Abstract
Background and aims
We investigated associations between body mass index (BMI) and hepatocellular carcinoma (HCC) in patients with hepatitis B (HBV) C (HCV) virus infection, alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), and liver cirrhosis (LC).
Methods
We followed 350,608 Korean patients with liver disease who underwent routine health examinations from 2003–2006 until December 2018 via national hospital discharge records. Multivariable adjusted hazard ratios (HRs) per 5-kg/m2 BMI increase (BMI ≥25 kg/m2) for HCC risk were calculated using Cox models. HCC developed in 17,752 patients.
Results
The HRs (95% CI) were 1.17 (1.06–1.28), 1.08 (0.87–1.34), 1.34 (1.14–1.58), 1.51 (1.17–1.94), and 1.11 (1.00–1.23) for HBV, HCV, ALD, NAFLD, and LC, respectively. The HRs for HBV were 1.45 (1.23–1.70) and 1.06 (0.95–1.19) in women and men, respectively; the corresponding HRs for LC were 1.27 (1.07–1.50) and 1.02 (0.90–1.16), respectively. In patients <65 years old with HBV, HCV, and NAFLD, the HRs were 1.17 (1.07–1.29), 1.33 (1.03–1.73), and 1.20 (0.87–1.64), respectively; the corresponding HRs were 1.05 (0.70–1.59), 0.74 (0.50–1.10), and 2.40 (1.62–3.54), respectively, in patients ≥65 years old. A BMI of 27.5–29.9 kg/m2 showed significantly higher HCC risks in patients with HBV, ALD, NAFLD, and LC.
Conclusions
Higher BMIs were associated with increased HCC risks in patients with HBV, ALD, NAFLD, and LC. Overweight status increased HCC risk. Women with HBV and LC had stronger BMI-HCC associations than men. The effect of high BMI was stronger in older patients with NAFLD and younger patients with viral hepatitis.
Introduction
Hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, and liver cirrhosis (LC) are well-known risk factors for hepatocellular carcinoma (HCC). The association between obesity and HCC in patients with these high-risk liver diseases (HLDs) is not well understood, and the role of a high body mass index (BMI) in HCC development and progression has not been clearly elucidated. Some studies have shown a positive association between BMI and HCC risk [1–5], whereas others have not [6, 7]. In patients with less severe liver diseases, such as non-alcoholic fatty liver disease (NAFLD) without LC, few studies have examined these associations; hence, the associations remain unclear. For example, in patients with NAFLD, obesity is not associated with a higher HCC risk [8].
We aimed to investigate the BMI-HCC association in patients with various liver diseases (HBV infection, HCV infection, LC, NAFLD, and alcoholic liver disease [ALD]). In addition, as previous studies have suggested potentially different BMI-HCC associations between men and women and between younger and older adults, we further investigated sex- and age-specific associations [6, 9–12].
Patients and methods
Study population and follow-up
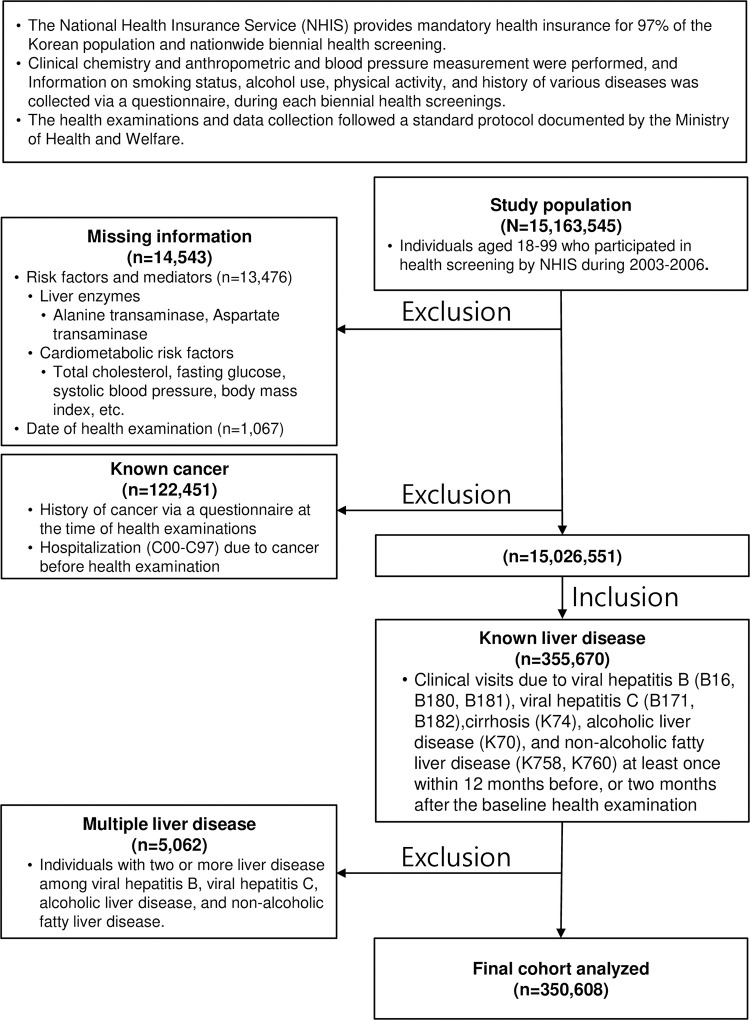
This population-based prospective cohort study enrolled 355,670 Koreans with liver diseases (HBV infection, HCV infection, LC, ALD, and NAFLD) aged 18–99 years who were examined between 2003 and 2006 and had no known cancer or missing information on variables and examination date. Baseline data were collected at the time of the health examination (index date). We excluded 5,062 patients with multiple liver diseases. We followed the remaining 350,608 included patients (Fig 1) until December 31, 2018, via record linkage to hospital discharge records from the National Health Insurance Service (NHIS), in which certified health information managers reviewed medical records and assigned standardized diagnosis codes. All patients discharged from the hospital due to HCC (International Classification of Diseases 10th Revision code C220) for the first time were considered incident cases. The authors were granted access to anonymized data from the NHIS (From June 1, 2022, to June 30, 2023). This study was approved by the Institutional Review Board of Gangneung Asan Hospital, Gangneung, Republic of Korea (GNAH 2022-04-005). Informed consent was waived owing to the use of anonymized data that were constructed and provided by the NHIS according to a strict confidentiality protocol. All research was conducted in accordance with both the Declarations of Helsinki and Istanbul.
