2018;9:2541. doi: 10.3389/fimmu.2018.02541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Q, Cao Y, Gao J. Serum calreticulin is a negative biomarker in patients with Alzheimer's disease. Int J Mol Sci. 2014;15:21740–53. doi: 10.3390/ijms151221740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma V, Ounallah-Saad H, Chakraborty D, Hleihil M, Sood R, Barrera I, et al. Local inhibition of PERK enhances memory and reverses age-related deterioration of cognitive and neuronal properties. J Neurosci. 2018;38:648–58. doi: 10.1523/JNEUROSCI.0628-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hugon J, Mouton-Liger F, Dumurgier J, Paquet C. PKR involvement in Alzheimer's disease. Alzheimers Res Ther. 2017;9:83. doi: 10.1186/s13195-017-0308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasini S, Corona C, Liu J, Greene LA, Shelanski ML. Specific downregulation of hippocampal ATF4 reveals a necessary role in synaptic plasticity and memory. Cell Rep. 2015;11:183–91. doi: 10.1016/j.celrep.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duran-Aniotz C, Cornejo VH, Espinoza S, Ardiles ÁO, Medinas DB, Salazar C, et al. IRE1 signaling exacerbates Alzheimer's disease pathogenesis. Acta Neuropathol. 2017;134:489–506. doi: 10.1007/s00401-017-1694-x. [DOI] [PubMed] [Google Scholar]
- 27.Martínez G, Vidal RL, Mardones P, Serrano FG, Ardiles AO, Wirth C, et al. Regulation of memory formation by the transcription factor XBP1. Cell Rep. 2016;14:1382–94. doi: 10.1016/j.celrep.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Cissé M, Duplan E, Checler F. The transcription factor XBP1 in memory and cognition: Implications in Alzheimer disease. Mol Med. 2017;22:905–17. doi: 10.2119/molmed.2016.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du Y, Liu X, Zhu X, Liu Y, Wang X, Wu X. Activating transcription factor 6 reduces Aβ1-42 and restores memory in Alzheimer's disease model mice. Int J Neurosci. 2020;130:1015–23. doi: 10.1080/00207454.2020.1715977. [DOI] [PubMed] [Google Scholar]
- 30.Santos LE, Ferreira ST. Crosstalk between endoplasmic reticulum stress and brain inflammation in Alzheimer's disease. Neuropharmacology. 2018;136:350–60. doi: 10.1016/j.neuropharm.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 2014;46:629–40. doi: 10.1093/abbs/gmu048. [DOI] [PubMed] [Google Scholar]
- 32.Sadleir KR, Popovic J, Vassar R. ER stress is not elevated in the 5XFAD mouse model of Alzheimer's disease. J Biol Chem. 2018;293:18434–43. doi: 10.1074/jbc.RA118.005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson's disease. Prog Neurobiol. 2013;106-107:17–32. doi: 10.1016/j.pneurobio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chai C, Lim KL. Genetic insights into sporadic Parkinson's disease pathogenesis. Curr Genomics. 2013;14:486–501. doi: 10.2174/1389202914666131210195808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corti O, Lesage S, Brice A. What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol Rev. 2011;91:1161–218. doi: 10.1152/physrev.00022.2010. [DOI] [PubMed] [Google Scholar]
- 36.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2013;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 37.Witt SN. Molecular chaperones, α-synuclein, and neurodegeneration. Mol Neurobiol. 2013;47:552–60. doi: 10.1007/s12035-012-8325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malek N, Swallow D, Grosset KA, Anichtchik O, Spillantini M, Grosset DG. Alpha-synuclein in peripheral tissues and body fluids as a biomarker for Parkinson's disease –A systematic review. Acta Neurol Scand. 2014;130:59–72. doi: 10.1111/ane.12247. [DOI] [PubMed] [Google Scholar]
- 39.Golbe LI, Di Iorio G, Bonavita V, Miller DC, Duvoisin RC. A large kindred with autosomal dominant Parkinson's disease. Ann Neurol. 1990;27:276–82. doi: 10.1002/ana.410270309. [DOI] [PubMed] [Google Scholar]
- 40.Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–73. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 41.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–8. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 42.Gerakis Y, Hetz C. Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer's disease. FEBS J. 2018;285:995–1011. doi: 10.1111/febs.14332. [DOI] [PubMed] [Google Scholar]
