Abstract
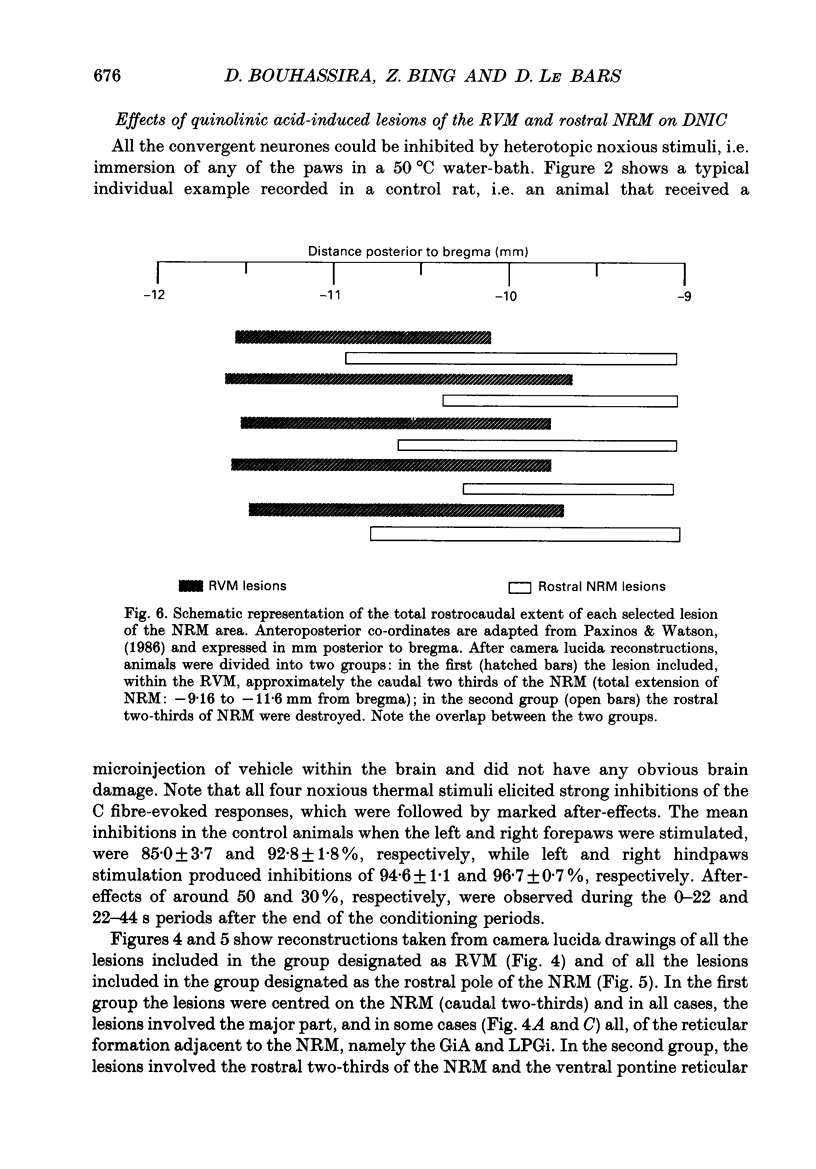
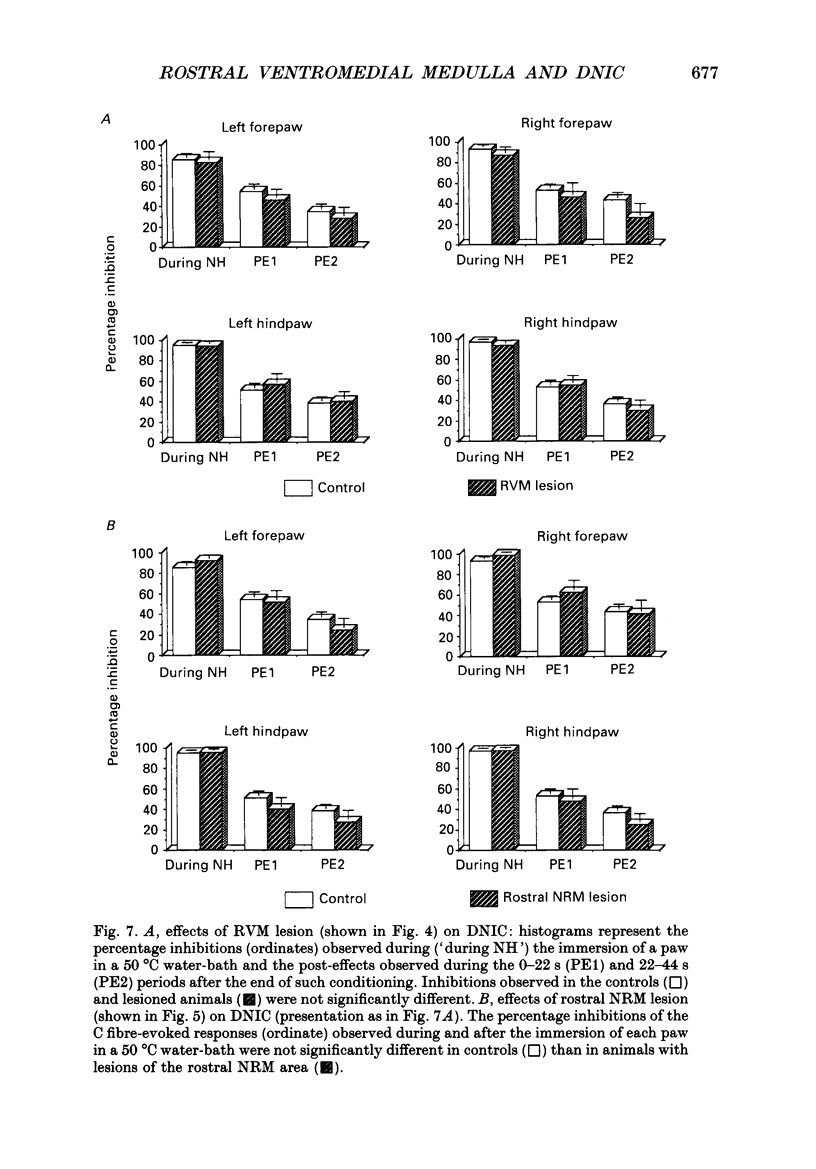
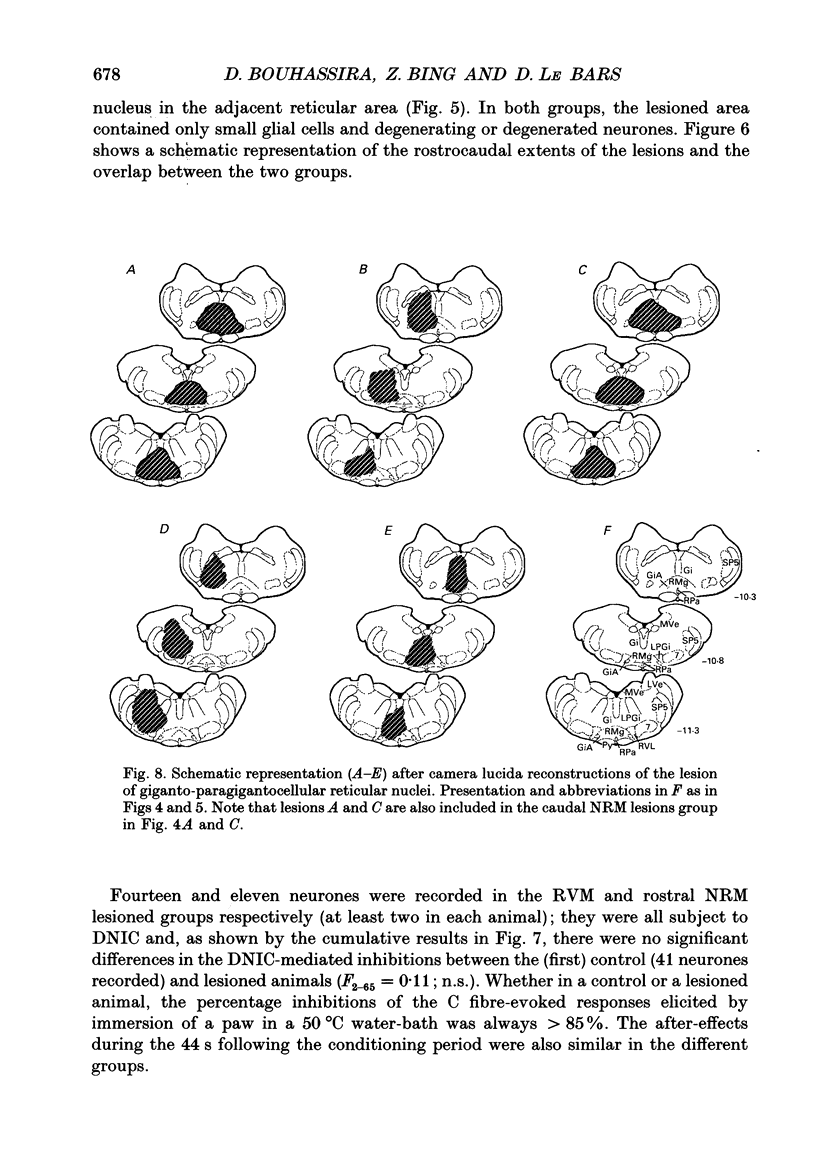
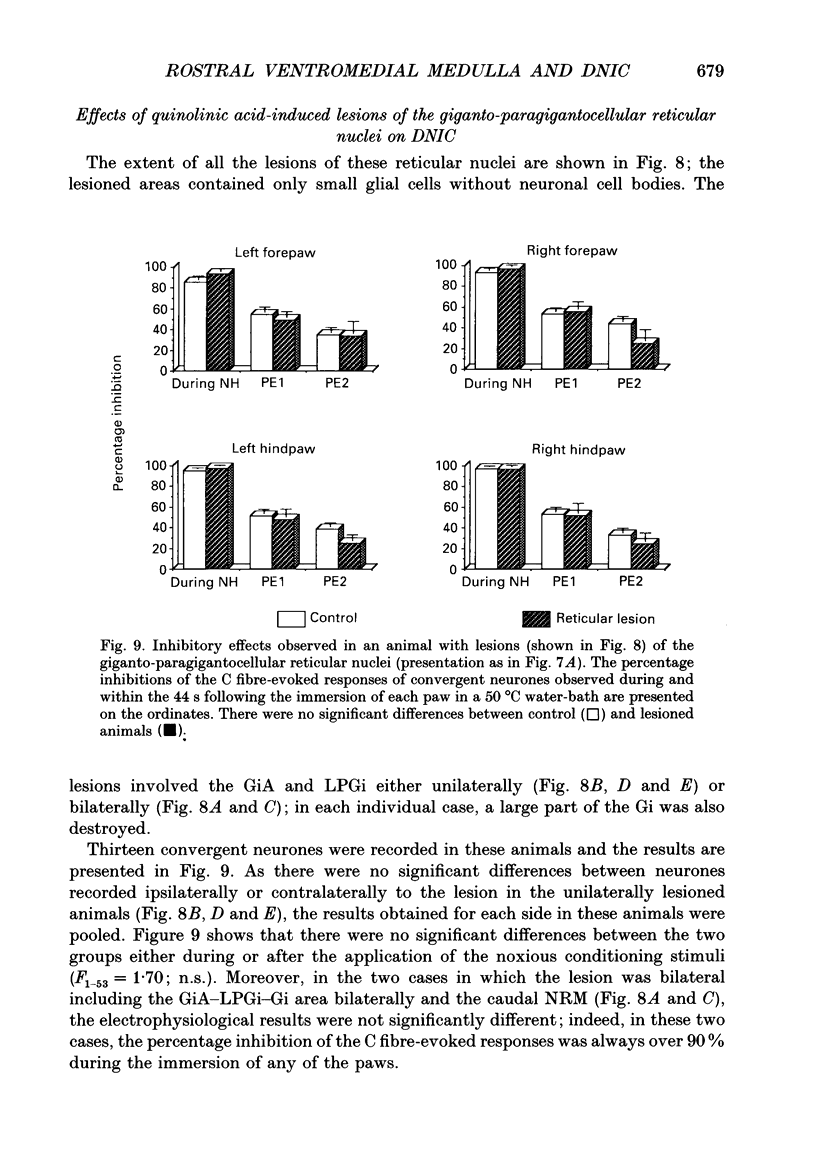
1. Previous electrophysiological, pharmacological and anatomical evidence had suggested a possible participation of rostral ventromedial medulla (RVM) in the supraspinal part of the loop underlying diffuse noxious inhibitory controls (DNICs). In order to test this hypothesis, two experimental series were performed during which DNICs were compared in control sham-operated rats and rats with lesions of the RVM and of an adjacent candidate for such a role, the nucleus gigantocellularis reticularis (Gi). 2. In the first experimental series, lesions were induced, in anaesthetized animals, by injections of quinolinic acid (0.3-0.8 microliter of a 360 nmol/microliter solution) into the RVM or Gi. In the control animals (n = 10), the vehicle alone (artificial cerebrospinal fluid) was injected. Histological lesion reconstructions were performed after each electrophysiological experiment. Three groups of animals were considered: in the first group (n = 5), the lesion was centered on the RVM, including the two caudal thirds of nucleus raphe magnus (NRM) and adjacent reticular areas; in the second group (n = 5), the lesions extended more rostrally and involved the rostral pole of NRM; in the third group (n = 5), the lesion extended more laterally and dorsally and included nucleus reticularis gigantocellularis pars alpha (GiA), nucleus reticularis paragigantocellularis lateralis (LPGi) and the Gi. In each case, all the neuronal cell bodies within the lesioned area were destroyed. 