Abstract
Fluorophores are essential tools for optical imaging and biomedical research. Their synthetic modification to incorporate new functions, however, remains a challenging task. Conventional strategies rely on linear synthesis in which a parent framework is gradually extended. We here designed and synthesized a versatile library of multi‐functional fluorophores via a scaffold‐based Ugi four‐component reaction (U‐4CR). The adaptability of the scaffold is achieved through modification of starting materials. This allows to use a small range of starting materials for the creation of fluorogenic probes that can detect reactive‐oxygen species and where the localization into subcellular organelles or membranes can be controlled. We present reaction yields ranging from 60 % to 90 % and discovered that some compounds can even function as imaging and therapeutic agents via Fenton chemistry inducing pyroptosis in living cancer cells. Our study underlines the potential of scaffold‐based synthesis for versatile creation of functional fluorophores and their applications.
Keywords: cell imaging, fluorescent probes, fluorogenic dyes, pyroptosis, scaffold synthesis
A modular approach is established to synthesize multi‐functional fluorophores via a scaffolding approach using the Ugi four‐component reaction. The characterization and applications of a small library of such probes with fluorogenic character for optical imaging and theranostics are shown. The probes can be equipped with distinct cellular localization signals and can induce programmed cell death.
Introduction
The development of advanced fluorescent materials with diverse functional properties has facilitated progress in bioimaging, molecular biology, biochemistry and biomedicine.[ 1 , 2 , 3 , 4 ] The used water‐soluble fluorescent dyes or fluorophores are not just signal units, but can act as local probes or might even participate in the regulation of biochemical processes.[ 5 , 6 , 7 , 8 ] They are capable of imaging specific molecules in living cells or tissue, greatly assisting in the ability to intervene diseases. [9] Recently, the development of theranostic systems that allow simultaneous diagnostic imaging and therapy has been presented.[ 10 , 11 ] For such applications, a fluorophore with multiple functions is required. [12] Hofkens et al. described a similar type of multifunctional fluorescent molecule with targeting, reporting, and anchoring ability. [13] Such probes are necessary when signal generation and modulation by specfic biochemical events, target binding, or theranostic activity are required. [14] Thus, preparing fluorophores with excellent optical properties, targetability, and specific functional properties is critical for applications, e.g., in disease identification or treatment. [15]
The synthesis and modifications of such multifunctional fluorophores, however, remains a challenging task. [16] Many conventional strategies rely on linear synthesis,[ 17 , 18 , 19 , 20 ] in which a parent framework (often a fluorophore) is gradually extended through the addition of functional groups (Figure 1, top) to allow bio‐recognition (targeting), signal regulation or biochemical interactions, e.g., via drug units. By using this strategy, several fluorogenic probes (fluorescent turn‐on probes) with other functional properties have been developed for bioimaging and diagnostics.[ 21 , 22 , 23 , 24 ] Examples are fluorescent probes with near‐infrared emission and high brightness for high signal‐to‐noise imaging‐guided diagnosis based on a cyanine dyes backbone.[ 25 , 26 ] Another example involves fluorogenic acedan dyes, which were coupled to triphenylphosphonium and morpholine as targeting groups. [27] These modifications require separate and complex synthetic procedures, both starting from the fluorophore. There are many other examples for biological applications of similar strategies[ 28 , 29 ] and it should be stressed that extensive optimization of reaction conditions is required before a functional dye, can be obtained which often hinders the application of such materials beyond basic research.
Figure 1.

Comparison of linear (top) and scaffold (middle) synthesis routes towards multi‐functional fluorophores with fluorogenic properties and the ability for cellular localization, imaging and pyroptosis (bottom).
An elegant solution to overcome these problems is to use a modular framework,[ 30 , 31 ] which directly integrates the fluorophore with other functional groups (Figure 1, middle). Such a scaffold‐based synthesis is much simpler than linear methods, it reduces the number of required steps and allows straightforward modification of functional groups within the framework. The scaffolding strategy has already facilitated the development of a variety of multifunctional light‐absorbing chromophores.[ 32 , 33 , 34 ] Its use for the design of functional fluorescent dyes, however, is still scarce. [35] The Urano group has established a modular platform for the detection of carboxypeptidases utilizing activatable fluorescent probes. [35] A linear synthesis was used with the necessity to subsequently tailor the fluorophore to match specific requirements. Here, we introduce an “editable” platform for scaffolded synthesis of multifunctional dyes via the Ugi four‐component reaction (U‐4CR). This strategy allows straightforward exchange of (commercially available) starting materials and resulting modification of fluorophore properties (Figure 1, middle). It can also be extended easily to other dye classes and functionalities than tested here (Figure 1, bottom). One key innovation we make here is to utilize the U‐4CR for the simplified synthesis of multi‐functional fluorophores in a single step, lifting the need of sequentially introducing functional moeities during fluorophore synthesis.
The U‐4CR offers superb scaffold diversity to obtain complex chromophore structures. [36] It combines four reaction partners in a single step and reaction vessel in an atom‐efficient manner without the need for catalysts. [37] Based on the U‐4CR fluorescent peptidomimetics, [38] pharmacophores, [39] and functional chromophores were prepared. [31] Müller and co‐workers pioneered the application of the U‐4CR for various chromophores including highly functional donor‐acceptor dyads. [33] Westermann and colleagues used the U‐4CR to synthesize rhodamine tags with clickable bioorthogonal groups for biolabeling. [40] Instead of utilizing four components, Vendrell et al. proposed a synthesis based on the Passerini three‐component reaction (P‐3CR) to create a BODIPY‐containing isonitrile fluorescent probe for low‐pH sensing in activated macrophages. [41] We recently utilized the U‐4CR to create a small library of linker molecules for labeling of biomacromolecules with fluorescent dyes in a two‐step protocol, an approach that allowed to convert commercial fluorophores into functional probes with high photostability or metal sensing ability, directly on the biological target. [42]
Building on the rising interest and success of scaffolded functional fluorophores, [43] we here introduce a modular synthesis platform for fluorogenic probes based on the U‐4CR. The obtained probes combine three distinct moeities, i.e., a fluorophore, a signal modulator and a unit used for cell labelling of specific targets (the cell membrane, mitochondria and lysosomes). Our approach uses the redox properties of ferrocene (Fc),[ 44 , 45 , 46 ] and its influence on organic dyes via photoinduced electron transfer (PeT)[ 47 , 48 , 49 ] for signal modulation. Furthermore, Fc is involved in a Fenton‐like reaction (the generation of hydroxyl radicals from hydrogen peroxide) that is stimulated by the microenvironment of cancer cells. [50] These hydroxyl radicals are reactive oxygen species capable of inducing cell death. [51] More specifically, in cancer cells, the presence of mild acidic conditions triggers the initiation of a Fenton‐like reaction, leading to the excessive consumption of hydrogen peroxide in order to generate hydroxyl radicals. This reaction provides a certain degree of safety to normal cells, as the Fenton reaction is significantly inhibited under slightly alkaline conditions and when there is an insufficient amount of hydrogen peroxide in a normal environment. [50] We demonstrate here that fluorogenic probes synthesized via the U‐4CR scafold strategy, which target either cytoplasmic lysosomes or cytoplasm mitochondria, can actually activate pyroptosis [51] pathways through photo‐Fenton chemistry. This is evidenced by the activation of caspase‐1 and caspase‐3 and the cleavage of gasdermin D and E. Our work underscores the versatility of the modular scaffold strategy, for modifying fluorescent probes not only for imaging but also for biomedical applications.