- 43.Bellani S, Mescola A, Ronzitti G, Tsushima H, Tilve S, Canale C, et al. GRP78 clustering at the cell surface of neurons transduces the action of exogenous alpha-synuclein. Cell Death Differ. 2014;21:1971–83. doi: 10.1038/cdd.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colla E, Coune P, Liu Y, Pletnikova O, Troncoso JC, Iwatsubo T, et al. Endoplasmic reticulum stress is important for the manifestations of α-synucleinopathy in vivo. JNeurosci. 2012;32:3306–20. doi: 10.1523/JNEUROSCI.5367-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoozemans JJ, van Haastert ES, Eikelenboom P, de Vos RA, Rozemuller JM, Scheper W. Activation of the unfolded protein response in Parkinson's disease. Biochem Biophys Res Commun. 2007;354:707–11. doi: 10.1016/j.bbrc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 46.Valdés P, Mercado G, Vidal RL, Molina C, Parsons G, Court FA, et al. Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc Natl Acad Sci U S A. 2014;111:6804–9. doi: 10.1073/pnas.1321845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan C, Liu J, Gao J, Sun Y, Zhang L, Song H, et al. IRE1 promotes neurodegeneration through autophagy-dependent neuron death in the Drosophila model of Parkinson's disease. Cell Death Dis. 2019;10:800. doi: 10.1038/s41419-019-2039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–8. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, et al. The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci U S A. 2008;105:145–50. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D. α-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci U S A. 2016;113:1931–6. doi: 10.1073/pnas.1520335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Credle JJ, Forcelli PA, Delannoy M, Oaks AW, Permaul E, Berry DL, et al. α-Synuclein-mediated inhibition of ATF6 processing into COPII vesicles disrupts UPR signaling in Parkinson's disease. Neurobiol Dis. 2015;76:112–25. doi: 10.1016/j.nbd.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 52.Betzer C, Lassen LB, Olsen A, Kofoed RH, Reimer L, Gregersen E, et al. Alpha-synuclein aggregates activate calcium pump SERCA leading to calcium dysregulation. EMBO Rep. 2018;19:e44617. doi: 10.15252/embr.201744617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung EM, Yoo YM, Park SY, Ahn C, Jeon BH, Hong EJ, et al. Calbindin-D (9k) is a novel risk gene for neurodegenerative disease. Cell Physiol Biochem. 2020;54:438–56. doi: 10.33594/000000229. [DOI] [PubMed] [Google Scholar]
- 54.Hai TW, Liu F, Coukos WJ, Green MR. Transcription factor ATF cDNA clones: An extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989;3:2083–90. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- 55.Vallejo M, Ron D, Miller CP, Habener JF. C/ATF, a member of the activating transcription factor family of DNA-binding proteins, dimerizes with CAAT/enhancer-binding proteins and directs their binding to cAMP response elements. Proc Natl Acad Sci U S A. 1993;90:4679–83. doi: 10.1073/pnas.90.10.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen M, Liu Y, Yang Y, Qiu Y, Wang Z, Li X, et al. Emerging roles of activating transcription factor (ATF) family members in tumourigenesis and immunity: Implications in cancer immunotherapy. Genes Dis. 2022;9:981–99. doi: 10.1016/j.gendis.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Persengiev SP, Green MR. The role of ATF/CREB family members in cell growth, survival and apoptosis. Apoptosis. 2003;8:225–8. doi: 10.1023/a:1023633704132. [DOI] [PubMed] [Google Scholar]
- 58.Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma C, Kang SC. Garcinol pacifies acrylamide induced cognitive impairments, neuroinflammation and neuronal apoptosis by modulating GSK signaling and activation of pCREB by regulating cathepsin B in the brain of zebrafish larvae. Food Chem Toxicol. 2020;138:111246. doi: 10.1016/j.fct.2020.111246. [DOI] [PubMed] [Google Scholar]