3. In the second experimental series, electrolytic lesions of the total rostrocaudal extent of the NRM (n = 5) were induced, in anesthetized animals, by passing cathodal current (5 mA, 8 s). In the control animals (n = 5), the electrode was lowered but current was not applied. 4. One week after lesioning, the animals were anaesthetized, paralysed, artificially ventilated and recordings were made from convergent neurones in trigeminal nucleus caudalis. These neurones gave responses due to activation of A and C fibres when percutaneous electrical stimuli were applied to their receptive fields. DNICs were triggered by immersion of each paw in a 50 degrees C water-bath. Both the general properties of the convergent neurones and the inhibitions of the C fibre-evoked responses produced by these heterotopic noxious stimuli were compared in the different groups of animals. 5. The sizes of receptive field, spontaneous activities, thresholds for C fibre-evoked responses and responses to C fibre activation were not different in the control and lesioned animals. The percentage inhibitions of the C fibre-evoked responses both during and in the 44s following the conditioning periods were also very similar in the different groups of animals.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



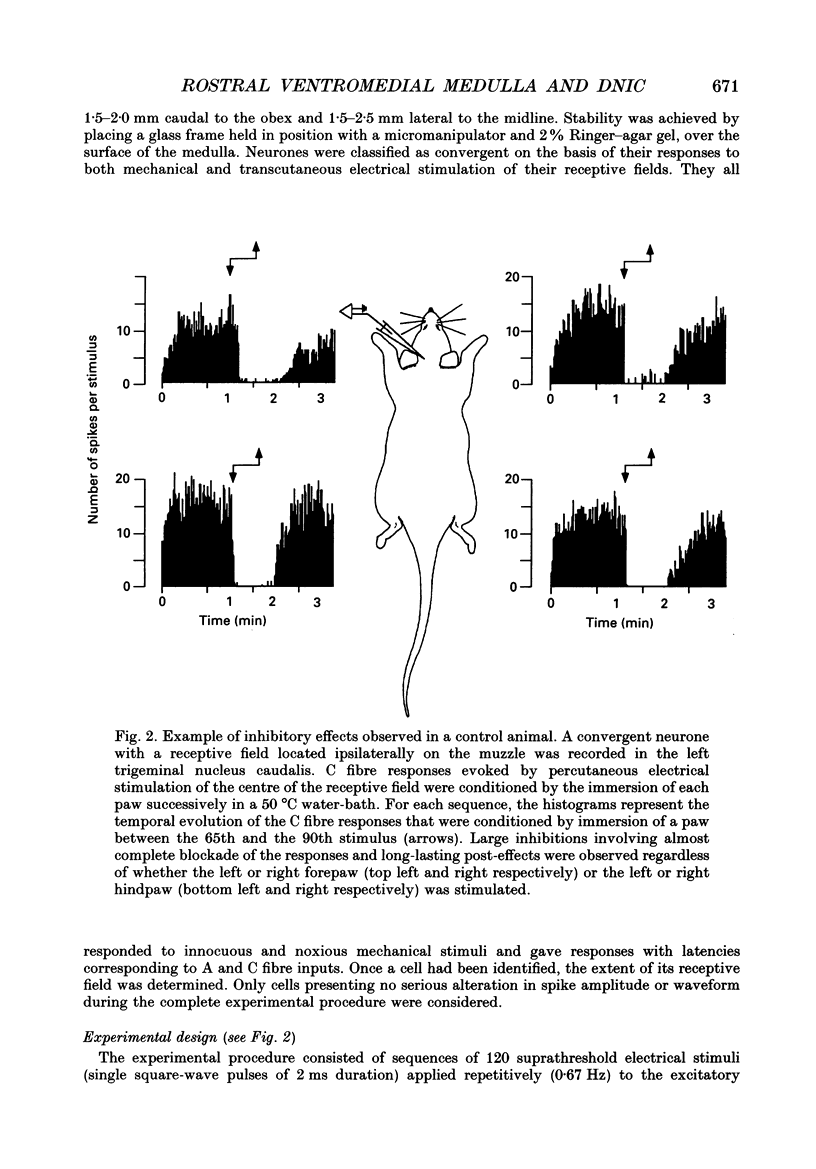

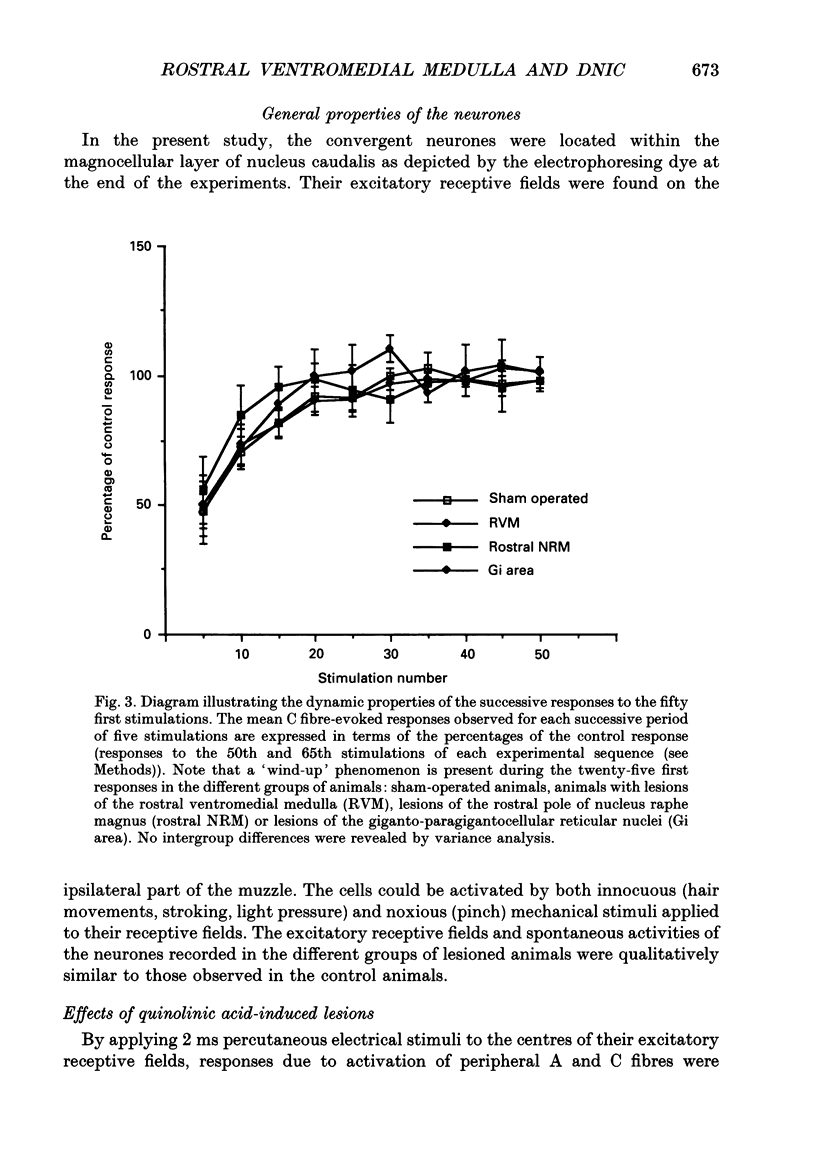
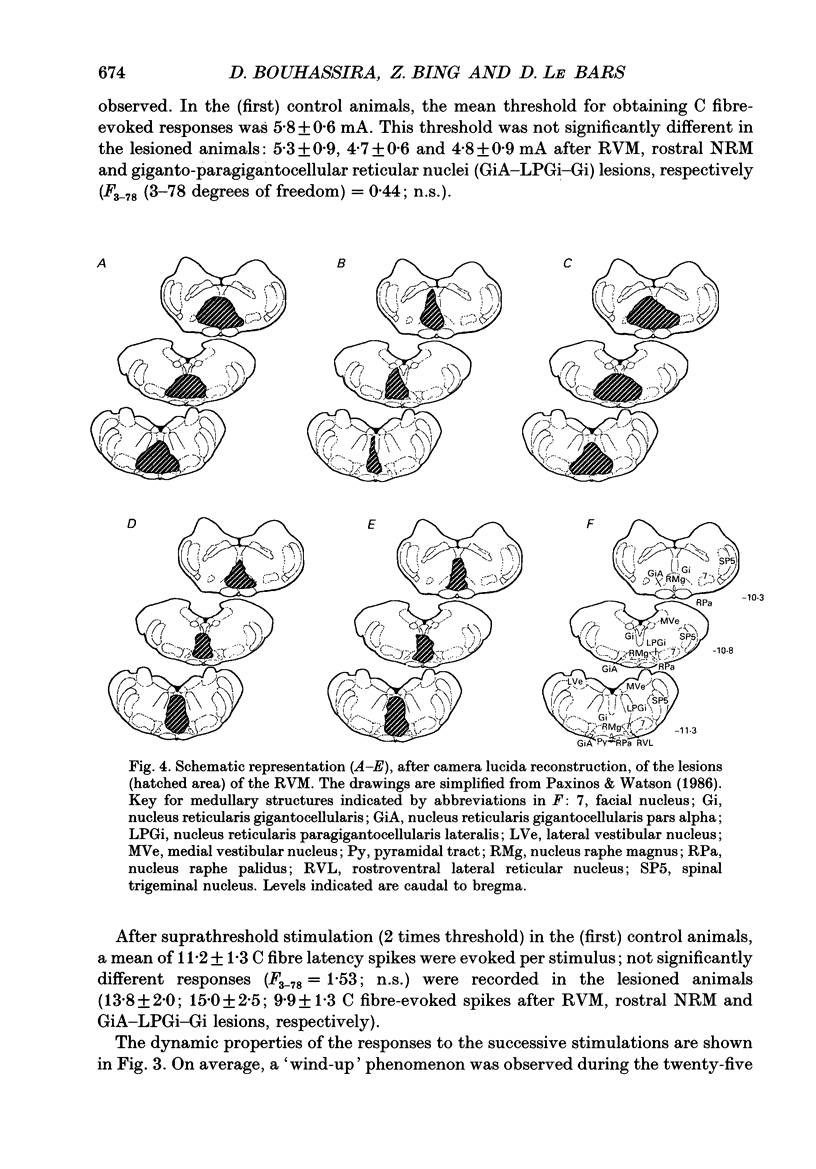
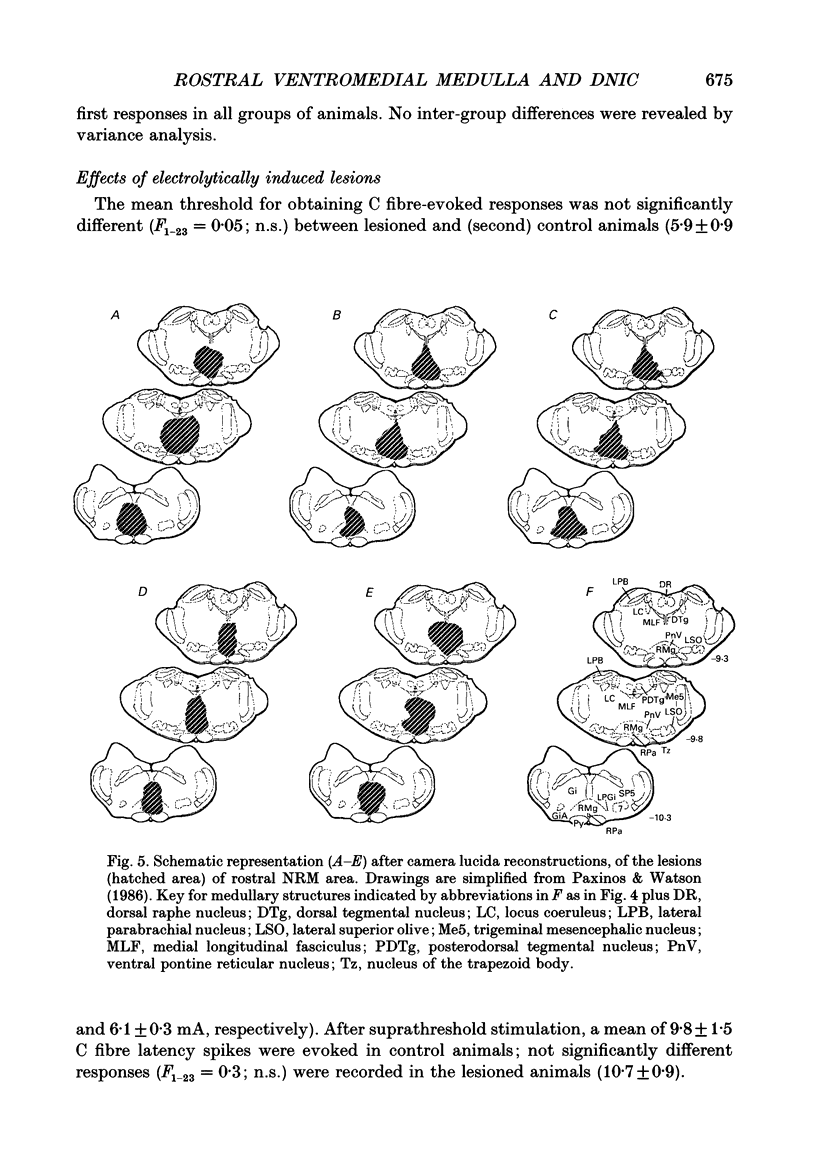
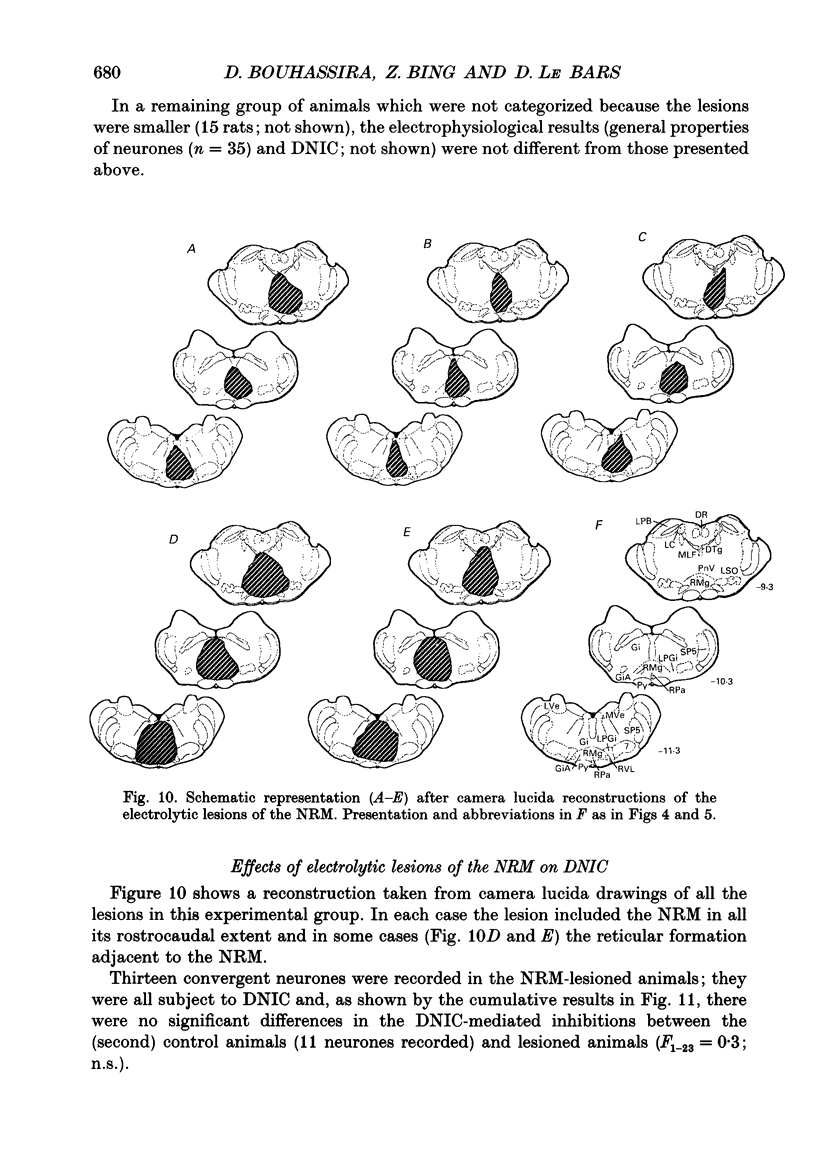
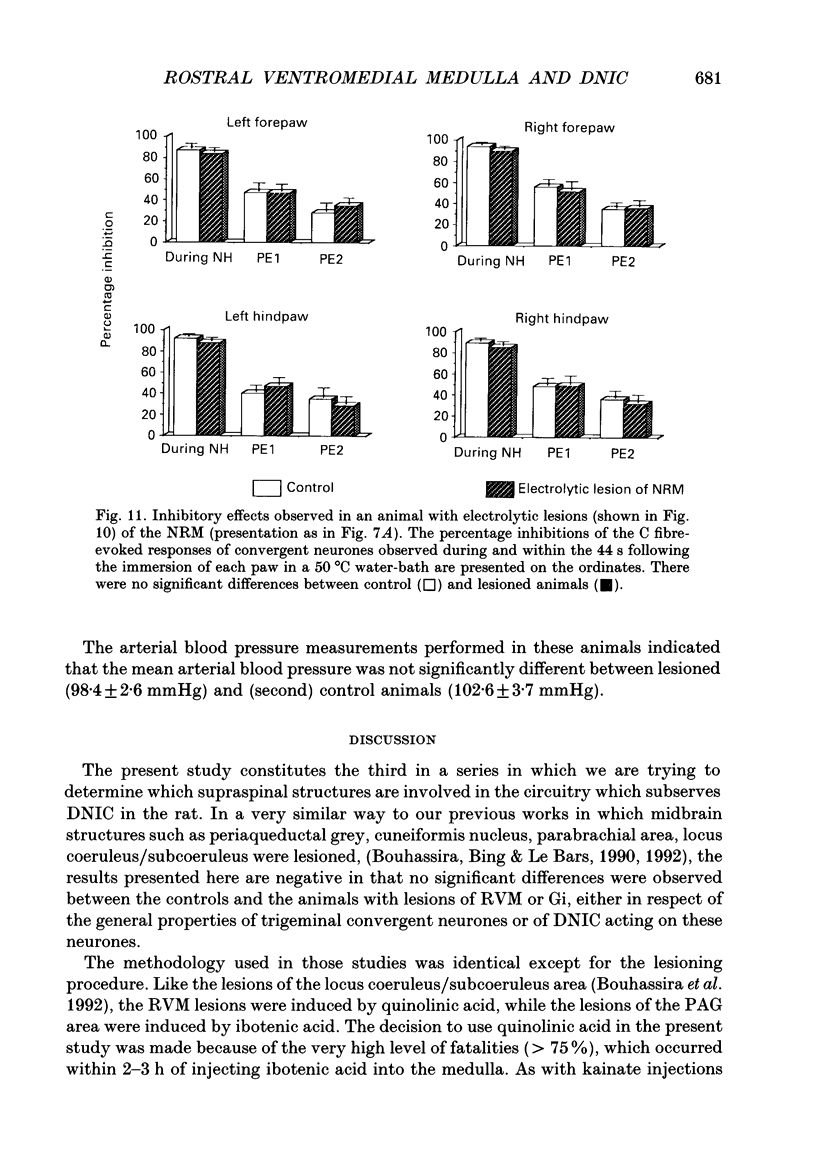






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott F. V., Melzack R. Brainstem lesions dissociate neural mechanisms of morphine analgesia in different kinds of pain. Brain Res. 1982 Nov 11;251(1):149–155. doi: 10.1016/0006-8993(82)91282-3. [DOI] [PubMed] [Google Scholar]