Results and Discussion
Scaffolding of Multi‐Functional Fluorophores via the U‐4CR
The U‐4CR method involves the one‐pot reaction of an aldehyde, an amine, an isocyanide, and a carboxylic acid to produce α‐N‐acetoamido carboxamide derivatives as shown in Scheme 1. This approach allows to combine up to four functional groups within the carboxamide scaffold. We employed this approach to obtain fluorogenic probes where fluorescence becomes activated (off→on) under specific chemical conditions in cellular organelles. Our goal was to obtain a series of these probes with a scaffold that can be readily modified by altering the starting materials to achieve distinct cellular localization. The probes included a localization moiety comprising either triphenylphosphonium, morpholine, or a hydrophobic aliphatic chain and a fluorophore.
Scheme 1.
Synthesis route of multi‐functional fluorophores starting with the fluorophore (4,10,11), targeting localization group (3, 5–7), Fc‐PeT units (2) and isocyanide (12 and 13). Compound 1 lacks the Fc moiety and was used as a control. Compounds 14–26 were obtained by one‐pot 4‐UCR. Compound 16–17 was used as a linker for compounds 27–30.
This enabled the fluorogenic probes to specifically anchor on the phospholipid membrane through hydrophobic interactions between alkane chain, [52] or to localize to mitochondria via triphenylphosphonium [53] and lysosomes via morpholine, [54] respectively. Additionally, a xanthene fluorophore and a functional ferrocenyl module were incorporated to allow fluorogenic imaging upon ferrocenyl oxidation or photo‐Fenton triggered pyroptosis in cancer cells. We used a hydrogen atom (compounds 14, 16, 18, 20, 22, 24, 27 and 29) as non‐functional negative control. Importantly, all starting materials used here were obtained commercially and did not have to be synthesized. For this, the reaction of four components (one of 1–3, one of 4–7, one of 8–11 with either 12 or 13) was performed in methanol for 48 hours, resulting in the formation of α‐N‐acylamino amides 14–26. The reaction yields for compounds 14 and 19 were high and exceeded 80 %, while those for compounds 15–18, 20, 21, 23–26 were between 60–80 %, with on exception of compound 22 (~50 %). Compounds 14–17 were purified by silica gel column chromatography, 18–25 were purified using a preparative HPLC, and 26 was purified by recrystallization. All compounds were subsequently characterized (Supporting Information, Part I/II, Figures S1–S26, S35–S58). While some functional dyes could be synthesized in one step (Scheme 1, 14–26), others (Scheme 1, 27–30) required additional steps, since some functional groups were not compatible with the U‐4CR reaction. For example, we observed that a mixture of a fluorophore with an amino group (compound 4), a cell‐targeting morpholine with a carboxylic acid, ferrocenecarboxaldehyde (compound 2), and n‐Butyl isocyanide (compound 13) did not undergo the U‐4CR reaction. The modularity of our approach, however, allows to swap the functional moieties in the precursors, illustrated in Scheme 1 (bottom, left).This approach allows an optimization of the conditions to obtain compounds such as 18 and 19 in a single step. Using a fluorophore with a carboxylic acid (compound 10) and a morpholino amino group (compound 5), under otherwise identical conditions, allowed the one‐pot reaction to proceed with good yields. Secondly, we used a more complex Scheme that started with compounds 16 and 17 and then introduced a linker allowing the isolation and purification of products 27–30 (Scheme 1, bottom, right) via column chromatography. The yields for these were 50–80 % (Figures S27–S34, S59–S66). These variations show that the scaffold synthesis based on U‐4CR allows a straightforward and effective optimization of reaction conditions by variation of the starting material.
Spectroscopic Properties of Multi‐Functional Fluorophores
Prior to the U‐4CR conjugation of relevant ferrocene (Fc) moieties to fluorophores, we investigated the quenching of dyes via hybridization in a double‐stranded DNA (Figure 2A) in bulk fluorescence measurements. The proximity of ferrocene induces fluorescence quenching for blue‐green‐absorbing fluorophores (Figures 2B, 2 C). The electron‐rich ferrocene moiety serves as an electron donor from the low‐spin iron (II) center for the photoexcited fluorophore [55] and efficiently suppresses fluorescence emission via photoinduced electron transfer (PeT).[ 56 , 57 ] Upon oxidation of the Fc moiety, the PeT mechanism is disrupted, leading to a recovery of fluorescence (Figures 2D, 2E). This effect was most pronounced for fluorescein (FAM) and tetramethylrhodamine (TMR) because these blue‐green‐absorbing fluorophores have higher lying excited states compared to red‐shifted fluorophores, [58] which increases the driving force for electron transfer with ferrocene. Therefore, FAM and TMR were selected as signal units in our dye U‐4CR conjugates (14–30). Among these, the conjugated triphenylphosphonium‐FAM−Fc compound 30 showed significantly lower fluorescence than the triphenylphosphonium‐FAM‐control (compound 29, Figure 2I). This trend was also observed in the morpholine‐FAM probes (compound 27–28, Figure 2F). Further support for the removal of PeT quenching is provided by fluorescence recovery of compounds 28 and 30 upon addition of H2O2 (Figures 2H, 2 K). A similar effect did not occur in the absence of Fc conjugation for compounds 27 and 29 (Figures 2G, 2 J).
Figure 2.
