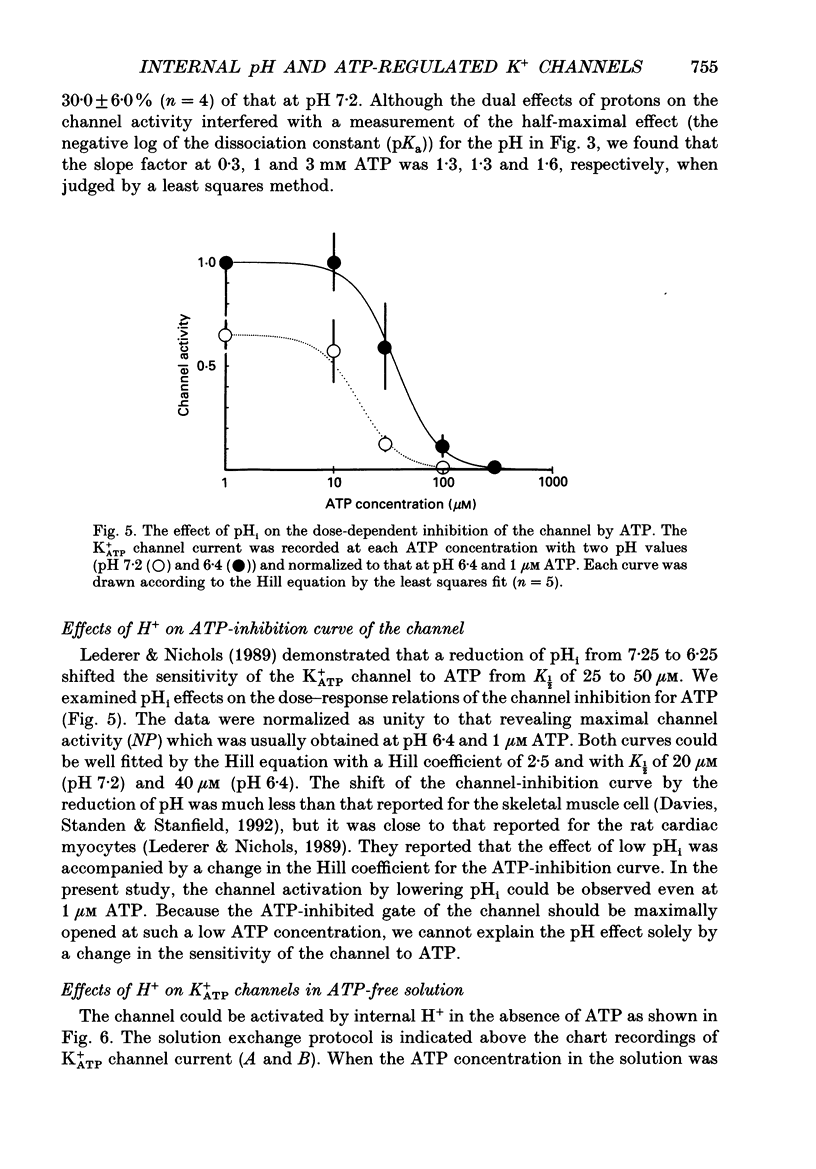
Abstract
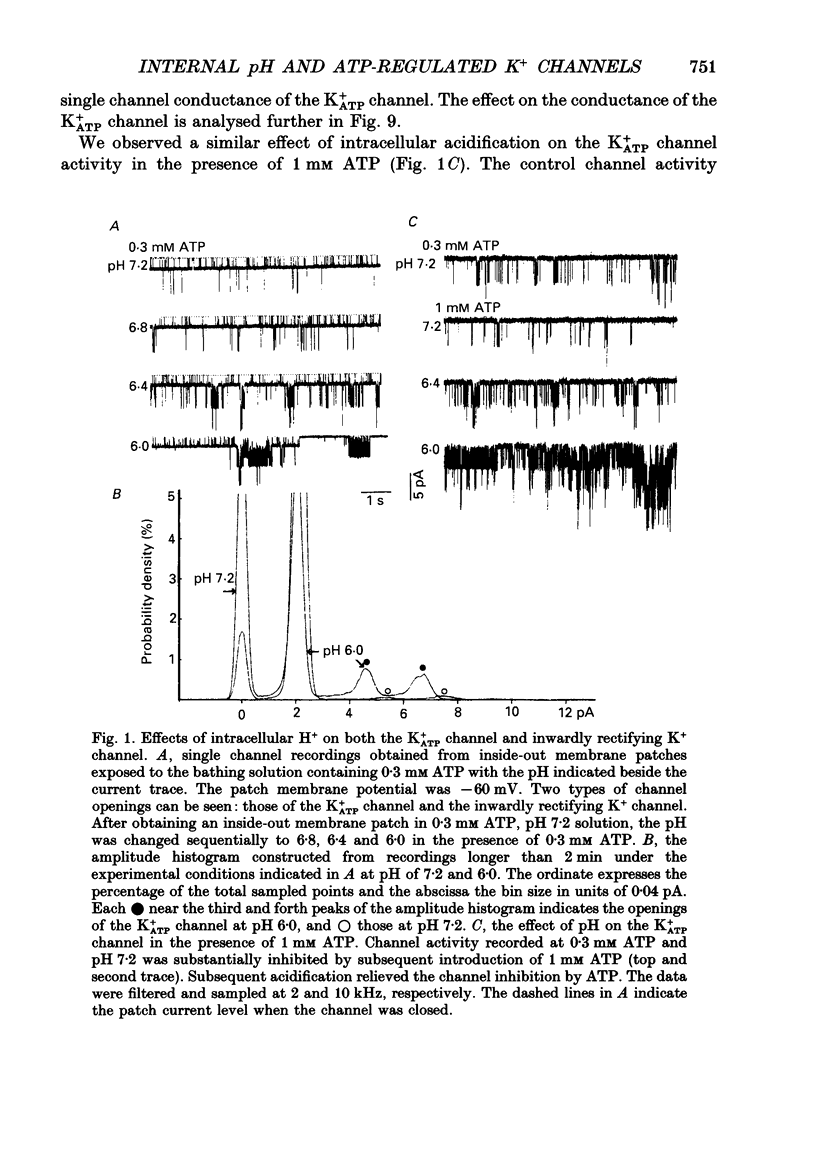
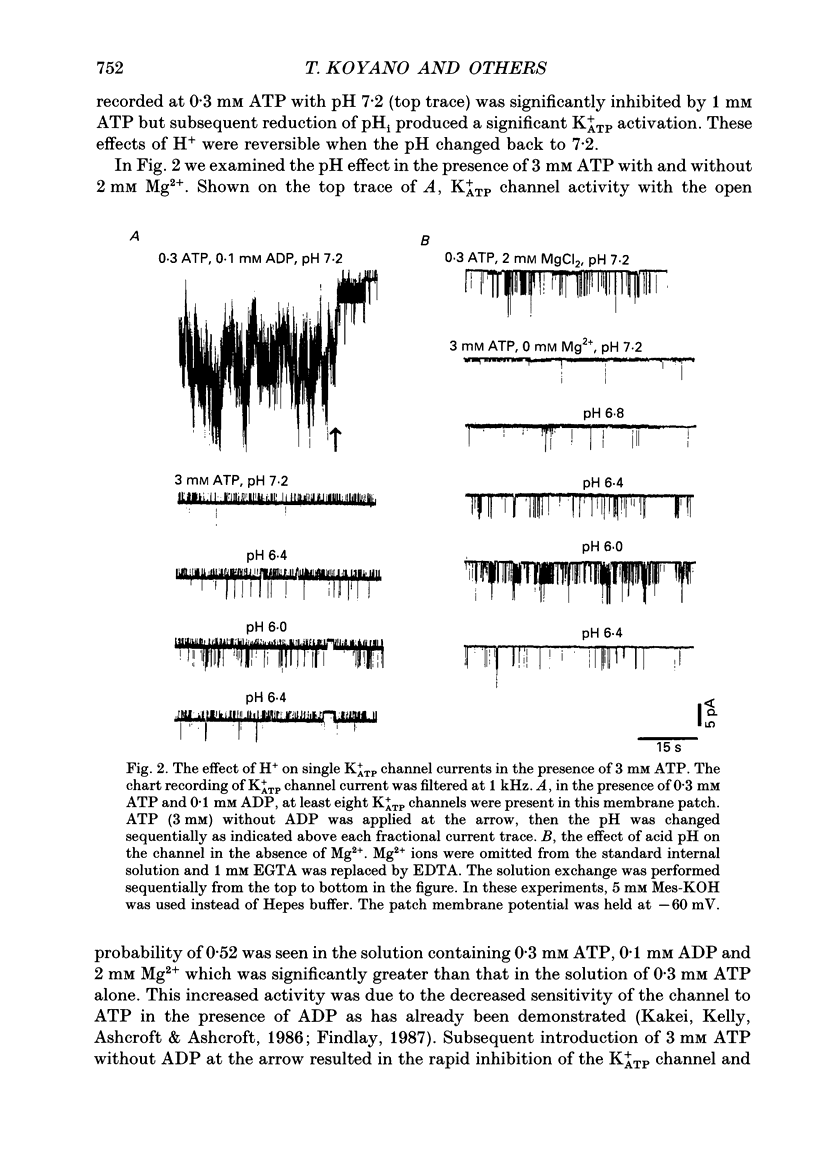
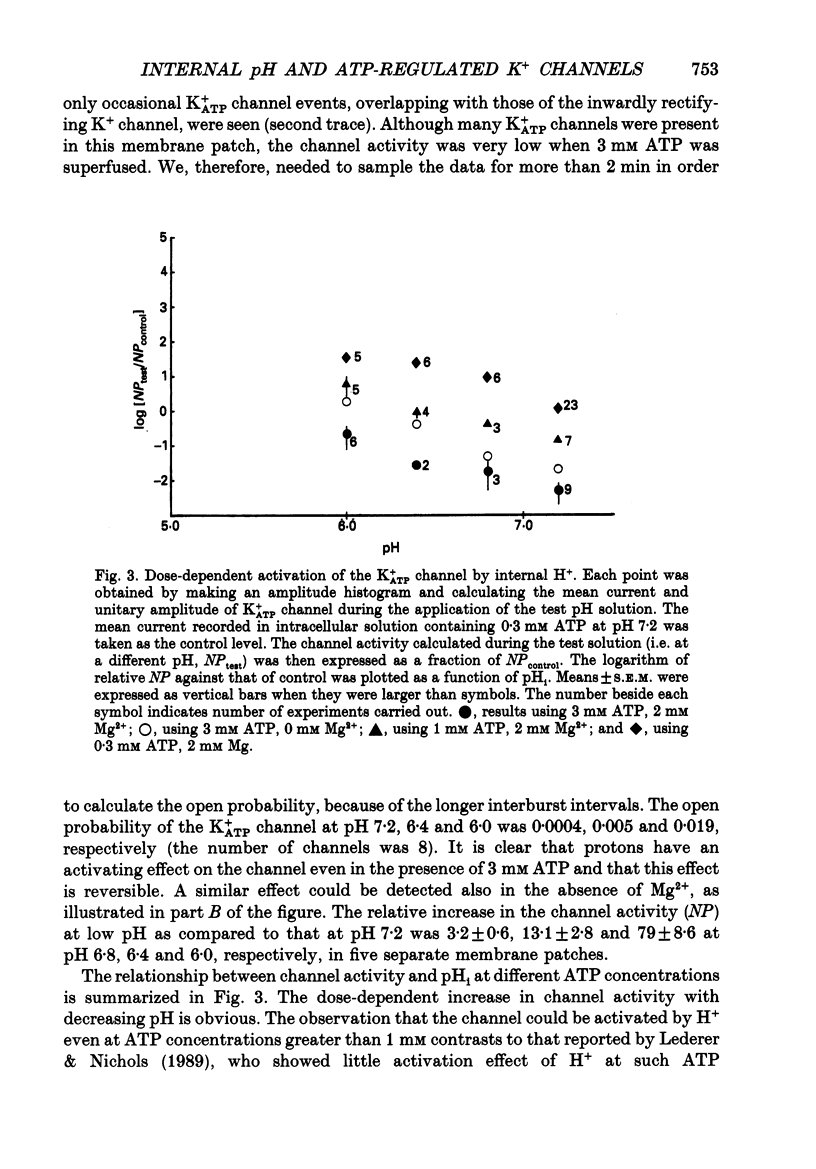
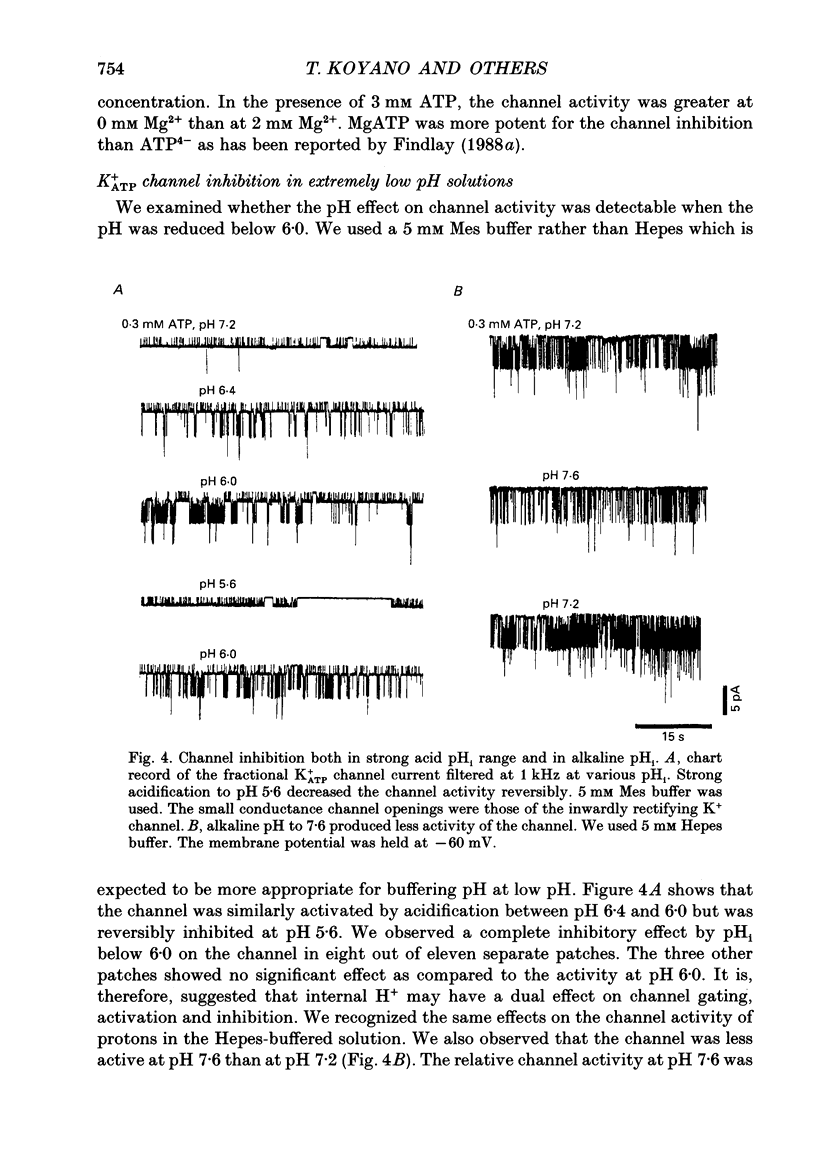
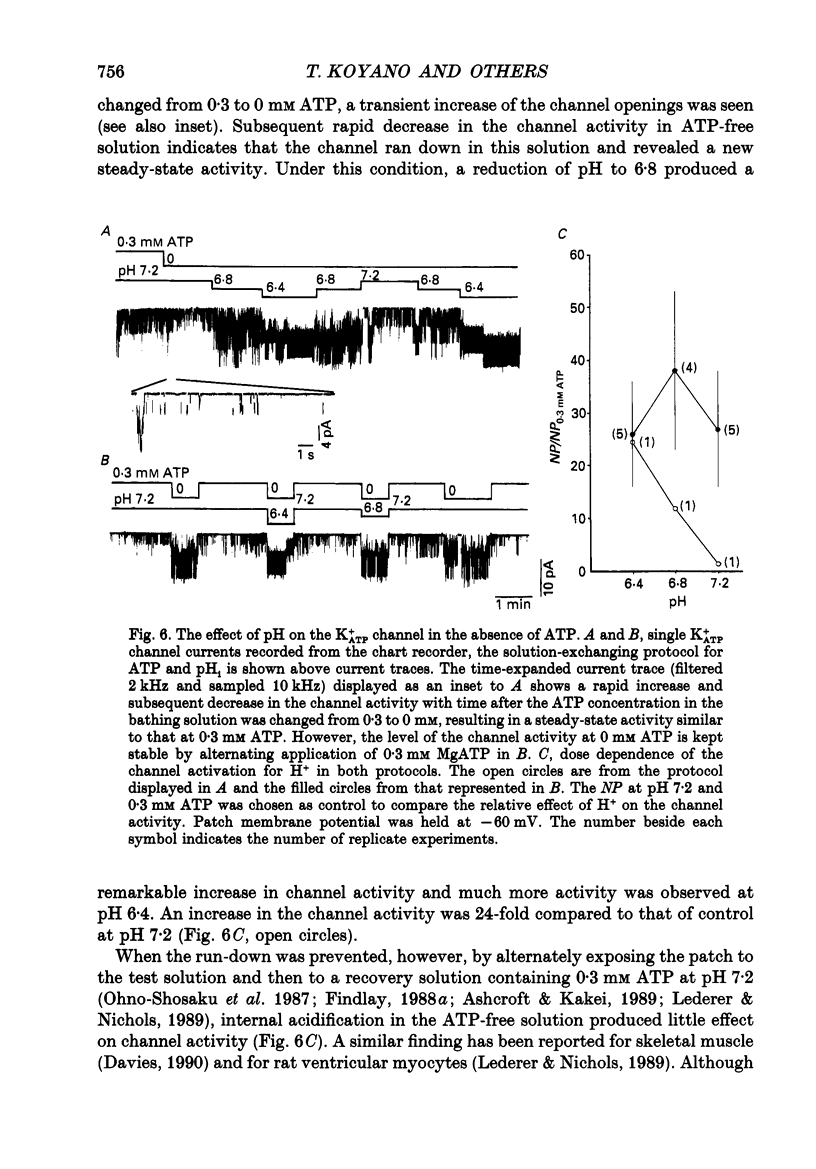
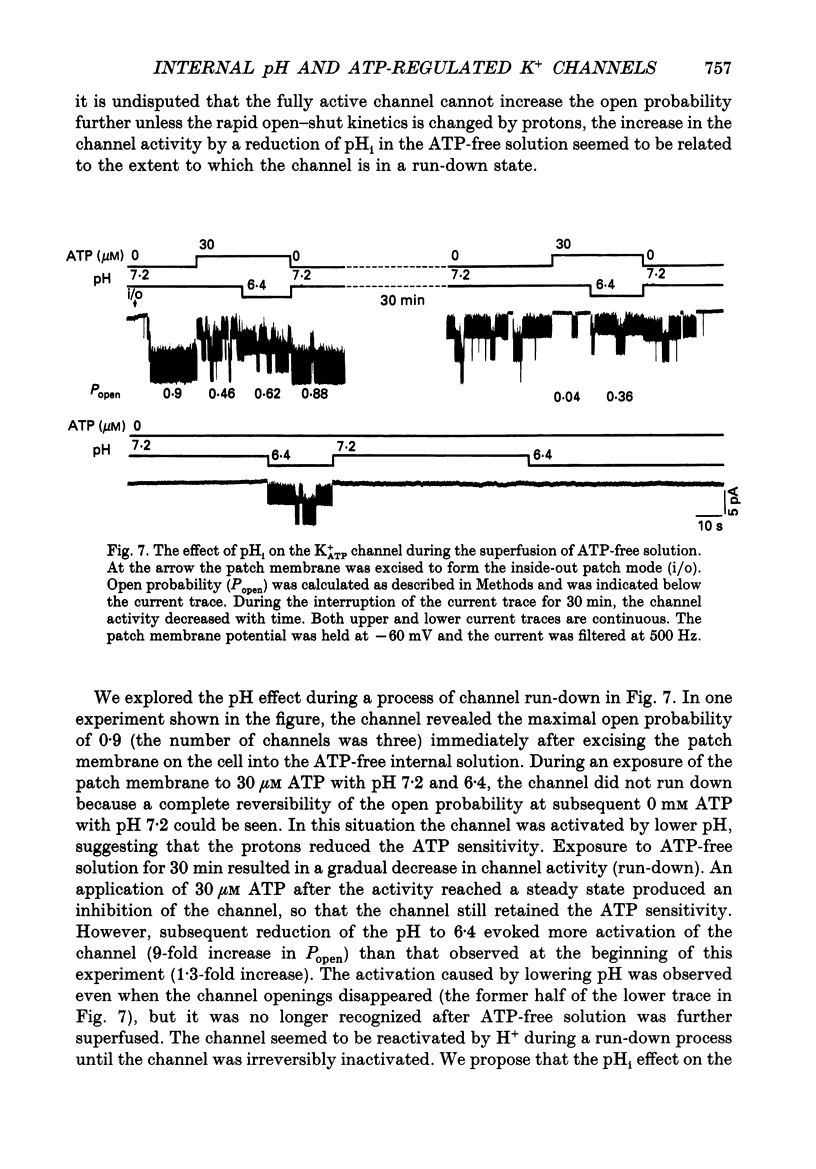