- Abbott F. V., Melzack R., Samuel C. Morphine analgesia in tail-flick and formalin pain tests is mediated by different neural systems. Exp Neurol. 1982 Mar;75(3):644–651. doi: 10.1016/0014-4886(82)90031-0. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Clanton C. H., Fields H. L. Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp Neurol. 1978 Mar 15;178(2):209–224. doi: 10.1002/cne.901780203. [DOI] [PubMed] [Google Scholar]
- Bernard J. F., Villanueva L., Carroué J., Le Bars D. Efferent projections from the subnucleus reticularis dorsalis (SRD): a Phaseolus vulgaris leucoagglutinin study in the rat. Neurosci Lett. 1990 Aug 24;116(3):257–262. doi: 10.1016/0304-3940(90)90083-l. [DOI] [PubMed] [Google Scholar]
- Besson J. M., Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987 Jan;67(1):67–186. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- Bing Z., Villanueva L., Le Bars D. Ascending pathways in the spinal cord involved in the activation of subnucleus reticularis dorsalis neurons in the medulla of the rat. J Neurophysiol. 1990 Mar;63(3):424–438. doi: 10.1152/jn.1990.63.3.424. [DOI] [PubMed] [Google Scholar]
- Bouhassira D., Bing Z., Le Bars D. Effects of lesions of locus coeruleus/subcoeruleus on diffuse noxious inhibitory controls in the rat. Brain Res. 1992 Jan 31;571(1):140–144. doi: 10.1016/0006-8993(92)90520-j. [DOI] [PubMed] [Google Scholar]
- Bouhassira D., Bing Z., Le Bars D. Studies of the brain structures involved in diffuse noxious inhibitory controls: the mesencephalon. J Neurophysiol. 1990 Dec;64(6):1712–1723. doi: 10.1152/jn.1990.64.6.1712. [DOI] [PubMed] [Google Scholar]