Spectroscopic characterization of compounds 27–30. (A) The influence of ferrocene on various fluorophores when terminally attached to dsDNA in (B, C) bulk fluorescence measurements (mean±SD, n=3). (D) Schematic illustration of PeT quenching and (E) fluorescence spectra of compound 30, showing the reversible nature of PeT quenching by the sequential addition of 1.0 equivalent of an oxidant and followed by a reductant. (F−K) Fluorescence spectra of compounds 27–30 at identical concentration and under varying concentrations of hydrogen peroxide (0, 0.03, 0.06, 0.12, 0.3, 0.6 and 1.2 %) in pH 6.5 PBS buffer solution.
Direct evidence for PeT quenching was obtained from pump‐probe transient absorption spectroscopy (TAS) with picosecond time resolution. [59] For compounds 27–30 (Figure 3A), the fluorescein fluorophore was consistently positioned at the N‐terminal end of the U‐4CR scaffold. Ferrocene was located at the carbon atom of the aldehyde functional group in compounds 28 and 30. Compounds 27 (Fc‐free control for 28) and 28 are characterized by the presence of a morpholine group, while compounds 29 (Fc‐free control for 30) and 30 incorporate a triphenylphosphonium group, respectively. According to the steady state absorption spectra (Figure S67, S68), the pump wavelength was set to 490 nm. These synthesized U‐4CR dyes based on fluorescein (Figures 3B, 3 C, 3F, 3G and Figures S69, S70) showed similar TAS absorbance difference spectra (ΔA) and clear spectral signatures of excited‐state absorption (ESA, positive signal around 410 nm) and two overlapping negative contributions of ground‐state bleaching (GSB) and stimulated emission (SE) with a minimum at around 498 nm. For 28 and 30 the complete ΔA signal vanished with a dominant decay time of 30 ps (single exponential fit y(t)=a*exp(−t/τ)) [60] indicating that the excited state is rapidly quenched by Fc. For 27 and 29, we used a double exponential fit function of the form y(t)=a1*exp(−t/τ1)+a2*exp(−t/τ2) [61] showing a fast 30 ps component and a slow decay with a time constant of 4 ns. The latter corresponds to the decay of the relaxed excited state matching the fluorescence lifetime of free fluorescein.[ 62 , 63 ] The appearance of the shorter‐lived component in the initial tens of picoseconds for 29 and 30 can be attributed to the presence of the anionic form of the fluorescein fluorophore, which coexists in a mixture with its dianion at pH 7.4.[ 64 , 65 ]
Figure 3.
Transient absorption spectroscopy of dye conjugates 27–30 with broad‐band probing and excitation at 490 nm.
A comparison of the normalized kinetics of compound 27/29 (without ferrocene conjugation) and 28/30 (with ferrocene conjugation), as shown in Figures 3D, 3E, 3H and 3I, allow an assessment of the electron transfer rate k eT. The rate k eT was calculated according to k eT=1/τFc‐1/τ based on both ESA and GSB/SE kinetics. We found values of k eT of 3.7×1010 s−1 for 28 and 3.5×1010 s−1 for 30. These high rates are indicative of fast electron transfer and are compatible with the large negative free energy difference ΔG for charge separation of −0.84 eV, calculated with the Rehm–Weller equation: [66] ΔG=e*[Eox−Ered]−E0,0+C; with the unit charge e−, the one‐electron oxidation potential of donor (Eox=0.45 V) and reduction potential of acceptor (Ered=−1.23 V) and the zero–zero energy (E0,0=2.52 eV). For the calculation of ΔG we neglected the solvent‐dependent coulombic attraction energy (C) due to the experiments being conducted in a polar solvent.
The comparison of electron transfer rates in different functional fluorophore constructs highlights the possibilities of the scaffold‐based strategy to optimize their photophysical properties. We could show that the U‐4CR scaffold allows for a simple exchange of the biotargeting modules, i.e., switching from morpholine to triphenylphosphonium, without significant alterations of the electron transfer rate (Figure 3). We can demonstrate, however, that the scaffold strategy also allows for the adjustment of the relative positions between the PeT donor and the fluorophore. Our TAS analysis of compounds 17/28/30, where the fluorophore is positioned at the N‐terminal, exhibit higher PeT rates compared to compound 19 (7.3×108 s−1), which has its fluorophore located at the C‐terminal of the scaffold (Figures S69–S72).
Cellular Imaging with Multi‐Functional Fluorophores
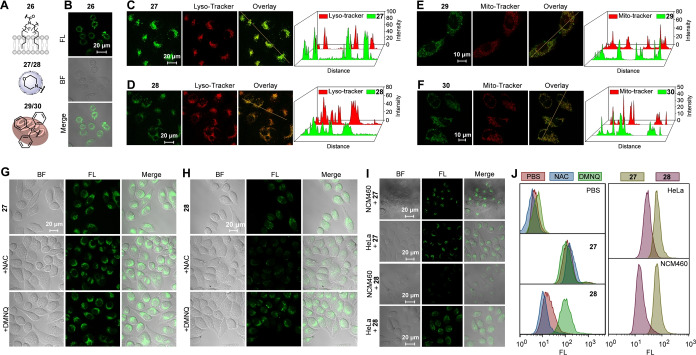
The resulting compounds 26–30 comprise distinct cellular localization groups on the scaffold. These include hydrophobic alkane chains, morpholine, and triphenylphosphonium moieties. Each of them is known for its selective cellular localization, ranging from the membrane to lysosomal targeting (morpholine group), and mitochondrial localization via the triphenylphosphonium group (Figure 4A). To verify the specific cellular localization of our compounds, we incubated them with living HeLa cells and imaged those with confocal microscopy. Figure 4B illustrates that HeLa cells treated with 26 showed uniform staining of the cytomembrane. This indicates a hydrophobic interaction between 26 and the plasma membrane. In contrast, 27–30 showed a distinct intracellular localization in HeLa cells. Since our compounds exhibit green fluorescence, we verified their co‐localization with the red fluorescence of commercially available Lyso‐ and Mito‐Tracker dyes (Figures 4C–F). These experiments show that compounds 27 and 28 specifically targeted lysosomal compartment, while compounds 29 and 30 are found in mitochondrial compartments. Importantly, the integration of the ferrocenyl module into these compounds does not affect their localization characteristics.
Figure 4.