Fig 1. Flow diagram of the study cohort.
Completeness of HCC incidence data by the NHIS
Ninety-seven percent of Koreans are NHIS-insured [13]. Additionally, patients with HLDs underwent liver cancer surveillance twice a year. Patients with high alpha-fetoprotein levels or a suspicious mass on ultrasonography were referred for HCC diagnosis using dynamic computed tomography or magnetic resonance imaging. All the procedures were covered by the NHIS. The completeness of cancer incidence data from the NHIS is comparable to that of the Korea National Cancer Incidence Database (> 95% for liver cancer) [14, 15].
Data collection
Data were collected during baseline examinations using measurements and questionnaires. Alanine transaminase and aspartate transaminase levels were measured using the nicotinamide adenine dinucleotide-ultraviolet or Reitman-Frankel method. Fasting serum glucose and total cholesterol levels were assessed using enzymatic methods [16]. Blood pressure was measured using a standard mercury sphygmomanometer. BMI was calculated as the measured weight (kg) divided by the square of measured height (m2). Smoking status, alcohol use, and history of cancer and cardiovascular disease were assessed using a questionnaire. Patients with a self-reported cancer history or patients admitted to a hospital for cancer before the baseline examination were considered to have preexisting cancer. Patient examinations and data collection followed a standard protocol documented by the government. The data collection methods for smoking and alcohol consumption were similar to those used in our previous study [7].
Prevalent diseases at baseline
We considered patients to have a baseline prevalent disease if they visited a hospital for the disease at least once within 12 months before or 2 months after the baseline examination. The diseases were selected using International Classification of Diseases 10th Revision codes: HBV infection (B16, B180, and B181), HCV infection (B171 and B182), diabetes (E10-E14), ALD (K70), LC (K74), and NAFLD (K758 and K760).
Statistical analysis
BMI was categorized into seven groups: <18.5, 18.5–20.9, 21–22.9, 23–24.9, 25–27.4, 27.5–29.9, and ≥30 kg/m2. BMI was also analyzed as a continuous variable (per 5 kg/m2 increase), assuming a linear association in the full, lower (<25 kg/m2), and upper (≥25 kg/m2) ranges. The effects of BMI on HCC were evaluated using stratified analysis across liver disease status. In the subgroup analysis, the BMI-HCC associations in patients with LC were examined according to LC etiology (HBV, HCV, ALD, or NAFLD).
The HRs for HCC incidence were obtained using Cox proportional hazards models stratified by age (years) at baseline (18–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, and 85–99 years, using the STRATA statement). The multivariable analysis was adjusted for age at baseline (continuous variable), sex, smoking status (never, former, or current smoker [<10, 10–19, or ≥20 cigarettes/day]), alcohol use (none, <10, 10–19, 20–39, and ≥40 g of ethanol/day; or missing information), physical activity (exercise with light sweating, none, 1–2 times/week, and 3–7 times/week), and income status (quartiles; 1 [low income], 2, 3, 4 [high income]). BMI effect mediators such as glycemic status (normoglycemia [<100 mg/dL], impaired fasting glucose levels [100–125 mg/dL], diabetes [≥126 mg/dL or prevalent diabetes]), total cholesterol levels (continuous variable) [17], and alanine transaminase levels (natural log-transformed levels) were further adjusted for in sensitivity analyses. Sex- and age-stratified analyses were performed.
Effect size differences between the sexes and age groups were evaluated using the Cochrane Q statistics test as an interaction test. All P-values were two-sided, and the analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
HBV, HCV, and LC were present in 120,994, 14,882, and 31,260 patients, respectively (Table 1). During a mean follow-up period of 13.7 years, HCC was diagnosed in 8,492 patients. The proportions of patients with HBV infection, HCV infection, and LC having a BMI ≥25 kg/m2 were 32.5%, 35.4%, and 34%, respectively. Patients with HBV infection were younger and had higher total cholesterol levels. Patients with LC were predominantly men and smokers, with higher glucose levels, more alcohol use, and less physical activity. HBV was the most common etiology of LC, followed by ALD (S1 Table).
