Abstract
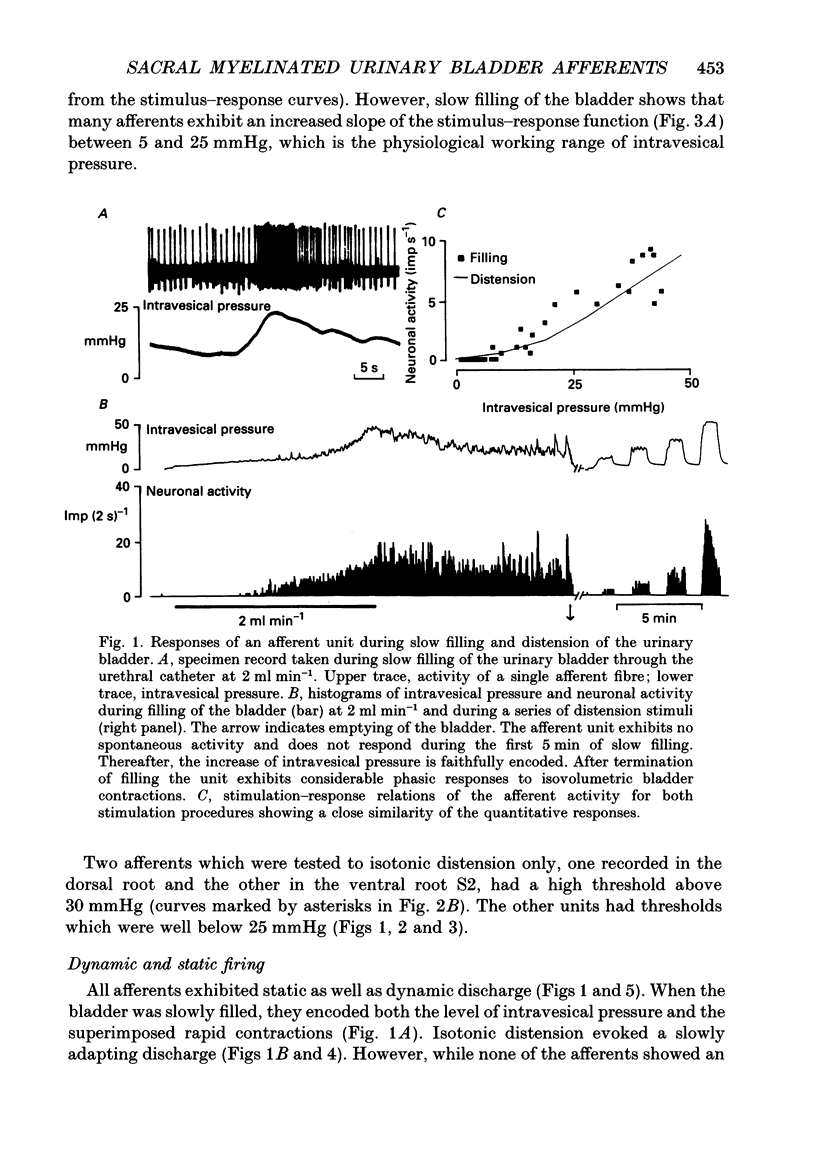
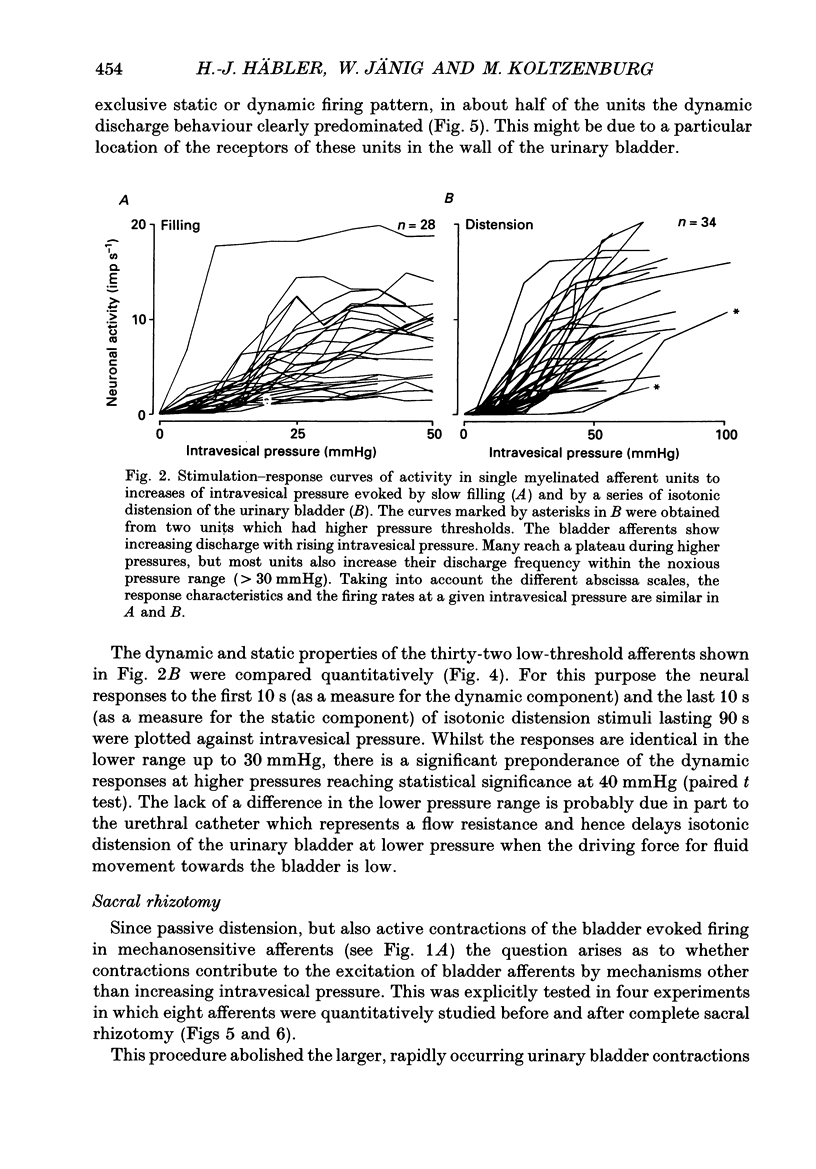
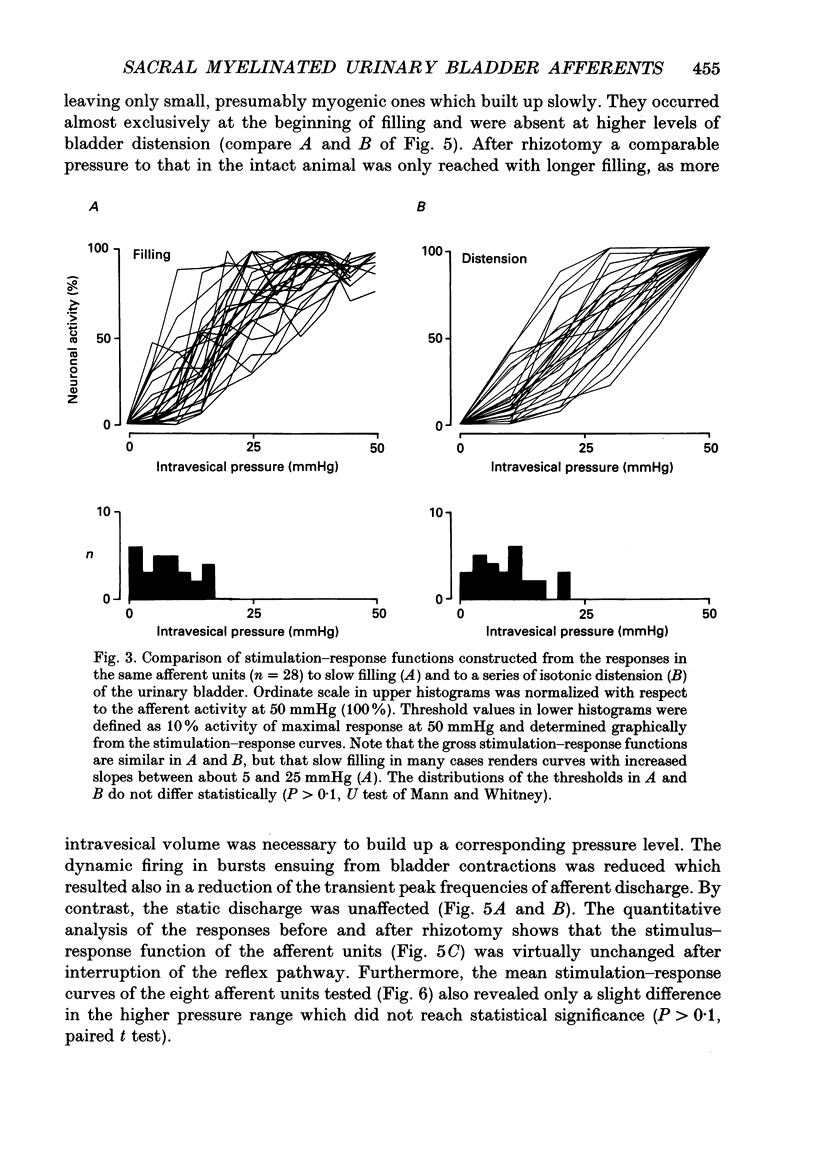
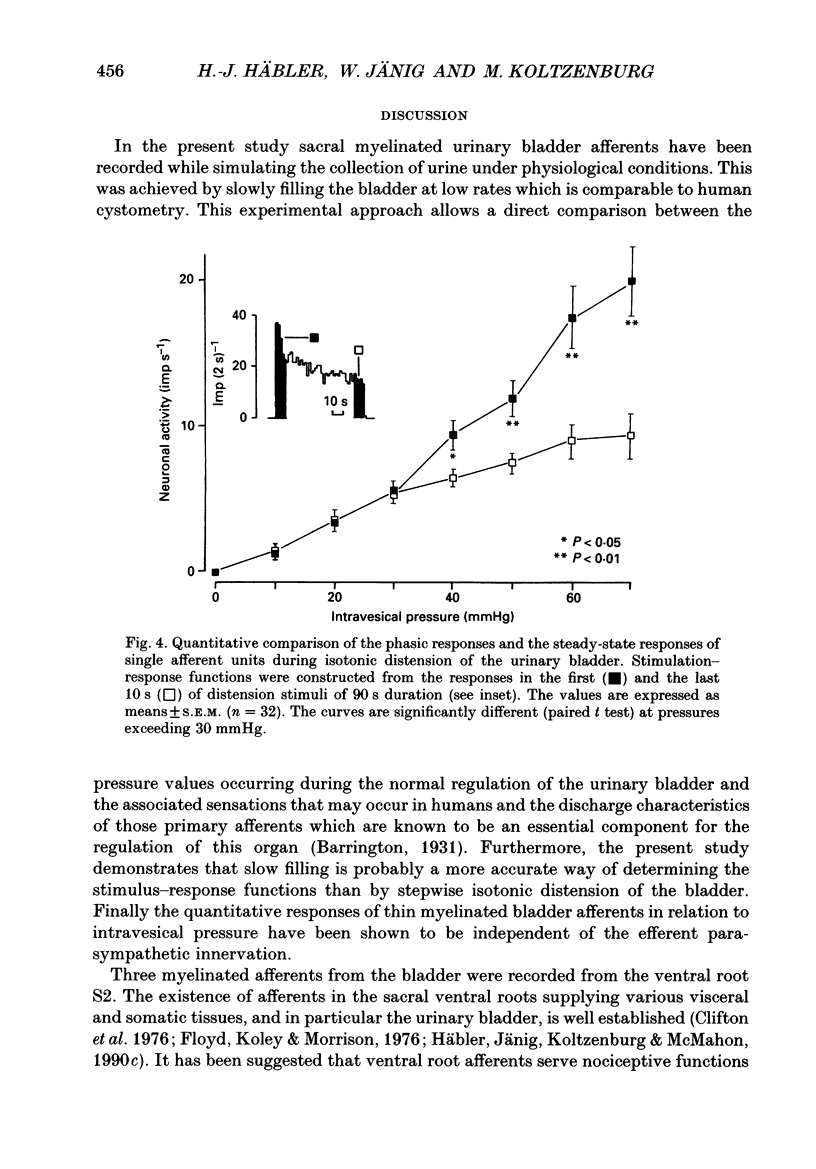
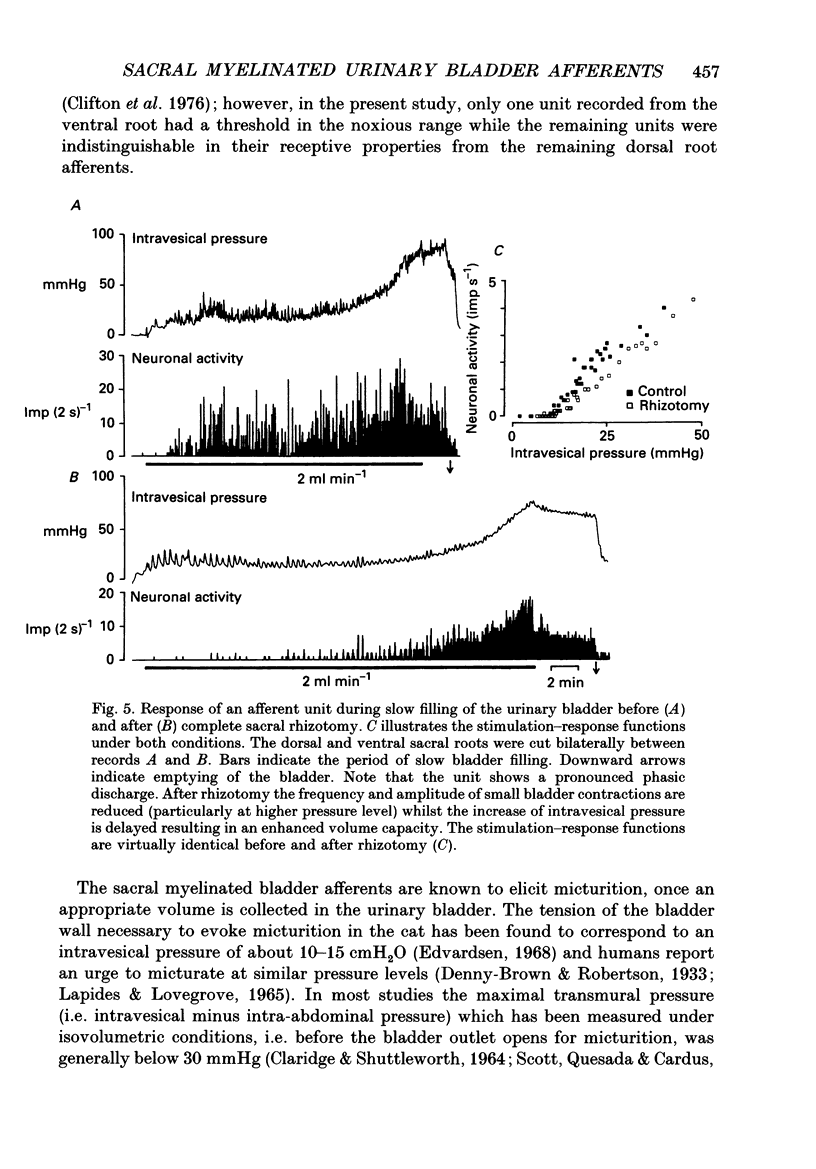
1. A total of sixty-five sacral afferent neurones with myelinated fibres supplying the urinary bladder was recorded from the sacral roots S2 in anaesthetized cats. All afferent units were identified with electrical stimulation of the pelvic nerve. The discharge properties were quantitatively evaluated using slow filling at rates of 1-2 ml min-1 and isotonic distension to preset pressure levels. Eight afferents were studied prior to and after acute sacral de-efferentation of the urinary bladder. 