Targeted localization of HeLa cells achieved by staining with compounds 26–30 via confocal microscopy. (A) An illustration of distinct compounds with customized cellular localization capabilities. (B) Confocal microscopy imaging of HeLa cells stained with compound 26 (10 μM). (C, D) Confocal microscopy images of HeLa cells labeled with 27/28 (1.0 μM); colocalization with Tracker Red (Lyso‐Tracker), Scale bar=20 μm. (E, F) Confocal microscopy images of HeLa cells labeled with 29/30 (1.0 μM); colocalization with Tracker Red (Mito‐Tracker), Scale bar=10 μm. (G, H) Confocal microscopy images of 27 (G) and 28 (H)‐incubated HeLa cells pretreated with different chemical agents: NAC (H2O2 inhibitor), DMNQ (H2O2 agonist), Scale bar: 20 μm. (I) Confocal microscopy images of 27‐ and 28‐incubated HeLa cancer and NCM460 cells, Scale bar: 20 μm. (J) Flow cytometry analysis of HeLa cells incubated with compound 27 and 28 after pre‐treatment with NAC or DMNQ, and comparative analysis between the treated HeLa cancer cells and NCM460 cells.
Furthermore, the inclusion of a ferrocenyl group in the main structure promotes a Fenton‐like response to intracellular hydrogen peroxide, from which we expect a removal of the PeT effect and subsequent recovery of fluorescence. To validate the specificity and responsiveness of this system towards hydrogen peroxide under cancerous conditions, comparative studies were conducted using NCM460, a normal cell line, and HeLa, a cancer cell line. Typically, the H2O2 concentration in normal cells is around 20 nM, whereas cancer cells can have values up to 0.4 mM. [67] Our analysis, conducted using confocal microscopy and flow cytometry (Figures 4I, 4 J), demonstrated a distinct behaviour of the compounds when tested across the two cell lines. Compound 27, lacking the ferrocene conjugation, exhibited uniform fluorescence across both cell types, indicating a lack of selective response to varying concentrations of H2O2. In stark contrast, compound 28, which contains the ferrocene moiety, demonstrated reduced fluorescence in the low‐H2O2 environment characteristic of normal cells. Conversely, it displayed significantly increased fluorescence in HeLa cells, where H2O2 levels are notably elevated. This differential fluorescence response underscores the sensitivity of 28 to H2O2, highlighting its potential for specific detection of cancer cells. Data obtained from flow cytometry analysis, after comprehensive cell staining, further corroborated the distinct behaviour of compounds 27 and 28 within cellular environments characterized by varying levels of H2O2. Additionally, the fluorescence of 28‐stained HeLa cells were markedly reduced upon pre‐incubation with the H2O2 inhibitor N‐acetylcysteine (NAC), [68] while pre‐treatment with an H2O2 oxidative stress inducer, 2,3‐dimethoxy‐1,4‐naphthoquinone (DMNQ) [69] enhanced the fluorescence of 28‐stained cells (Figure 4H). Notably, this oxidative stress treatment did not alter the fluorescence of 27‐stained HeLa cells (Figure 4G). Importantly, independent application of either NAC or DMNQ directly to the cell culture medium did not affect the fluorescence of compounds 27 and 28 (Figure S73). These results suggest that compound 28 is capable of responding specifically to H2O2 through Fenton‐like chemistry within cancer cells.
Biomedical Applications
Hydroxyl radicals produced by Fenton‐like chemistry from H2O2 have potential applications in impeding cancer cell proliferation.[ 50 , 70 , 71 ] We here employed a CCK‐8 assay as a benchmark method for evaluating cell viability in the presence of our compounds (Figure 5A). Specifically, HeLa cells were co‐incubated with compound 30 for 24 hours, resulting in a substantial reduction in cell viability to approximately 44 %. The half‐maximal inhibitory concentration (IC50) value of 30 against the HeLa cell line was 22±2 μM, demonstrating a moderate inhibitory effect. Compound 30, when tested independently of cellular incubation, did not mediate the CCK‐8 reaction (Figure S74). Exposing 30‐incubated cells to a blue LED for 30 minutes, however, decreased cell viability significantly, yielding a lower IC50 value of 8.5 0.5 μM. In contrast, under control conditions, either with light irradiation in the absence of any compound or with the inclusion of compound 29 (without Fc conjugation), we observed no cytotoxicity on the timescale of imaging. This indicates that light exposure, in combination with 30, enhances Fenton‐Like reactions, thereby generating an increased number of hydroxyl radicals. [72] This increase of intracellular hydroxyl radicals was directly observed using the Cell Meter™ detection kit, featuring a commercially available fluorescent probe designed for the specific detection of intracellular hydroxyl radicals. Figure 5B shows the red signal of the Cell Meter™ dye, which is indicative on the presence of hydroxyl radicals in the cytoplasm. To substantiate that these radicals were indeed localized in the cytoplasm, DAPI, [73] a near‐UV dye, was employed to stain and define the cytoplasmic area. Notably, the Cell Meter™ signal increased for prolonged light exposure, in the presence of compound 30, while compound 29 did not show similar effects.
Figure 5.
Cancer cellular lysosome‐/mitochondria‐targeted pyroptosis induced by compounds 28 and 30. (A) Cell viability analysis of compound 29‐ and 30‐mediated toxicity against HeLa cells measured by CCK‐8. The data are expressed as mean±standard deviation (n=3), based on three independent experiments for each group. (B) Fluorescent imaging of intracellular hydroxyl radicals using the Cell Meter™ hydroxyl radical detection kit in the absence and presence of light irradiation. (C) Representative phase‐contrast images of HeLa cells after treatment with 29 and 30. White arrows indicate cells with pyroptotic morphology. (D) ELISA analysis of IL‐1β and IL‐18 secretion into the supernatant of HeLa cells treated with 27, 28, 29 and 30 with and without light exposure (mean±SD, n=3). (E) Correlation between the concentrations of compounds 28 and 30 and the secretion levels of IL‐1β and IL‐18 into the supernatant of treated HeLa cells (mean±SD, n=3). (F) western blots of cleavage of GSDM and Caspase in response to 27,28,29 and 30‐treated HeLa cells. The uncropped blots are shown in Figure S75. (G) Relative cell viability of HeLa cells treated with 27–30 in presence of either YVAD (caspase‐1 inhibitor) or/and DEVD (caspase‐3 inhibitor); mean±SD, n=3. (H) A schematic representation elucidates the pyroptosis pathway induced in HeLa cells by treatment with compound 28 and 30. Statistical significance was determined using one‐way analysis of variance (ANOVA) followed by Tukey's test. *p<0.05, **p<0.01, ***p<0.001, ****p <0.0001.