Table 1. Baseline demographic and clinical characteristics.
| Characteristic | HBV n = 120,994 |
HCV n = 14,882 |
ALD n = 106,112 |
NAFLD n = 88,353 |
LC n = 31,260 |
|---|---|---|---|---|---|
| HCC | 9,043 | 1,654 | 2,659 | 650 | 6,506 |
| Sex | |||||
| Men | 76,067 (62.9) | 8,274 (55.6) | 95,767 (90.3) | 53,870 (61.0) | 22,316 (71.4) |
| Women | 44,927 (37.1) | 6,608 (44.4) | 10,345 (9.7) | 34,483 (39.0) | 8,944 (28.6) |
| BMI, kg/m2 | |||||
| <18.5 | 3,824 (3.2) | 383 (2.6) | 2,817 (2.7) | 1,303 (1.5) | 933 (3.0) |
| 18.5–20.9 | 18,815 (15.6) | 1,889 (12.7) | 12,981 (12.2) | 7,001 (7.9) | 4,384 (14.0) |
| 21–22.9 | 27,659 (22.9) | 3,190 (21.4) | 20,412 (19.2) | 14,422 (16.3) | 7,133 (22.8) |
| 23–24.9 | 31,441 (26.0) | 4,150 (27.9) | 27,028 (25.5) | 22,751 (25.8) | 8,184 (26.2) |
| 25–27.4 | 26,392 (21.8) | 3,466 (23.3) | 27,419 (25.8) | 25,432 (28.8) | 6,987 (22.4) |
| 27.5–29.9 | 9,389 (7.8) | 1,302 (8.7) | 11,257 (10.6) | 11,995 (13.6) | 2,668 (8.5) |
| ≥30 | 3,474 (2.9) | 502 (3.4) | 4,198 (4.0) | 5,449 (6.2) | 971 (3.1) |
| Glycemic status | |||||
| Normoglycemia | 87,980 (72.7) | 9,505 (63.9) | 61,990 (58.4) | 56,414 (63.9) | 18,449 (59.0) |
| IFG | 23,302 (19.3) | 3,278 (22.0) | 28,530 (26.9) | 21,181 (24.0) | 6,855 (21.9) |
| Diabetes | 9,712 (8.0) | 2,099 (14.1) | 15,592 (14.7) | 10,758 (12.2) | 5,956 (19.1) |
| Smoking, pack/day | |||||
| Never | 76,565 (63.3) | 10,087 (67.8) | 302 (0.3) | 157 (0.2) | 19,013 (60.8) |
| Former | 12,537 (10.4) | 1,447 (9.7) | 42,255 (39.8) | 55,046 (62.3) | 3,430 (11.0) |
| <0.5 | 7,777 (6.4) | 872 (5.9) | 15,196 (14.3) | 9,758 (11.0) | 2,765 (8.8) |
| 0.5–0.9 | 15,999 (13.2) | 1,494 (10.0) | 9,684 (9.1) | 4,885 (5.5) | 3,857 (12.3) |
| 1–1.9 | 5,267 (4.4) | 592 (4.0) | 24,181 (22.8) | 11,196 (12.7) | 1,394 (4.5) |
| ≥2 | 2,634 (2.2) | 373 (2.5) | 12,818 (12.1) | 5,436 (6.2) | 737 (2.4) |
| Unknown | 215 (0.2) | 17 (0.1) | 1,676 (1.6) | 1,875 (2.1) | 64 (0.2) |
| Alcohol, ethanol (g)/day | |||||
| None | 68,917 (57.0) | 9,676 (65.0) | 1,833 (1.7) | 1,739 (2.0) | 20,007 (64.0) |
| <10 | 27,974 (23.1) | 2,581 (17.3) | 28,734 (27.1) | 46,244 (52.3) | 4,763 (15.2) |
| 10–19 | 13,670 (11.3) | 1,330 (8.9) | 21,144 (19.9) | 18,797 (21.3) | 2,561 (8.2) |
| 20–39 | 3,908 (3.2) | 399 (2.7) | 22,447 (21.2) | 12,014 (13.6) | 1,078 (3.4) |
| ≥40 | 3,972 (3.3) | 492 (3.3) | 11,479 (10.8) | 4,378 (5.0) | 2,039 (6.5) |
| Unknown | 2,553 (2.1) | 404 (2.7) | 20,475 (19.3) | 5,181 (5.9) | 812 (2.6) |
| Physical activity, times/week | |||||
| None | 62,244 (51.4) | 7,994 (53.7) | 59,009 (55.6) | 46,538 (52.7) | 18,044 (57.7) |
| 1–2 | 34,625 (28.6) | 3,532 (23.7) | 27,337 (25.8) | 23,294 (26.4) | 7,137 (22.8) |
| ≥3 | 24,125 (19.9) | 3,356 (22.6) | 19,766 (18.6) | 18,521 (21.0) | 6,079 (19.4) |
| Income status, quartile | |||||
| Q1 (low) | 24,235 (20.0) | 2,788 (18.7) | 20,164 (19.0) | 16,790 (19.0) | 6,076 (19.4) |
| Q2 | 24,333 (20.1) | 2,689 (18.1) | 22,816 (21.5) | 16,654 (18.8) | 5,914 (18.9) |
| Q3 | 31,972 (26.4) | 3,866 (26.0) | 30,046 (28.3) | 23,338 (26.4) | 7,890 (25.2) |
| Q4 | 40,454 (33.4) | 5,539 (37.2) | 33,086 (31.2) | 31,571 (35.7) | 11,380 (36.4) |
| Age groups, years | |||||
| <65 | 11,4728 (94.8) | 12,008 (80.7) | 93,860 (88.5) | 79,551 (90.0) | 25,686 (82.2) |
| ≥65 | 6,266 (5.2) | 2,874 (19.3) | 12,252 (11.5) | 8,802 (10.0) | 5,574 (17.8) |
| Total cholesterol, mg/dL | |||||
| <200 | 79,873 (66.0) | 10,385 (69.8) | 59,626 (56.2) | 43,981 (49.8) | 23,348 (74.7) |
| 200–239 | 31,497 (26.0) | 3,401 (22.9) | 32,149 (30.3) | 30,222 (34.2) | 5,975 (19.1) |
| ≥240 | 9,624 (8.0) | 1,096 (7.4) | 14,337 (13.5) | 14,150 (16.0) | 1,937 (6.2) |
Data are expressed as numbers and percentages.