- Bouhassira D., Villanueva L., Le Bars D. Effects of systemic morphine on diffuse noxious inhibitory controls: role of the periaqueductal grey. Eur J Pharmacol. 1992 Jun 5;216(2):149–156. doi: 10.1016/0014-2999(92)90355-8. [DOI] [PubMed] [Google Scholar]
- Bouhassira D., Villanueva L., Le Bars D. Intracerebroventricular morphine decreases descending inhibitions acting on lumbar dorsal horn neuronal activities related to pain in the rat. J Pharmacol Exp Ther. 1988 Oct;247(1):332–342. [PubMed] [Google Scholar]
- Carstens E. Inhibition of spinal dorsal horn neuronal responses to noxious skin heating by medial hypothalamic stimulation in the cat. J Neurophysiol. 1982 Sep;48(3):808–822. doi: 10.1152/jn.1982.48.3.808. [DOI] [PubMed] [Google Scholar]
- Casey K. L., Morrow T. J. Effect of medial bulboreticular and raphe nuclear lesions on the excitation and modulation of supraspinal nocifensive behaviors in the cat. Brain Res. 1989 Oct 30;501(1):150–161. doi: 10.1016/0006-8993(89)91036-6. [DOI] [PubMed] [Google Scholar]
- Casey K. L. Somatosensory responses of bulboreticular units in awake cat: relation to escape-producing stimuli. Science. 1971 Jul 2;173(3991):77–80. doi: 10.1126/science.173.3991.77. [DOI] [PubMed] [Google Scholar]
- Chan S. H., Chong A. P., Ong B. T., Chong O. K., Lim H. C. Pulmonary edema as a cause for fulminant death following microinjection of kainic acid into the rat medulla oblongata. Neurosci Lett. 1985 Dec 18;62(3):341–346. doi: 10.1016/0304-3940(85)90572-5. [DOI] [PubMed] [Google Scholar]
- Chance W. T., Krynock G. M., Rosecrans J. A. Effects of medial raphe and raphe magnus lesions on the analgesic activity of morphine and methadone. Psychopharmacology (Berl) 1978 Mar 1;56(2):133–137. doi: 10.1007/BF00431838. [DOI] [PubMed] [Google Scholar]