Cell viability was further refined through phase‐contrast imaging (Figure 5C). Cells treated with 29, designated as the positive control, uniformly presented intact cell membranes, indicating no discernible damage. Treatment with 30 induced morphological alterations, characterized by significant swelling and a unique cellular morphology: a centralized nucleus encircled by an expansive cytoplasmic zone. After 15 minutes of light exposure, these morphological changes were clearly visible with multiple protrusions on the cell membrane with diameters ranging from approximately 2 to 5 μm. Such morphological features are reminiscent of those observed in pyroptosis,[ 74 , 75 ] a form of programmed cell death recently delineated by the caspase‐mediated cleavage of proteins from the gasdermin family.[ 76 , 77 , 78 ]
To substantiate the hypothesis that our compounds can induce pyroptosis, we quantified key biomarkers associated with this form of cell death, specifically the inflammatory cytokines interleukin‐1β (IL‐1β) and interleukin‐18 (IL‐18). This quantification was conducted on cancer cells treated with compounds 27–30, utilizing ELISA kits (Figure 5D). The results showed an increased release of IL‐1β and IL‐18, especially when exposed to compounds 28 and 30 in conjunction with light irradiation. Importantly, a positive correlation between the levels of IL‐1β and IL‐18 and the concentrations of the compounds was observed (Figure 5E), reinforcing the potential link between these compounds and the activation of pyroptotic pathways. In stark contrast, cells treated with compounds 27 and 29, which lack Fc, exhibited negligible release of these cytokines, even when exposed to light. This supports the role of Fc present in compounds 28 and 30 for pyroptosis.
A western blot analysis further supported these findings, offering insights into the mechanism by which these compounds induce pyroptosis (Figure 5F and S75). In SDS‐PAGE, a significant alteration in gasdermin D (GSDMD) dynamics was observed in cells treated with compound 28 or 30. Specifically, there was a noticeable reduction in the levels of full‐length GSDMD and a concurrent increase in the GSDMD−N fragment, which was cleaved by activated caspase‐1. This change was corroborated by the upregulation of cleaved caspase‐1 (c‐caspase‐1). Both compounds 28 (lysosome‐targeting) and 30 (mitochondria‐targeting) were shown to induce pyroptosis via a classic caspase‐1‐dependent pathway. However, in the case of pyroptosis induced by compound 30 with mitochondrial targeting, caspase‐3 activation was also responsible for the cleavage of gasdermin E (GSDME). Such complexity underscores the potential of differential regulatory mechanisms in pyroptosis initiated at these subcellular sites (Figure 5H). Importantly, the modular design of our experimental approach offers an advantage. It enables the preservation of a unique functional while permitting alterations of cellular localization groups. This methodology not only underscores the importance of precise molecular control in elucidating complex cellular processes, but also enhances our understanding of the role of the caspase family in pyroptosis.
To elucidate the role of the caspase family in the pyroptosis pathway initiated by compounds 27–30, we conducted caspase inhibition studies. Specifically, HeLa cells underwent pretreatment with z‐YVAD‐FMK (YVAD), a caspase‐1 inhibitor, and/or DEVD, a selective inhibitor of caspase‐3, before being exposed to these compounds (Figure 5G). Cells pretreated with YVAD exhibited a significant enhancement in resistance to cell death following exposure to compounds 28 and 30. In contrast, DEVD pretreatment afforded a protective effect specifically in response to compound 30. The concurrent administration of YVAD and DEVD yielded notable cytoprotective efficacy, substantially attenuating cytotoxicity induced by compound 30. These results indicate the critical involvement of caspase‐1 in the pyroptosis pathway triggered by compound 28 and delineate the synergistic roles of caspase‐1 and caspase‐3 in the cell death process elicited by compound 30. Conversely, in control experiments, neither inhibitor improved cell viability in the presence of compound 27, which targets lysosomes yet lacks a functional ferrocene unit, or compound 29, which targets mitochondria without incorporating a functional ferrocene unit. This lack of a protective effect from the inhibitors against the effects of compounds 27 and 29 suggests the specificity of the caspase‐mediated pyroptosis pathway for compounds equipped with a functional ferrocene unit. Our observations suggest a nuanced relationship between the molecule localization and their ability to modulate cell death pathways, emphasizing the potential for targeted therapeutic strategies in the modulation of pyroptosis.
Conclusions
The synthesis of new fluorophores with multiple functionalities has become an essential aspect in bioimaging and biomedical research. Traditional synthesis methods of such fluorophores rely on linear synthesis routes, which require extensive optimization of reaction conditions before a functional dye can be obtained. In previous work, [42] we developed ′linker′ molecules that can serve as a scaffold for connecting a biological target, a fluorophore and a functional group. Such linkers provide a new means for biolabeling, facilitating the attachment of the linker, followed by the incorporation of the fluorophore in a second step. The synthesis of these linkers was done using a scaffold‐based synthesis using the U‐4CR.
We here considered the possibility of directly integrating the fluorophore as a moiety into a functional scaffold. Based on the strategy, we obtained multi‐functional fluorophores combining three distinct moeities, i.e., a fluorophore, a signal modulator and a unit for labelling of specific cellular targets. Our approach uses the redox properties of Fc and the resulting fluorescence signal modulation for turn‐on fluorescence following Fc oxidation. Besides specific cellular localization we found that our probes are involved in a Fenton‐like reaction which can activate pyroptosis, a form of programmed cell death.
The modular synthesis approach used here can simplify the production of multi‐functional fluorophores, not only with fluorogenic character, from a common scaffold. We render it feasible to extend the range of functionalities to self‐blinking,[ 79 , 80 ] antifading (self‐healing dyes),[ 81 , 82 ] metal sensing [83] and many others.[ 84 , 85 , 86 ] This approach will thus facilitate the exploration of (bio)molecular imaging and therapeutic applications for imaging, biomedicine and beyond.
Supporting Information
The Supporting Information file encompasses comprehensive experimental details, including chemical characterizations and spectral analyses. This documentation contains all NMR and MS spectra, along with detailed descriptions of the methodologies employed in spectroscopy, microscopy, and biochemical assays. The authors have cited additional references in the methods part. [53]
Conflict of Interests
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgments
This work was funded by the National Natural Science Foundation of China (Grant Nos. 22374075 to L.Z. and 22175090 to S.J.), the Primary Research & Development Plan of the Jiangsu Province (BE2021712 to S.J.), China Postdoctoral Science Foundation (2022 M721408 to H.Y.W.), and the Jiangsu Provincial Excellent Postdoctoral Program (H.Y.W.). Fluorophore development in the lab of T.C. is supported via the German Science Foundation (Sachbeihilfe DFG/CO879‐6‐1). We thank Menghui Jia from the Materials Characterization Center and East China Normal University for support of TAS measurements and data analysis. Open Access funding enabled and organized by Project Deal. Open Access funding enabled and organized by Projekt DEAL.