2. All afferent units were silent when the bladder was empty and responded in a graded manner to an increase of intravesical pressure. During slow filling the level of afferent activity correlated closely with the level of the intravesical pressure. All afferents behaved like slowly adapting mechanoreceptors with both a dynamic and static component of their discharge. With the exception of two units the intraluminal pressure threshold was below 25 mmHg. Thus virtually all myelinated afferents respond in the pressure range that is reached during a non-painful micturition cycle. 3. The stimulus-response functions of the afferents were similar regardless of whether intravesical pressure was increased by slow filling or by distension. However, during slow filling stimulation response functions often exhibited steeper slopes between 5 and 25 mmHg indicating that relatively small changes of intravesical pressure result in large changes of afferent activity. Nevertheless, all units displayed monotonically increasing stimulus response functions throughout the innocuous and noxious pressure level. 4. The stimulus-response functions of the afferent neurones did not change after acute de-efferentation of the urinary bladder, although the rapid phasic fluctuations of afferent activity that are produced by small contractions of the urinary bladder under normal conditions largely disappeared. This means that contractions and distension activate the afferent endings by a common mechanism. 5. It is concluded that the myelinated sacral afferents of the urinary bladder form a homogeneous population which encodes all information necessary for the normal regulation of this organ. Furthermore, this set of afferents mediates all sensations which may reach consciousness within a normal micturition cycle.