Abbreviations: ALD, alcoholic liver disease; LC, liver cirrhosis; HCC, hepatocellular carcinoma; BMI, body mass index; IFG, impaired fasting glucose
Categorical analyses
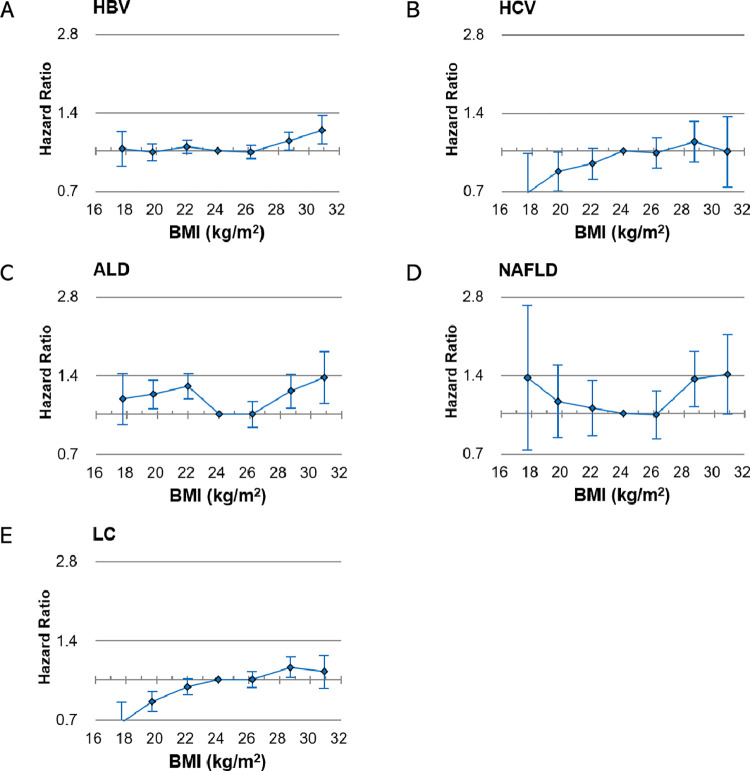
HRs for HCC were compared based on a BMI range of 23–24.9 kg/m2. In patients with HBV infection, the HRs of HCC were increased in the BMI ranges of 27.5–29.9 (HR 1.09, 95% CI 1.01–1.18) and ≥30 kg/m2 (HR 1.20, 95% CI 1.06–1.36) (Fig 2; S2 Table). In patients with HCV, the HRs were 0.99 (95% CI 0.86–1.13), 1.09 (95% CI 0.91–1.31), and 1.00 (95% CI 0.73–1.36) for BMI ranges of 25–27.4, 27.5–29.9, and ≥30 kg/m2, respectively. In patients with ALD, the HRs of HCC were significantly increased in the BMI ranges of 27.5–29.9 (HR 1.22, 95% CI 1.06–1.42) and ≥30 kg/m2 (HR 1.38, 95% CI 1.10–1.74). Similarly, in patients with NAFLD, the HRs of HCC were increased in the BMI ranges of 27.5–29.9 (HR 1.36, 95% CI 1.06–1.74) and ≥30 kg/m2 (HR 1.42, 95% CI 1.00–2.01). In patients with LC, the HRs of HCC were 1.00 (95% CI 0.93–1.07), 1.11 (95% CI 1.02–1.22), and 1.07 (95% CI 0.93–1.24) in BMI ranges of 25–27.4, 27.5–29.9, and ≥30 kg/m2, respectively. In the subgroup analysis of LC etiology, patients with HBV-LC co-occurrence showed increased HCC risks in the BMI range of 27.5–29.9 kg/m2 (HR 1.35, 95% CI 1.15–1.59).
Fig 2. Hepatocellular carcinoma risk according to liver disease, for each 5-kg/m2 BMI increase in patients with BMI ≥25 kg/m2.
HBV, hepatitis B virus; HCV, hepatitis C virus; ALD, alcoholic liver disease; NAFLD, nonalcoholic fatty liver disease; LC, liver cirrhosis; BMI, body mass index.
Linear analyses
In linear analyses according to BMI, for each 5 kg/m2 increase from a BMI ≥25 kg/m2, the multivariable-adjusted HRs were 1.17 (P = 0.001), 1.08 (P = 0.486), 1.11 (P = 0.047), 1.34 (P<0.001), and 1.51 (P = 0.001) for HBV infection, HCV infection, LC, ALD, and NAFLD, respectively (Fig 2). Overall, positive BMI-HCC associations were shown in patients with chronic liver disease (CLD). In the subgroup analysis for patients with LC (BMI ≥25 kg/m2), the HRs for HCC per 5 kg/m2 increase in BMI were 1.19 (0.98–1.45, P = 0.075), 1.38 (0.75–2.51, P = 0.297), 1.36 (0.92–2.02, P = 0.125), and 1.59 (0.53–4.76, P = 0.410) in patients with HBV infection, HCV infection, ALD, and NAFLD, respectively (S3 Table).