Zhang L., Wang C., Li Y., Wang H., Sun K., Lu S., Wang Y., Jing S., Cordes T., Angew. Chem. Int. Ed. 2025, 64, e202415627. 10.1002/anie.202415627
Contributor Information
Dr. Lei Zhang, Email: ias_lzhang@njtech.edu.cn.
Prof. Dr. Su Jing, Email: sjing@njtech.edu.cn.
Prof. Dr. Thorben Cordes, Email: thorben.cordes@tu-dortmund.de.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Mertes N., Busch M., Huppertz M.-C., Hacker C. N., Wilhelm J., Gürth C.-M., Kühn S., Hiblot J., Koch B., Johnsson K., J. Am. Chem. Soc. 2022, 144, 6928–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li D.-H., Gamage R. S., Oliver A. G., Patel N. L., Muhammad Usama S., Kalen J. D., Schnermann M. J., Smith B. D., Angew. Chem. Int. Ed. 2023, 62, e202305062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qiao Q., Liu W., Chen J., Wu X., Deng F., Fang X., Xu N., Zhou W., Wu S., Yin W., Liu X., Xu Z., Angew. Chem. Int. Ed. 2022, 61, e202202961. [DOI] [PubMed] [Google Scholar]
- 4. Numasawa K., Hanaoka K., Saito N., Yamaguchi Y., Ikeno T., Echizen H., Yasunaga M., Komatsu T., Ueno T., Miura M., Nagano T., Urano Y., Angew. Chem. Int. Ed. 2020, 59, 6015–6020. [DOI] [PubMed] [Google Scholar]
- 5. Li H., Kim D., Yao Q., Ge H., Chung J., Fan J., Wang J., Peng X., Yoon J., Angew. Chem. Int. Ed. 2021, 60, 17268–17289. [DOI] [PubMed] [Google Scholar]
- 6. Hüll K., Morstein J., Trauner D., Chem. Rev. 2018, 118, 10710–10747. [DOI] [PubMed] [Google Scholar]
- 7. Dong M., Babalhavaeji A., Samanta S., Beharry A. A., Woolley G. A., Acc. Chem. Res. 2015, 48, 2662–2670. [DOI] [PubMed] [Google Scholar]
- 8. Schönberger M., Althaus M., Fronius M., Clauss W., Trauner D., Nat. Chem. 2014, 6, 712–719. [DOI] [PubMed] [Google Scholar]
- 9. Fujita K., Urano Y., Chem. Rev. 2024, 124, 4021–4078. [DOI] [PubMed] [Google Scholar]
- 10. Figliola C., Anton H., Sutter C., Chériaux C., Sutter A., Mazan V., Elhabiri M., Didier P., Jacquemin D., Ulrich G., ChemBioChem 2023, 24, e202300139. [DOI] [PubMed] [Google Scholar]
- 11. Sharma A., Verwilst P., Li M., Ma D., Singh N., Yoo J., Kim Y., Yang Y., Zhu J.-H., Huang H., Hu X.-L., He X.-P., Zeng L., James T. D., Peng X., Sessler J. L., Kim J. S., Chem. Rev. 2024, 124, 2699–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wen G., Leen V., Jia Y., Rohand T., Hofkens J., Chem. Eur. J. 2022, 28, e202202404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wen G., Leen V., Rohand T., Sauer M., Hofkens J., Chem. Rev. 2023, 123, 3299–3323. [DOI] [PubMed] [Google Scholar]
- 14. Celli J. P., Spring B. Q., Rizvi I., Evans C. L., Samkoe K. S., Verma S., Pogue B. W., Hasan T., Chem. Rev. 2010, 110, 2795–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang R. R., Schroeder A. B., Grudzinski J. J., Rosenthal E. L., Warram J. M., Pinchuk A. N., Eliceiri K. W., Kuo J. S., Weichert J. P., Nat. Rev. Clin. Oncol. 2017, 14, 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wender P. A., Verma V. A., Paxton T. J., Pillow T. H., Acc. Chem. Res. 2008, 41, 40–49. [DOI] [PubMed] [Google Scholar]
- 17. Ducharme G. T., LaCasse Z., Sheth T., Nesterova I. V., Nesterov E. E., Angew. Chem. Int. Ed. 2020, 59, 8440–8444. [DOI] [PubMed] [Google Scholar]
- 18. Zheng Q., Ayala A. X., Chung I., Weigel A. V., Ranjan A., Falco N., Grimm J. B., Tkachuk A. N., Wu C., Lippincott-Schwartz J., Singer R. H., Lavis L. D., ACS Cent. Sci. 2019, 5, 1602–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grimm J. B., Lavis L. D., Nat. Methods 2022, 19, 149–158. [DOI] [PubMed] [Google Scholar]
- 20. Kolmakov K., Wurm C. A., Hennig R., Rapp E., Jakobs S., Belov V. N., Hell S. W., Chem. Eur. J. 2012, 18, 12986–12998. [DOI] [PubMed] [Google Scholar]
- 21. Li H., Kim H., Xu F., Han J., Yao Q., Wang J., Pu K., Peng X., Yoon J., Chem. Soc. Rev. 2022, 51, 1795–1835. [DOI] [PubMed] [Google Scholar]
- 22. Lukinavičius G., Reymond L., Umezawa K., Sallin O., D'Este E., Göttfert F., Ta H., Hell S. W., Urano Y., Johnsson K., J. Am. Chem. Soc. 2016, 138, 9365–9368. [DOI] [PubMed] [Google Scholar]
- 23. Wang X., Ding Q., Groleau R. R., Wu L., Mao Y., Che F., Kotova O., Scanlan E. M., Lewis S. E., Li P., Tang B., James T. D., Gunnlaugsson T., Chem. Rev. 2024, 124, 7106–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Usama S. M., Inagaki F., Kobayashi H., Schnermann M. J., J. Am. Chem. Soc. 2021, 143, 5674–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He Y., Wang S., Yu P., Yan K., Ming J., Yao C., He Z., El-Toni A. M., Khan A., Zhu X., Sun C., Lei Z., Zhang F., Chem. Sc. 2021, 12, 10474–10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X., Chen Y., He H., Wang S., Lei Z., Zhang F., Angew. Chem. Int. Ed. 2021, 60, 26337–26341. [DOI] [PubMed] [Google Scholar]