Full text
PDF



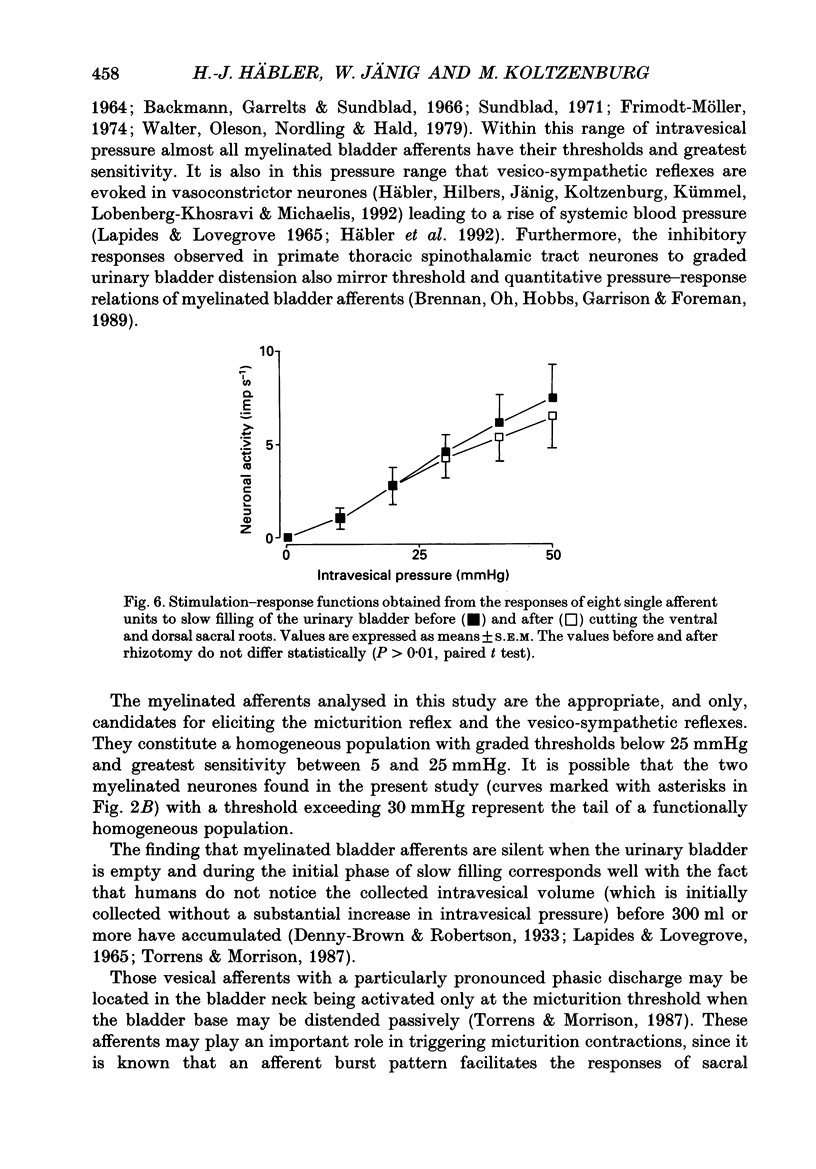


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K. A., von Garrelts B., Sundblad R. Micturition in normal women. Studies of pressure and flow. Acta Chir Scand. 1966 Oct;132(4):403–412. [PubMed] [Google Scholar]
- Bahns E., Halsband U., Jänig W. Responses of sacral visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflugers Arch. 1987 Oct;410(3):296–303. doi: 10.1007/BF00580280. [DOI] [PubMed] [Google Scholar]
- Brennan T. J., Oh U. T., Hobbs S. F., Garrison D. W., Foreman R. D. Urinary bladder and hindlimb afferent input inhibits activity of primate T2-T5 spinothalamic tract neurons. J Neurophysiol. 1989 Mar;61(3):573–588. doi: 10.1152/jn.1989.61.3.573. [DOI] [PubMed] [Google Scholar]
- CLARIDGE M., SHUTTLEWORTH K. E. THE DYNAMICS OF OBSTRUCTED MICTURITION. Invest Urol. 1964 Sep;2:188–199. [PubMed] [Google Scholar]
- Clifton G. L., Coggeshall R. E., Vance W. H., Willis W. D. Receptive fields of unmyelinated ventral root afferent fibres in the cat. J Physiol. 1976 Apr;256(3):573–600. doi: 10.1113/jphysiol.1976.sp011340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C. Nervous control of the urinary bladder of the cat. Brain Res. 1975 Apr 11;87(2-3):201–211. doi: 10.1016/0006-8993(75)90417-5. [DOI] [PubMed] [Google Scholar]
- Edvardsen P. Nervous control of urinary bladder in cats. I. The collecting phase. Acta Physiol Scand. 1968 Jan-Feb;72(1):157–171. doi: 10.1111/j.1748-1716.1968.tb03838.x. [DOI] [PubMed] [Google Scholar]
- Floyd K., Koley J., Morrison J. F. Proceedings: Afferent discharges in the sacral ventral roots of cats. J Physiol. 1976 Jul;259(1):37P–38P. [PubMed] [Google Scholar]
- Floyd K., Lawrenson G. Mechanosensitive afferents in the cat pelvic nerve [proceedings]. J Physiol. 1979 May;290(2):51P–52P. [PubMed] [Google Scholar]
- Frimodt-Moller C. A urodynamic study of micturition in healthy men and women. Dan Med Bull. 1974 Apr;21(2):41–48. [PubMed] [Google Scholar]