- Craig A. D., Jr, Burton H. Spinal and medullary lamina I projection to nucleus submedius in medial thalamus: a possible pain center. J Neurophysiol. 1981 Mar;45(3):443–466. doi: 10.1152/jn.1981.45.3.443. [DOI] [PubMed] [Google Scholar]
- Dostrovsky J. O., Shah Y., Gray B. G. Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus, and adjacent reticular formation. II. Effects on medullary dorsal horn nociceptive and nonnociceptive neurons. J Neurophysiol. 1983 Apr;49(4):948–960. doi: 10.1152/jn.1983.49.4.948. [DOI] [PubMed] [Google Scholar]
- Fields H. L., Heinricher M. M., Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Giesler G. J., Jr, Yezierski R. P., Gerhart K. D., Willis W. D. Spinothalamic tract neurons that project to medial and/or lateral thalamic nuclei: evidence for a physiologically novel population of spinal cord neurons. J Neurophysiol. 1981 Dec;46(6):1285–1308. doi: 10.1152/jn.1981.46.6.1285. [DOI] [PubMed] [Google Scholar]
- Goldman P. L., Collins W. F., Taub A., Fitzmartin J. Evoked bulbar reticular unit activity following delta fiber stimulation of peripheral somatosensory nerve in cat. Exp Neurol. 1972 Dec;37(3):597–606. doi: 10.1016/0014-4886(72)90102-1. [DOI] [PubMed] [Google Scholar]
- Gray B. G., Dostrovsky J. O. Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus, and adjacent reticular formation. I. Effects on lumbar spinal cord nociceptive and nonnociceptive neurons. J Neurophysiol. 1983 Apr;49(4):932–947. doi: 10.1152/jn.1983.49.4.932. [DOI] [PubMed] [Google Scholar]
- Guldin W. O., Markowitsch H. J. No detectable remote lesions following massive intrastriatal injections of ibotenic acid. Brain Res. 1981 Nov 30;225(2):446–451. doi: 10.1016/0006-8993(81)90852-0. [DOI] [PubMed] [Google Scholar]