- 27. Yuan L., Wang L., Agrawalla B. K., Park S.-J., Zhu H., Sivaraman B., Peng J., Xu Q.-H., Chang Y.-T., J. Am. Chem. Soc. 2015, 137, 5930–5938. [DOI] [PubMed] [Google Scholar]
- 28. Hirano T., Kikuchi K., Urano Y., Higuchi T., Nagano T., J. Am. Chem. Soc. 2000, 122, 12399–12400. [Google Scholar]
- 29. Hirano T., Kikuchi K., Urano Y., Nagano T., J. Am. Chem. Soc. 2002, 124, 6555–6562. [DOI] [PubMed] [Google Scholar]
- 30. Volkov A., Mi J., Lalit K., Chatterjee P., Jing D., Carnahan S. L., Chen Y., Sun S., Rossini A. J., Huang W., Stanley L. M., J. Am. Chem. Soc. 2023, 145, 6230–6239. [DOI] [PubMed] [Google Scholar]
- 31. Levi L., Müller T. J. J., Chem. Soc. Rev. 2016, 45, 2825–2846. [DOI] [PubMed] [Google Scholar]
- 32. Rocha R. O., Rodrigues M. O., Neto B. A. D., ACS Omega 2020, 5, 972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bay S., Makhloufi G., Janiak C., Müller T. J. J., Beilstein J. Org. Chem. 2014, 10, 1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brandner L., Müller T. J. J., Front. Chem. 2023, 11, 1124209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuriki Y., Sogawa M., Komatsu T., Kawatani M., Fujioka H., Fujita K., Ueno T., Hanaoka K., Kojima R., Hino R., Ueo H., Ueo H., Kamiya M., Urano Y., J. Am. Chem. Soc. 2024, 146, 521–531. [DOI] [PubMed] [Google Scholar]
- 36. de Moliner F., Kielland N., Lavilla R., Vendrell M., Angew. Chem. Int. Ed. 2017, 56, 3758–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ugi I., Angew. Chem. Int. Ed. 1959, 71, 386–386. [Google Scholar]
- 38. Kunig V. B. K., Potowski M., Akbarzadeh M., Klika Škopić M., dos Santos Smith D., Arendt L., Dormuth I., Adihou H., Andlovic B., Karatas H., Shaabani S., Zarganes-Tzitzikas T., Neochoritis C. G., Zhang R., Groves M., Guéret S. M., Ottmann C., Rahnenführer J., Fried R., Dömling A., Brunschweiger A., Angew. Chem. Int. Ed. 2020, 59, 20338–20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burchak O. N., Mugherli L., Ostuni M., Lacapère J. J., Balakirev M. Y., J. Am. Chem. Soc. 2011, 133, 10058–10061. [DOI] [PubMed] [Google Scholar]
- 40. Brauch S., Henze M., Osswald B., Naumann K., Wessjohann L. A., van Berkel S. S., Westermann B., Org. Biomol. Chem. 2012, 10, 958–965. [DOI] [PubMed] [Google Scholar]
- 41. Vázquez-Romero A., Kielland N., Arévalo M. J., Preciado S., Mellanby R. J., Feng Y., Lavilla R., Vendrell M., J. Am. Chem. Soc. 2013, 135, 16018–16021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang L., Isselstein M., Köhler J., Eleftheriadis N., Huisjes N. M., Guirao-Ortiz M., Narducci A., Smit J. H., Stoffels J., Harz H., Leonhardt H., Herrmann A., Cordes T., Angew. Chem. Int. Ed. 2022, 61, e202112959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reguera L., Méndez Y., Humpierre A. R., Valdés O., Rivera D. G., Acc. Chem. Res. 2018, 51, 1475–1486. [DOI] [PubMed] [Google Scholar]
- 44. Patra M., Gasser G., Nat. Chem. Rev. 2017, 1, 0066. [Google Scholar]
- 45. Zhang H., Hu J., Qu D., Org. Lett. 2012, 14, 2334–2337. [DOI] [PubMed] [Google Scholar]
- 46. Wu G., Liu F., Li N., Fu Q., Wang C., Yang S., Xiao H., Tang L., Wang F., Zhou W., Wang W., Kang Q., Li Z., Lin N., Wu Y., Chen G., Tan X., Yang Q., Adv. Sci. 2023, 10, 2304104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Karmakar M., Bhatta S. R., Giri S., Thakur A., Inorg. Chem. 2020, 59, 4493–4507. [DOI] [PubMed] [Google Scholar]
- 48. Zhang R., Wang Z., Wu Y., Fu H., Yao J., Org. Lett. 2008, 10, 3065–3068. [DOI] [PubMed] [Google Scholar]
- 49. Li M., Guo Z., Zhu W., Marken F., James T. D., Chem. Commun. 2015, 51, 1293–1296. [DOI] [PubMed] [Google Scholar]
- 50. Tang Z., Liu Y., He M., Bu W., Angew. Chem. Int. Ed. 2019, 58, 946–956. [DOI] [PubMed] [Google Scholar]
- 51. Cao C., Wang X., Yang N., Song X., Dong X., Chem. Sci. 2022, 13, 863–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang T., Ménard-Moyon C., Bianco A., Chem. Soc. Rev. 2022, 51, 3535–3560. [DOI] [PubMed] [Google Scholar]
- 53. Kim H., Shin M., Han S., Kwon W., Hahn S. K., Biomacromolecules 2019, 20, 2889–2903. [DOI] [PubMed] [Google Scholar]
- 54. Shchepinova M. M., Cairns A. G., Prime T. A., Logan A., James A. M., Hall A. R., Vidoni S., Arndt S., Caldwell S. T., Prag H. A., Pell V. R., Krieg T., Mulvey J. F., Yadav P., Cobley J. N., Bright T. P., Senn H. M., Anderson R. F., Murphy M. P., Hartley R. C., Cell Chem. Biol. 2017, 24, 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fukuzumi S., Yoshida Y., Okamoto K., Imahori H., Araki Y., Ito O., J. Am. Chem. Soc. 2002, 124, 6794–6795. [DOI] [PubMed] [Google Scholar]
- 56. Martínez R., Ratera I., Tárraga A., Molina P., Veciana J., Chem. Commun. 2006, 3809–3811. [DOI] [PubMed] [Google Scholar]