Age- and sex-stratified analyses
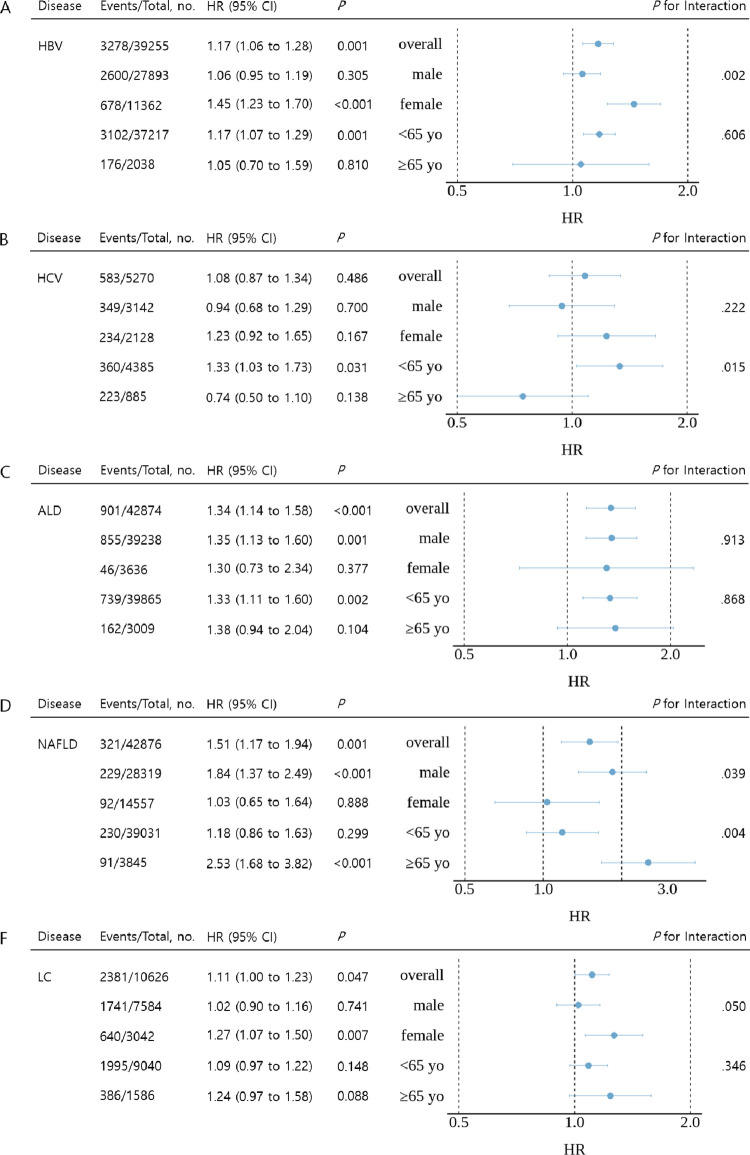
In the sex-stratified analyses (BMI ≥25 kg/m2), women with HBV infection (1.45 vs. 1.06, P for interaction = 0.002) and LC (1.27 vs. 1.02, P for interaction = 0.050) showed stronger positive BMI-HCC associations than in men (Fig 3). However, for patients with NAFLD, the association was stronger in men than in women (1.84 vs. 1.03, P for interaction = 0.039). The association showed no sex difference in patients with ALD (1.35 vs. 1.30, P for interaction = 0.913). In the age-stratified analyses (patients divided into groups aged <65 years and ≥65 years; BMI ≥25 kg/m2), younger patients with HBV infection (1.17 vs. 1.05, P for interaction = 0.606) and HCV infection (1.33 vs. 0.77, P for interaction = 0.015) had a higher HCC risk than older patients (Fig 3). In the subgroup analysis of patients with LC, women with HBV infection (1.73 vs. 0.95, P for interaction = 0.003) and older patients with NAFLD (11.08 vs. 0.76, P for interaction = 0.036) had significantly higher risks of HCC associated with a higher BMI than men with HBV and younger patients with NAFLD, respectively (S4 Table).
Fig 3. Cox regression analysis of hepatocellular carcinoma risk according to liver disease, for each 5-kg/m2 BMI increase in patients with BMI ≥25 kg/m2.
HBV, hepatitis B virus; HCV, hepatitis C virus; ALD, alcoholic liver disease; NAFLD, nonalcoholic fatty liver disease; LC, liver cirrhosis; BMI, body mass index.
Discussion
This prospective cohort study of more than 350,000 patients with CLDs showed that obesity increased HCC risk. In overweight (BMI ≥25 and <30 kg/m2) and obese (BMI ≥30 kg/m2) patients with HBV infection, LC, ALD, or NALFD, BMI increase was associated with higher HCC risks. In patients with a BMI ≥25 kg/m2, HCC risks associated with higher BMIs were more prominent in women than in men with HBV infection, HCV infection, or LC. Age-specific BMI-HCC associations differed by CLDs; the associations were stronger in younger adults than in older adults with HBV and HCV infections but stronger in older adults than in younger adults with NAFLD.
In our study, obesity increased the HCC risk in patients with HBV infection. Some previous studies have shown inconsistent associations between obesity and HCC risk in Western and Taiwanese patients with HBV infection [3, 18, 19], although the association was consistent in other studies [4, 20–22]. Recently, a large-scale Korean study reported an association between a high BMI and HCC risk [23]. Our study confirmed that obesity increased HCC incidence in Koreans with HBV infection. Previous studies may not have found a significant BMI-HCC association, mainly because of the relatively small study sample sizes and the modest nature of true associations. Notably, our study showed that overweight status (especially a BMI of 27.5–29.9 kg/m2) was associated with higher HCC risks.
In our study, patients with HCV infection showed a modest obesity-HCC association. Some previous studies showed increased obesity-associated HCC risks [4], although others did not [24–26]. In the current study, higher BMIs were significantly associated with HCC risk in patients with HBV infection but not in patients with HCV infection. Associations related to HCV than HBV were weaker in HBV-endemic areas [7], whereas the opposite findings have been reported in other areas. These findings might be partially explained by the fact that antiHCV medication efficacy was lower than anti-HBV medication efficacy in the early 2000s. The formal heterogeneity test showed no difference in the BMI-HCC association between HBV and HCV infections (P for heterogeneity = 0.509) in the current study. The modest association in patients with HCV infection was not significant because of the relatively small number of patients with HCV infection compared with the number of patients with HBV infection in the present study.