- Hall J. G., Duggan A. W., Johnson S. M., Morton C. R. Medullary raphé lesions do not reduce descending inhibition of dorsal horn neurones of the cat. Neurosci Lett. 1981 Aug 7;25(1):25–29. doi: 10.1016/0304-3940(81)90095-1. [DOI] [PubMed] [Google Scholar]
- Halpern B. P., Halverson J. D. Modification of escape from noxious stimuli after bulbar reticular formation lesions. Behav Biol. 1974 Jun;11(2):215–229. doi: 10.1016/s0091-6773(74)90385-x. [DOI] [PubMed] [Google Scholar]
- Köhler C., Schwarcz R., Fuxe K. Hippocampal lesions indicate differences between the excitotoxic properties of acidic amono acids. Brain Res. 1979 Oct 19;175(2):366–371. doi: 10.1016/0006-8993(79)91018-7. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Villanueva L. Electrophysiological evidence for the activation of descending inhibitory controls by nociceptive afferent pathways. Prog Brain Res. 1988;77:275–299. doi: 10.1016/s0079-6123(08)62795-8. [DOI] [PubMed] [Google Scholar]
- Lumb B. M. Hypothalamic influences on viscero-somatic neurones in the lower thoracic spinal cord of the anaesthetized rat. J Physiol. 1990 May;424:427–444. doi: 10.1113/jphysiol.1990.sp018075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K., Ohta Y., Mori S. Ascending and descending projections of the nucleus reticularis gigantocellularis in the cat demonstrated by the anterograde neural tracer, Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res. 1988 Sep 13;460(1):124–141. doi: 10.1016/0006-8993(88)91212-7. [DOI] [PubMed] [Google Scholar]
- Mohrland J. S., McManus D. Q., Gebhart G. F. Lesions in nucleus reticularis gigantocellularis: effect on the antinociception produced by micro-injection of morphine and focal electrical stimulation in the periaqueductal gray matter. Brain Res. 1982 Jan 7;231(1):143–152. doi: 10.1016/0006-8993(82)90014-2. [DOI] [PubMed] [Google Scholar]