- 57. Liu J.-Y., El-Khouly M. E., Fukuzumi S., Ng D. K. P., ChemPhysChem 2012, 13, 2030–2036. [DOI] [PubMed] [Google Scholar]
- 58. Escudero D., Acc. Chem. Res. 2016, 49, 1816–1824. [DOI] [PubMed] [Google Scholar]
- 59. Chi W., Chen J., Liu W., Wang C., Qi Q., Qiao Q., Tan T. M., Xiong K., Liu X., Kang K., Chang Y.-T., Xu Z., Liu X., J. Am. Chem. Soc. 2020, 142, 6777–6785. [DOI] [PubMed] [Google Scholar]
- 60. Li S., Zhang H., Lu R., Yu A., Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 184, 204–210. [DOI] [PubMed] [Google Scholar]
- 61. Zhang X., Photochem. Photobiol. Sci. 2010, 9, 1261–1268. [DOI] [PubMed] [Google Scholar]
- 62. Ramakrishna G., Ghosh H. N., J. Phys. Chem. B 2001, 105, 7000–7008. [Google Scholar]
- 63. Sjöback R., Nygren J., Kubista M., Spectrochim. Acta A Mol. Biomol. Spectrosc. 1995, 51, 7–21. [Google Scholar]
- 64. Hanczyc P., Mikhailovsky A., Boyer D. R., Sawaya M. R., Heeger A., Eisenberg D., J. Phys. Chem. B 2018, 122, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Martin M. M., Lindqvist L., J. Lumin. 1975, 10, 381–390. [Google Scholar]
- 66. Weller A., Pure Appl. Chem. 1968, 16, 115–124. [Google Scholar]
- 67. Ranji-Burachaloo H., Gurr P. A., Dunstan D. E., Qiao G. G., ACS Nano 2018, 12, 11819–11837. [DOI] [PubMed] [Google Scholar]
- 68. Cloonan S. M., Elmes R. B. P., Erby M., Bright S. A., Poynton F. E., Nolan D. E., Quinn S. J., Gunnlaugsson T., Williams D. C., J. Med. Chem. 2015, 58, 4494–4505. [DOI] [PubMed] [Google Scholar]
- 69. Poole L. B., Free Radical Biol. Med. 2015, 80, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xu R., Yang J., Qian Y., Deng H., Wang Z., Ma S., Wei Y., Yang N., Shen Q., Nanoscale Horiz. 2021, 6, 348–356. [DOI] [PubMed] [Google Scholar]
- 71. Tang Z., Zhao P., Wang H., Liu Y., Bu W., Chem. Rev. 2021, 121, 1981–2019. [DOI] [PubMed] [Google Scholar]
- 72. Zhou J., Zhu X., Cheng Q., Wang Y., Wang R., Cheng X., Xu J., Liu K., Li L., Li X., He M., Wang J., Xu H., Jing S., Huang L., Inorg. Chem. 2020, 59, 9177–9187. [DOI] [PubMed] [Google Scholar]
- 73. Kapuscinski J., Biotech. Histochem. 1995, 70, 220–233. [DOI] [PubMed] [Google Scholar]
- 74. Wu M., Liu X., Chen H., Duan Y., Liu J., Pan Y., Liu B., Angew. Chem. Int. Ed. 2021, 60, 9093–9098. [DOI] [PubMed] [Google Scholar]
- 75. Sborgi L., Rühl S., Mulvihill E., Pipercevic J., Heilig R., Stahlberg H., Farady C. J., Müller D. J., Broz P., Hiller S., EMBO J. 2016, 35, 1766–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yi Z., Qin X., Zhang L., Chen H., Song T., Luo Z., Wang T., Lau J., Wu Y., Toh T. B., Lee C.-S., Bu W., Liu X., J. Am. Chem. Soc. 2024, 146, 9413–9421. [DOI] [PubMed] [Google Scholar]
- 77. Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F., Nature 2015, 526, 660–665. [DOI] [PubMed] [Google Scholar]
- 78. Wang K., Sun Q., Zhong X., Zeng M., Zeng H., Shi X., Li Z., Wang Y., Zhao Q., Shao F., Ding J., Cell 2020, 180, 941–955. [DOI] [PubMed] [Google Scholar]
- 79. Lardon N., Wang L., Tschanz A., Hoess P., Tran M., D'Este E., Ries J., Johnsson K., J. Am. Chem. Soc. 2021, 143, 14592–14600. [DOI] [PubMed] [Google Scholar]
- 80. Uno S.-N., Kamiya M., Yoshihara T., Sugawara K., Okabe K., Tarhan M. C., Fujita H., Funatsu T., Okada Y., Tobita S., Urano Y., Nat. Chem. 2014, 6, 681–689. [DOI] [PubMed] [Google Scholar]
- 81. Isselstein M., Zhang L., Glembockyte V., Brix O., Cosa G., Tinnefeld P., Cordes T., J. Phys. Chem. Lett. 2020, 11, 4462–4480. [DOI] [PubMed] [Google Scholar]
- 82. Pati A. K., El Bakouri O., Jockusch S., Zhou Z., Altman R. B., Fitzgerald G. A., Asher W. B., Terry D. S., Borgia A., Holsey M. D., Batchelder J. E., Abeywickrama C., Huddle B., Rufa D., Javitch J. A., Ottosson H., Blanchard S. C., Proc. Natl. Acad. Sci. USA 2020, 117, 24305–24315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schwering M., Kiel A., Kurz A., Lymperopoulos K., Sprödefeld A., Krämer R., Herten D.-P., Angew. Chem. Int. Ed. 2011, 50, 2940–2945. [DOI] [PubMed] [Google Scholar]
- 84. Deen J., Vranken C., Leen V., Neely R. K., Janssen K. P. F., Hofkens J., Angew. Chem. Int. Ed. 2017, 56, 5182–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lukinavičius G., Mitronova G. Y., Schnorrenberg S., Butkevich A. N., Barthel H., Belov V. N., Hell S. W., Chem. Sci. 2018, 9, 3324–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Eiring P., McLaughlin R., Matikonda S. S., Han Z., Grabenhorst L., Helmerich D. A., Meub M., Beliu G., Luciano M., Bandi V., Zijlstra N., Shi Z.-D., Tarasov S. G., Swenson R., Tinnefeld P., Glembockyte V., Cordes T., Sauer M., Schnermann M. J., Angew. Chem. Int. Ed. 2021, 60, 26685–26693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.