In previous studies, obesity was associated with HCC development, mostly in patients in Western countries with ALD-LC and HCV-LC co-occurrences [1, 5, 24, 27, 28]. The HCC risk associated with obesity was more pronounced in patients with ALD-LC co-occurrence than in patients with viral hepatitis-LC co-occurrence [5, 24]. In the current study, the BMI-HCC association was stronger in patients with ALD-LC and HCV-LC co-occurrences than in patients with HBV-LC co-occurrence. However, the association was not significant owing to the smaller number of participants with HCV infection.
In our study, NAFLD patients with overweight and obesity showed an increased HCC risk; moreover, a 5-kg/m2 BMI increment increased HCC risk by 51%. NAFLD is a known risk factor for HCC, and a BMI increase is associated with a higher NAFLD prevalence; nevertheless, few studies have reported an obesity-related increase in HCC risk in patients with NAFLD [24, 27, 29, 30]. The absence of a BMI-HCC association may be explained, at least partially, by crude BMI classification into two groups ([≥30 vs. <30 kg/m2] [8, 29] and [≥25 vs. <25 kg/m2] [30]), a retrospective study design [8, 24, 27], including patients with cirrhosis only [24, 27], and adjustment for the effect modifiers of BMI [24, 27]. We may have identified this association because of the prospective study design, the inclusion of patients with and without cirrhosis, the detailed BMI classification into seven groups, and the lack of adjustment for effect modifiers of BMI.
Regarding ALD, previous studies were conducted mostly in patients with cirrhosis, and few studies examined the BMI-HCC association in patients without LC [5, 27]. In the current study, patients with ALD without LC showed higher HCC risks associated with overweight and obesity, probably because of the synergistic interaction between alcohol intake and BMI [31]. The associations were similar in both sexes, albeit statistically insignificant in women due to the small number of women with ALD.
Furthermore, our study showed a higher relative risk of HCC associated with BMI in women than men with viral hepatitis-LC co-occurrence. Few studies have examined sex-specific BMI-HCC associations in patients with viral hepatitis-LC co-occurrence [6, 10, 23, 32–35]. In the current study, the BMI-HCC associations were similar between men and women with ALD; nevertheless, potentially stronger associations were observed in men than in women with NAFLD. Our study suggests different associations according to sex for each CLD. Unfortunately, except for the presumption of sex-specific differences in the hepatocarcinogenic mechanisms of high BMIs and each liver disease, proposing a mechanism that can adequately explain the sex differences in the obesity-related HCC risk observed in our study is difficult. Further studies are needed to clarify the potentially different associations according to sex for each CLD.
In the age-specific analysis, relative HCC risks associated with a higher BMI were higher in younger (<65 years) than in older adults with HBV and HCV infections, whereas the risks were higher in older than in younger adults with NAFLD. Younger patients with viral hepatitis may experience a stronger synergistic effect between viral infection and obesity due to lower comorbidity rates [13, 36]. Regarding NAFLD, our finding of stronger associations in older adults may reflect the fact that high BMI affects HCC development in the long run. For HCC prevention, comorbid obesity may not be ignored in younger patients with viral hepatitis; however, more attention may be needed in NAFLD patients with obesity, especially in patients aged ≥65 years.
The role of obesity in hepatocarcinogenesis in patients with HLD is not well understood. Patients with HBV/HCV infections and LC have a 10–100-fold higher HCC risk than patients without HLDs, whereas obesity is associated with a 2–3-fold higher HCC risk in the general population. If HLDs and obesity are independent risk factors for HCC, the effect of obesity on HCC would be negligible. The current study showed that a higher BMI increased HCC risk in patients with CLDs, including HLDs. Our results indicate a strong synergistic interaction between the hepatocarcinogenic mechanisms of CLDs (including HLDs) and obesity [37]. Detailed mechanisms underlying these synergistic interactions are yet to be elucidated.
This study had some strengths. We examined the relationship between HCC risk and obesity in patients with major liver diseases, such as HBV infection, HCV infection, LC, and NAFLD. Previous studies examined the relationship between obesity and HCC risk in patients with a single liver disease. However, we analyzed HCC risk in patients with each type of HLD in a nationwide cohort. Furthermore, this was a large-scale study that analyzed the relationship between HCC risk and obesity by age and sex. We also tested our hypothesis by adjusting for the main confounding variables but not for the effect mediators of obesity [38]. We used a prospective cohort design to minimize recall and selection biases related to retrospective studies.
Our study had several limitations. First, obesity was defined in terms of BMI only; other factors, such as visceral obesity (evaluated using the waist-to-hip ratio), could not be investigated owing to the nature of the raw data. Second, important data such as fibrosis score, antiviral therapies, and detailed viral factors for HBV and HCV were not available. For example, antiviral therapies are known to reduce the risk of HBV- and HCV-related HCC. Third, the proportion of Koreans with a BMI >30 kg/m2 (especially >35 kg/m2) was small. Therefore, the relationship between obesity and HCC incidence may have been underestimated. Fourth, we could not analyze the impact of the change in BMI on HCC risk. Only a few studies examined the association between BMI (or weight change) and HCC in persons with liver diseases [39]. The results were inconsistent. Our analysis based on a single measurement of BMI may underestimate the true association of dynamic change in BMI. Finally, the study population included only Koreans, which may limit its interpretation and applicability. For example, most cases of LC in Korea are caused by HBV infection. The potential differences in associations across LC etiologies suggest that the BMI-HCC association in patients with LC may depend on the population-specific distribution of the LC etiology.