- Mokha S. S., Goldsmith G. E., Hellon R. F., Puri R. Hypothalamic control of nocireceptive and other neurons in the marginal layer of the dorsal horn of the medulla (trigeminal nucleus caudalis) in the rat. Exp Brain Res. 1987;65(2):427–436. doi: 10.1007/BF00236316. [DOI] [PubMed] [Google Scholar]
- Pretel S., Guinan M. J., Carstens E. Inhibition of the responses of cat dorsal horn neurons to noxious skin heating by stimulation in medial or lateral medullary reticular formation. Exp Brain Res. 1988;72(1):51–62. doi: 10.1007/BF00248500. [DOI] [PubMed] [Google Scholar]
- Proudfit H. K., Anderson E. G. Morphine analgesia: blockade by raphe magnus lesions. Brain Res. 1975 Nov 21;98(3):612–618. doi: 10.1016/0006-8993(75)90380-7. [DOI] [PubMed] [Google Scholar]
- Proudfit H. K. Reversible inactivation of raphe magnus neurons: effects on nociceptive threshold and morphine-induced analgesia. Brain Res. 1980 Nov 17;201(2):459–464. doi: 10.1016/0006-8993(80)91053-7. [DOI] [PubMed] [Google Scholar]
- Satoh M., Akaike A., Nakazawa T., Takagi H. Evidence for involvement of separate mechanisms in the production of analgesia by electrical stimulation of the nucleus reticularis paragigantocellularis and nucleus raphe magnus in the rat. Brain Res. 1980 Aug 4;194(2):525–529. doi: 10.1016/0006-8993(80)91236-6. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Whetsell W. O., Jr, Mangano R. M. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983 Jan 21;219(4582):316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Villanueva L., Bing Z., Bouhassira D., Le Bars D. Encoding of electrical, thermal, and mechanical noxious stimuli by subnucleus reticularis dorsalis neurons in the rat medulla. J Neurophysiol. 1989 Feb;61(2):391–402. doi: 10.1152/jn.1989.61.2.391. [DOI] [PubMed] [Google Scholar]