- 60.Gao J, Davidson MK, Wahls WP. Distinct regions of ATF/CREB proteins Atf1 and Pcr1 control recombination hotspot ade6-M26 and the osmotic stress response. Nucleic Acids Res. 2008;36:2838–51. doi: 10.1093/nar/gkn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huebner K, Procházka J, Monteiro AC, Mahadevan V, Schneider-Stock R. The activating transcription factor 2: An influencer of cancer progression. Mutagenesis. 2019;34:375–89. doi: 10.1093/mutage/gez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhoumik A, Lopez-Bergami P, Ronai Z. ATF2 on the double –Activating transcription factor and DNA damage response protein. Pigment Cell Res. 2007;20:498–506. doi: 10.1111/j.1600-0749.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li M, Zhang D, Ge X, Zhu X, Zhou Y, Zhang Y, et al. TRAF6-p38/JNK-ATF2 axis promotes microglial inflammatory activation. Exp Cell Res. 2019;376:133–48. doi: 10.1016/j.yexcr.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Hsu CC, Hu CD. Critical role of N-terminal end-localized nuclear export signal in regulation of activating transcription factor 2 (ATF2) subcellular localization and transcriptional activity. J Biol Chem. 2012;287:8621–32. doi: 10.1074/jbc.M111.294272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang H, Khang R, Ham S, Jeong GR, Kim H, Jo M, et al. Activation of the ATF2/CREB-PGC-1α pathway by metformin leads to dopaminergic neuroprotection. Oncotarget. 2017;8:48603–18. doi: 10.18632/oncotarget.18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Q, Du X, He X, Yu Q, Hu K, Breitwieser W, et al. JNK-mediated activation of ATF2 contributes to dopaminergic neurodegeneration in the MPTP mouse model of Parkinson's disease. Exp Neurol. 2016;277:296–304. doi: 10.1016/j.expneurol.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 67.Hai T, Wolford CC, Chang YS. ATF3, a hub of the cellular adaptive-response network, in the pathogenesis of diseases: Is modulation of inflammation a unifying component? Gene Expr. 2010;15:1–11. doi: 10.3727/105221610x12819686555015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takii R, Inouye S, Fujimoto M, Nakamura T, Shinkawa T, Prakasam R, et al. Heat shock transcription factor 1 inhibits expression of IL-6 through activating transcription factor 3. J Immunol. 2010;184:1041–8. doi: 10.4049/jimmunol.0902579. [DOI] [PubMed] [Google Scholar]
- 69.Hunt D, Hossain-Ibrahim K, Mason MR, Coffin RS, Lieberman AR, Winterbottom J, et al. ATF3 upregulation in glia during Wallerian degeneration: Differential expression in peripheral nerves and CNS white matter. BMC Neurosci. 2004;5:9. doi: 10.1186/1471-2202-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gey M, Wanner R, Schilling C, Pedro MT, Sinske D, Knöll B. Atf3 mutant mice show reduced axon regeneration and impaired regeneration-associated gene induction after peripheral nerve injury. Open Biol. 2016;6:160091. doi: 10.1098/rsob.160091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahlgren H, Bas-Orth C, Freitag HE, Hellwig A, Ottersen OP, Bading H. The nuclear calcium signaling target, activating transcription factor 3 (ATF3), protects against dendrotoxicity and facilitates the recovery of synaptic transmission after an excitotoxic insult. J Biol Chem. 2014;289:9970–82. doi: 10.1074/jbc.M113.502914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.López-Cerdán A, Andreu Z, Hidalgo MR, Grillo-Risco R, Català-Senent JF, Soler-Sáez I, et al. Unveiling sex-based differences in Parkinson's disease: A comprehensive meta-analysis of transcriptomic studies. Biol Sex Differ. 2022;13:68. doi: 10.1186/s13293-022-00477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Q, Yang X, Cai D, Ye L, Hou Y, Zhang L, et al. Echinacoside protects against MPP(+)-induced neuronal apoptosis via ROS/ATF3/CHOP pathway regulation. Neurosci Bull. 2016;32:349–62. doi: 10.1007/s12264-016-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Francis JS, Dragunow M, During MJ. Over expression of ATF-3 protects rat hippocampal neurons from in vivo injection of kainic acid. Brain Res Mol Brain Res. 2004;124:199–203. doi: 10.1016/j.molbrainres.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 75.Gachon F, Gaudray G, Thébault S, Basbous J, Koffi JA, Devaux C, et al. The cAMP response element binding protein-2 (CREB-2) can interact with the C/EBP-homologous protein (CHOP) FEBS Lett. 2001;502:57–62. doi: 10.1016/s0014-5793(01)02646-1. [DOI] [PubMed] [Google Scholar]