In conclusion, in patients with CLD, obesity and overweight (specifically a BMI of 27.5–29.9 kg/m2) were associated with an increased HCC risk. In overweight and obese patients with CLDs, a BMI increase was associated with a higher HCC risk. The risk of HCC associated with a higher BMI was more pronounced in women than in men with viral hepatitis and LC. In patients with NAFLD, the impact of a high BMI on HCC was greater in older patients (≥65 years).
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This research was supported by data from the National Health Insurance Service of Korea (NHIS-2024-1-264).
Data Availability
The data that support the findings of this study are available from the National Health Insurance Service (NHIS) [http://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do], but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Funding Statement
This study was supported by The Research Supporting Program of The Korean Association for the Study of the Liver and The Korean Liver Foundation (KASLKLF2021-08) and the Medical Research Promotion Program through Gangneung Asan Hospital funded by the Asan Foundation (2022II0016).
References
- 1.N’Kontchou G, Paries J, Htar MTT, Ganne-Carrie N, Costentin L, Grando-Lemaire V, et al. Risk factors for hepatocellular carcinoma in patients with alcoholic or viral C cirrhosis. Clin Gastroenterol Hepatol. 2006;4: 1062–1068. doi: 10.1016/j.cgh.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 2.Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP, et al. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5: 938–945, 945.e1-4. doi: 10.1016/j.cgh.2007.02.039 [DOI] [PubMed] [Google Scholar]
- 3.Yu MW, Shih WL, Lin CL, Liu CJ, Jian JW, Tsai KS, et al. Body-mass index and progression of hepatitis B: a population-based cohort study in men. J Clin Oncol. 2008;26: 5576–5582. doi: 10.1200/JCO.2008.16.1075 [DOI] [PubMed] [Google Scholar]
- 4.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135: 111–121. doi: 10.1053/j.gastro.2008.03.073 [DOI] [PubMed] [Google Scholar]
- 5.Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36: 150–155. doi: 10.1053/jhep.2002.33713 [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Wang X, Wang J, Yan Z, Luo J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur J Cancer. 201248: 2137–2145. doi: 10.1016/j.ejca.2012.02.063 [DOI] [PubMed] [Google Scholar]
- 7.Yi SW, Choi JS, Yi JJ, Lee YH, Han KJ. Risk factors for hepatocellular carcinoma by age, sex, and liver disorder status: a prospective cohort study in Korea. Cancer. 2018;124: 2748–2757. doi: 10.1002/cncr.31406 [DOI] [PubMed] [Google Scholar]
- 8.Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155: 1828–1837.e2. doi: 10.1053/j.gastro.2018.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A, Das A, Majumder K, Arora N, Mayo HG, Singh PP, et al. Obesity is independently associated with increased risk of hepatocellular cancer-related mortality: a systematic review and meta-analysis. Am J Clin Oncol. 2018;41: 874. doi: 10.1097/COC.0000000000000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jee SH, Yun JE, Park EJ, Cho ER, Park IS, Sull JW, et al. Body mass index and cancer risk in Korean men and women. Int J Cancer. 2008;123: 1892–1896. doi: 10.1002/ijc.23719 [DOI] [PubMed] [Google Scholar]
- 11.Liu YY, Lin CL, Weng CH, Chang PH, Chien CH, Huang KC, et al. Older age and high α-fetoprotein predict higher risk of hepatocellular carcinoma in chronic hepatitis-B-related cirrhotic patients receiving long-term nucleos(t)ide analogue therapy. Diagnostics (Basel). 2022;12: 2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancebo A, González-Diéguez ML, Cadahía V, Varela M, Pérez R, Navascués CA, et al. Annual incidence of hepatocellular carcinoma among patients with alcoholic cirrhosis and identification of risk groups. Clin Gastroenterol Hepatol. 2013;11: 95–101. doi: 10.1016/j.cgh.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 13.Yi SW, Ohrr H, Shin SA, Yi JJ. Sex-age-specific association of body mass index with all-cause mortality among 12.8 million Korean adults: a prospective cohort study. Int J Epidemiol. 2015;44: 1696–1705. doi: 10.1093/ije/dyv138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016;48: 436–450. doi: 10.4143/crt.2016.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seo HJ, Oh IH, Yoon SJ. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac J Cancer Prev. 2012;13: 6163–6168. doi: 10.7314/apjcp.2012.13.12.6163 [DOI] [PubMed] [Google Scholar]
- 16.Lee EY, Lee YH, Yi SW, Shin SA, Yi JJ. BMI and all-cause mortality in normoglycemia, impaired fasting glucose, newly diagnosed diabetes, and prevalent diabetes: a cohort study. Diabetes Care. 2017;40: 1026–1033. doi: 10.2337/dc16-1458 [DOI] [PubMed] [Google Scholar]
- 17.Yi SW, Kim SH, Han KJ, Yi JJ, Ohrr H. Higher cholesterol levels, not statin use, are associated with a lower risk of hepatocellular carcinoma. Br J Cancer. 2020;122: 630–633. doi: 10.1038/s41416-019-0691-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brichler S, Nahon P, Zoulim F, Layese R, Bourcier V, Audureau E, et al. Non-virological factors are drivers of hepatocellular carcinoma in virosuppressed hepatitis B cirrhosis: results of ANRS CO12 CirVir cohort. J Viral Hepat. 2019;26: 384–396. doi: 10.1111/jvh.13029 [DOI] [PubMed] [Google Scholar]