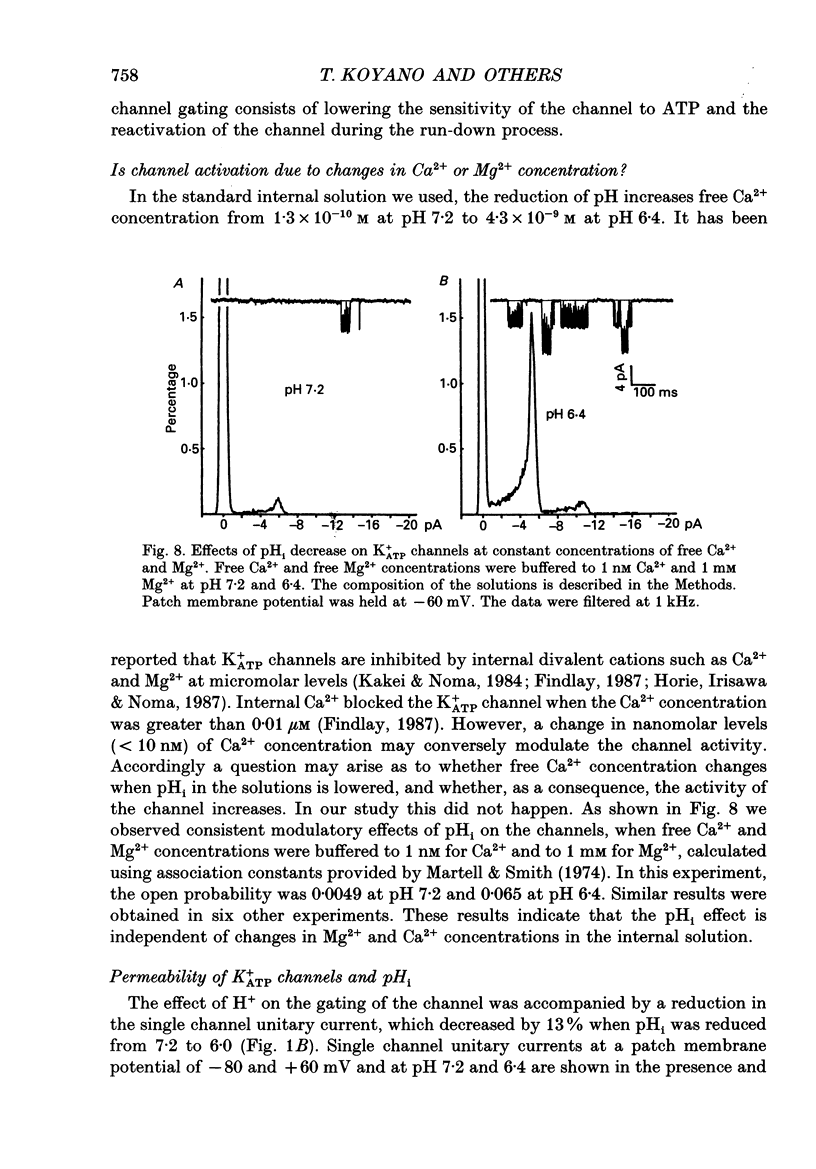
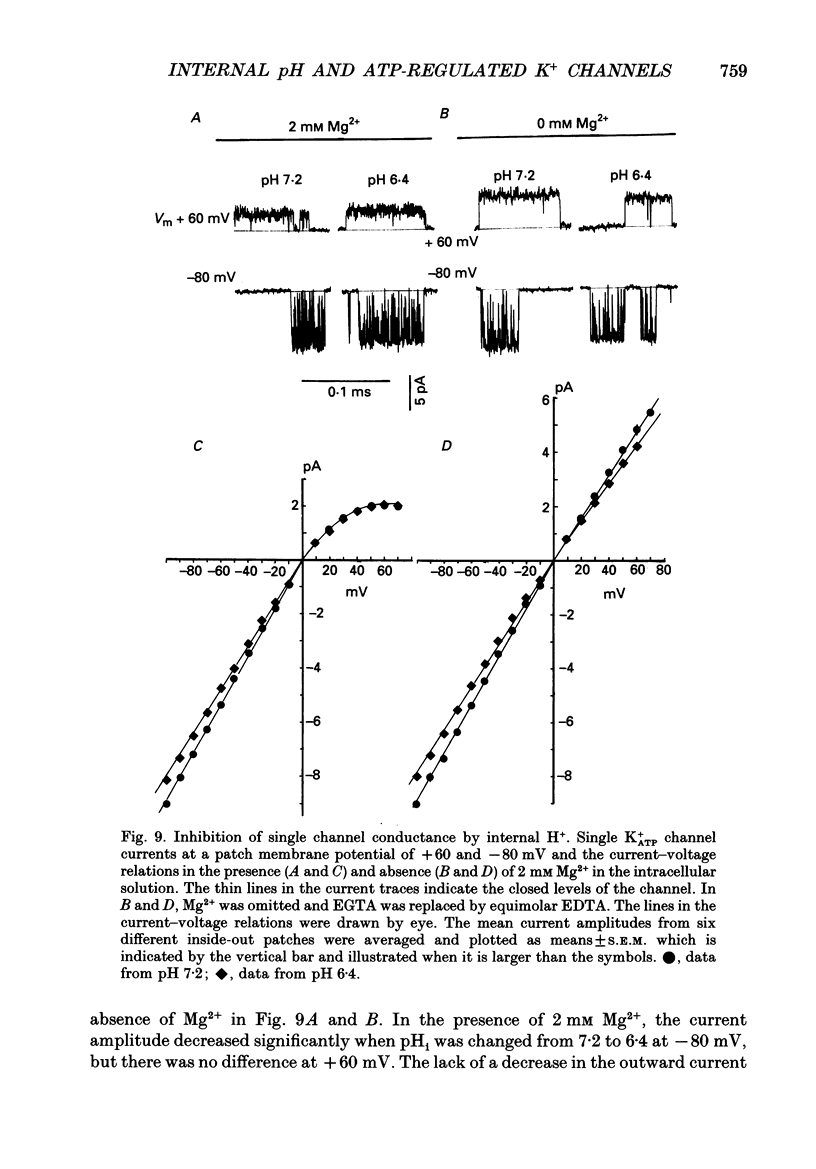
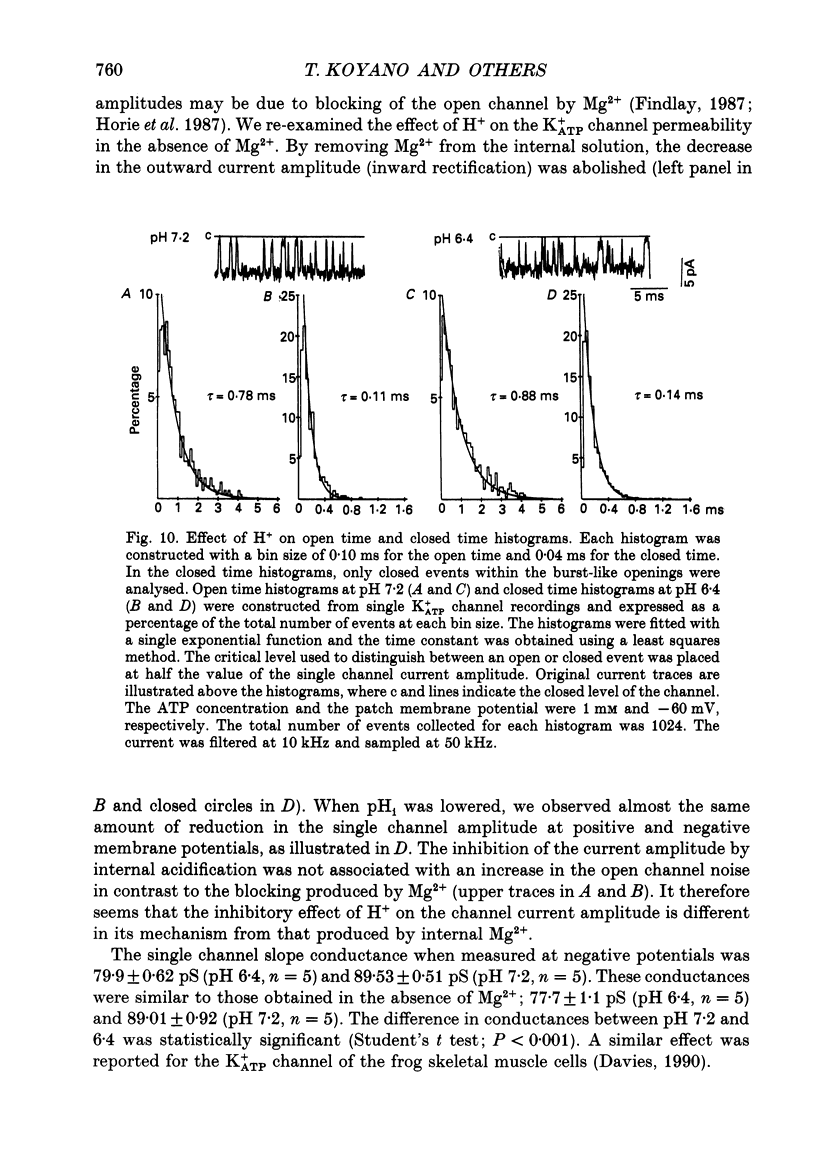
1. The ATP-regulated potassium channel (K+ATP) was investigated with respect to modulation by intracellular pH (pHi) by using the inside-out