- Villanueva L., Bouhassira D., Bing Z., Le Bars D. Convergence of heterotopic nociceptive information onto subnucleus reticularis dorsalis neurons in the rat medulla. J Neurophysiol. 1988 Sep;60(3):980–1009. doi: 10.1152/jn.1988.60.3.980. [DOI] [PubMed] [Google Scholar]
- Villanueva L., Peschanski M., Calvino B., Le Bars D. Ascending pathways in the spinal cord involved in triggering of diffuse noxious inhibitory controls in the rat. J Neurophysiol. 1986 Jan;55(1):34–55. doi: 10.1152/jn.1986.55.1.34. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Plant R. L., Rudy T. A. Studies on the antagonism by raphe lesions of the antinociceptive action of systemic morphine. Eur J Pharmacol. 1977 Feb 21;41(4):399–408. doi: 10.1016/0014-2999(77)90260-6. [DOI] [PubMed] [Google Scholar]
- Yezierski R. P. Effects of midbrain and medullary stimulation on spinomesencephalic tract cells in the cat. J Neurophysiol. 1990 Feb;63(2):240–255. doi: 10.1152/jn.1990.63.2.240. [DOI] [PubMed] [Google Scholar]
- Zemlan F. P., Behbehani M. M., Beckstead R. M. Ascending and descending projections from nucleus reticularis magnocellularis and nucleus reticularis gigantocellularis: an autoradiographic and horseradish peroxidase study in the rat. Brain Res. 1984 Feb 6;292(2):207–220. doi: 10.1016/0006-8993(84)90757-1. [DOI] [PubMed] [Google Scholar]
- Zorman G., Hentall I. D., Adams J. E., Fields H. L. Naloxone-reversible analgesia produced by microstimulation in the rat medulla. Brain Res. 1981 Aug 24;219(1):137–148. doi: 10.1016/0006-8993(81)90273-0. [DOI] [PubMed] [Google Scholar]