- 19.Chao LT, Wu CF, Sung FY, Lin CL, Liu CJ, Huang CJ, et al. Insulin, glucose and hepatocellular carcinoma risk in male hepatitis B carriers: results from 17-year follow-up of a population-based cohort. Carcinogenesis. 2011;32: 876–881. doi: 10.1093/carcin/bgr058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chayanupatkul M, Omino R, Mittal S, Kramer JR, Richardson P, Thrift AP, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017; 66:355–362. doi: 10.1016/j.jhep.2016.09.013 [DOI] [PubMed] [Google Scholar]
- 21.Fu SC, Huang YW, Wang TC, Hu JT, Chen DS, Yang SS. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with new onset diabetes: a nationwide cohort study. Aliment Pharmacol Ther. 2015;41: 1200–1209. doi: 10.1111/apt.13191 [DOI] [PubMed] [Google Scholar]
- 22.Loomba R, Yang HI, Su J, Brenner D, Iloeje U, Chen CJ. Obesity and alcohol synergize to increase the risk of incident hepatocellular carcinoma in men. Clin Gastroenterol Hepatol. 2010;8: 891–898.e1-2. doi: 10.1016/j.cgh.2010.06.027 [DOI] [PubMed] [Google Scholar]
- 23.Kim K, Choi S, Park SM. Association of high body mass index and hepatocellular carcinoma in patients with chronic hepatitis B virus infection: a Korean population-based cohort study. JAMA Oncol. 2018;4: 737–739. doi: 10.1001/jamaoncol.2018.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ioannou GN, Green P, Lowy E, Mun EJ, Berry K. Differences in hepatocellular carcinoma risk, predictors and trends over time according to etiology of cirrhosis. PLoS One. 2018;13: e0204412. doi: 10.1371/journal.pone.0204412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohki T, Tateishi R, Sato T, Masuzaki R, Imamura J, Goto T, et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol. 2008;6: 459–464. doi: 10.1016/j.cgh.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 26.Veldt BJ, Chen W, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47: 1856–1862. doi: 10.1002/hep.22251 [DOI] [PubMed] [Google Scholar]
- 27.Ioannou GN, Green P, Kerr KF, Berry K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol. 2019;71: 523–533. doi: 10.1016/j.jhep.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pais R, Lebray P, Rousseau G, Charlotte F, Esselma G, Savier E, et al. Non-alcoholic fatty liver disease increases the risk of hepatocellular carcinoma in patients with alcohol-associated cirrhosis awaiting liver transplants. Clin Gastroenterol Hepatol. 2015;13: 992–999.e2. doi: 10.1016/j.cgh.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 29.Grimaudo S, Pipitone RM, Pennisi G, Celsa C, Cammà C, Di Marco V, et al. Association between PNPLA3 rs738409 C>G variant and liver-related outcomes in patients with non-alcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2020;18: 935–944.e3. [DOI] [PubMed] [Google Scholar]
- 30.Ito T, Ishigami M, Ishizu Y, Kuzuya T, Honda T, Ishikawa T, et al. Serum nutritional markers as prognostic factors for hepatic and extrahepatic carcinogenesis in Japanese patients with non-alcoholic fatty liver disease. Nutr Cancer. 2020;72: 884–891. doi: 10.1080/01635581.2019.1653474 [DOI] [PubMed] [Google Scholar]
- 31.Loomba R, Yang HI, Su J, Brenner D, Barrett-Connor E, Iloeje U, et al. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177: 333–342. doi: 10.1093/aje/kws252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arano T, Nakagawa H, Tateishi R, Ikeda H, Uchino K, Enooku K, et al. Serum level of adiponectin and the risk of liver cancer development in chronic hepatitis C patients. Int J Cancer. 2011;129: 2226–2235. doi: 10.1002/ijc.25861 [DOI] [PubMed] [Google Scholar]
- 33.Hassan MM, Abdel-Wahab R, Kaseb A, Shalaby A, Phan AT, El-Serag HB, et al. Obesity early in adulthood increases risk but does not affect outcomes of hepatocellular carcinoma. Gastroenterology. 2015;149: 119–129. doi: 10.1053/j.gastro.2015.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loosen SH, Roderburg C, Jördens MS, Fluegen G, Luedde T, Kostev K. Overweight and obesity determine the risk for gastrointestinal cancer in a sex-dependent manner: a retrospective cohort study of 287,357 outpatients in Germany. Cancers (Basel). 2022;14: 931. doi: 10.3390/cancers14040931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sung H, Siegel RL, Torre LA, Pearson-Stuttard J, Islami F, Fedewa SA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2019;69: 88–112. doi: 10.3322/caac.21499 [DOI] [PubMed] [Google Scholar]
- 36.Jung MH, Yi SW, An SJ, Yi JJ. Age-specific associations between systolic blood pressure and cardiovascular mortality. Heart. 2019;105: 1070–1077. doi: 10.1136/heartjnl-2019-314697 [DOI] [PubMed] [Google Scholar]
- 37.Shin HS, Jun BG, Yi SW. Impact of diabetes, obesity, and dyslipidemia on the risk of hepatocellular carcinoma in patients with chronic liver diseases. Clin Mol Hepatol. 2022;28: 773–789. doi: 10.3350/cmh.2021.0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013309: 71–82. doi: 10.1001/jama.2012.113905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Yatsuya H, Yamagishi K, Wakai K, Tamakoshi A, Iso H, et al. Body mass index and weight change during adulthood are associated with increased mortality from liver cancer: the JACC Study. J Epidemiol. 2013;23: 219–226. doi: 10.2188/jea.je20120199 [DOI] [PMC free article] [PubMed] [Google Scholar]