- 76.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–55. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 78.Wei M, Li C, Yan Z, Hu Z, Dong L, Zhang J, et al. Activated microglia exosomes mediated miR-383-3p promotes neuronal necroptosis through inhibiting ATF4 expression in intracerebral hemorrhage. Neurochem Res. 2021;46:1337–49. doi: 10.1007/s11064-021-03268-3. [DOI] [PubMed] [Google Scholar]
- 79.Iurlaro R, Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283:2640–52. doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- 80.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–90. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ohno M. Roles of eIF2α kinases in the pathogenesis of Alzheimer's disease. Front Mol Neurosci. 2014;7:22. doi: 10.3389/fnmol.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei N, Zhu LQ, Liu D. ATF4: A novel potential therapeutic target for Alzheimer's disease. Mol Neurobiol. 2015;52:1765–70. doi: 10.1007/s12035-014-8970-8. [DOI] [PubMed] [Google Scholar]
- 83.Sun X, Liu J, Crary JF, Malagelada C, Sulzer D, Greene LA, et al. ATF4 protects against neuronal death in cellular Parkinson's disease models by maintaining levels of parkin. J Neurosci. 2013;33:2398–407. doi: 10.1523/JNEUROSCI.2292-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aimé P, Karuppagounder SS, Rao A, Chen Y, Burke RE, Ratan RR, et al. The drug adaptaquin blocks ATF4/CHOP-dependent pro-death Trib3 induction and protects in cellular and mouse models of Parkinson's disease. Neurobiol Dis. 2020;136:104725. doi: 10.1016/j.nbd.2019.104725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang C, Cai X, Wang R, Zhai S, Zhang Y, Hu W, et al. Neuroprotective effects of verbascoside against Alzheimer's disease via the relief of endoplasmic reticulum stress in Aβ-exposed U251 cells and APP/PS1 mice. J Neuroinflammation. 2020;17:309. doi: 10.1186/s12974-020-01976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Angelastro JM, Ignatova TN, Kukekov VG, Steindler DA, Stengren GB, Mendelsohn C, et al. Regulated expression of ATF5 is required for the progression of neural progenitor cells to neurons. J Neurosci. 2003;23:4590–600. doi: 10.1523/JNEUROSCI.23-11-04590.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mason JL, Angelastro JM, Ignatova TN, Kukekov VG, Lin G, Greene LA, et al. ATF5 regulates the proliferation and differentiation of oligodendrocytes. Mol Cell Neurosci. 2005;29:372–80. doi: 10.1016/j.mcn.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 88.Persengiev SP, Devireddy LR, Green MR. Inhibition of apoptosis by ATFx: A novel role for a member of the ATF/CREB family of mammalian bZIP transcription factors. Genes Dev. 2002;16:1806–14. doi: 10.1101/gad.992202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salminen A, Kaarniranta K. ER stress and hormetic regulation of the aging process. Ageing Res Rev. 2010;9:211–7. doi: 10.1016/j.arr.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 90.Ren J, Bi Y, Sowers JR, Hetz C, Zhang Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat Rev Cardiol. 2021;18:499–521. doi: 10.1038/s41569-021-00511-w. [DOI] [PubMed] [Google Scholar]
- 91.Shen J, Prywes R. ER stress signaling by regulated proteolysis of ATF6. Methods. 2005;35:382–9. doi: 10.1016/j.ymeth.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 92.Kohno K. How transmembrane proteins sense endoplasmic reticulum stress. Antioxid Redox Signal. 2007;9:2295–303. doi: 10.1089/ars.2007.1819. [DOI] [PubMed] [Google Scholar]
- 93.Huang J, Wan L, Lu H, Li X. High expression of active ATF6 aggravates endoplasmic reticulum stress-induced vascular endothelial cell apoptosis through the mitochondrial apoptotic pathway. Mol Med Rep. 2018;17:6483–9. doi: 10.3892/mmr.2018.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu L, Liu C, Lu Y, Liu L, Jiang Y. ER stress related factor ATF6 and caspase-12 trigger apoptosis in neonatal hypoxic-ischemic encephalopathy. Int J Clin Exp Pathol. 2015;8:6960–6. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.