- Häbler H. J., Hilbers K., Jänig W., Koltzenburg M., Kümmel H., Lobenberg-Khosravi N., Michaelis M. Viscero-sympathetic reflex responses to mechanical stimulation of pelvic viscera in the cat. J Auton Nerv Syst. 1992 May 1;38(2):147–158. doi: 10.1016/0165-1838(92)90234-8. [DOI] [PubMed] [Google Scholar]
- Häbler H. J., Jänig W., Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990 Jun;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häbler H. J., Jänig W., Koltzenburg M., McMahon S. B. A quantitative study of the central projection patterns of unmyelinated ventral root afferents in the cat. J Physiol. 1990 Mar;422:265–287. doi: 10.1113/jphysiol.1990.sp017983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955 Jun 28;128(3):593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., Morrison J. F. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res. 1986;67:87–114. doi: 10.1016/s0079-6123(08)62758-2. [DOI] [PubMed] [Google Scholar]
- Klevmark B. Motility of the urinary bladder in cats during filling at physiological rates. I. Intravesical pressure patterns studied by a new method of cystometry. Acta Physiol Scand. 1974 Mar;90(3):565–577. doi: 10.1111/j.1748-1716.1974.tb05621.x. [DOI] [PubMed] [Google Scholar]
- Lapides J., Lovegrove R. H. Urinary vesicovascular reflex. J Urol. 1965 Oct;94(4):397–401. doi: 10.1016/S0022-5347(17)63638-3. [DOI] [PubMed] [Google Scholar]
- SCOTT F. B., QUESADA E. M., CARDUS D. STUDIES ON THE DYNAMICS OF MICTURITION: OBSERVATIONS ON HEALTHY MEN. J Urol. 1964 Nov;92:455–463. doi: 10.1016/S0022-5347(17)63987-9. [DOI] [PubMed] [Google Scholar]
- Walter S., Olesen K. P., Nordling J., Hald T. Bladder function in urologically normal middle aged females. A urodynamic and radiological investigation. Scand J Urol Nephrol. 1979;13(3):249–258. doi: 10.3109/00365597909179533. [DOI] [PubMed] [Google Scholar]
- de Groat W. C., Ryall R. W. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J Physiol. 1969 Jan;200(1):87–108. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]