membrane patch clamp technique in ventricular cells isolated from the heart of the guinea-pig. Channels which had been closed by internal ATP (0.3-3 mM) were dose-dependently activated by decreasing the pHi over the range of pH 7.6-6.0. However, the channel was conversely inhibited when the pHi was further decreased below 6.0. Inwardly rectifying K+ channels were also decreased in activity when pHi fell from 7.2 to 6.0. 2. The channel activation was also observed with constant concentration of free Ca2+ (1 nM) and Mg2+ (1 mM) in the bathing solution, suggesting that a change in divalent cation concentration is not involved in channel modulation by pHi. 3. When the dose-response relations of the channel activity for ATP concentrations at different pHi were examined, the channel activity obtained at 1 microM ATP was increased by decreasing pH from 7.2 to 6.4. The half-maximal inhibition for ATP concentration at pH 7.2 and 6.4 was 20 and 40 microM, respectively, and the Hill coefficient was 2.5 in both curves. 4. In the absence of ATP, internal H+ was able to reactivate run-down channels but it had less effect on the channel as long as the activity was maintained at a higher level. The increase in the channel activity by H+ was facilitated with a proceeding of the run-down. However, after the channel was completely inactivated by a long exposure of the membrane patch to ATP-free solution, a reduction of pH could not activate the channel. 5. The decrease of pH from 7.2 to 6.4 reduced single channel conductance from 89.0 to 77.7 pS in the absence of Mg2+, whereas it reduced the conductance only at the negative membrane potentials in the presence of 2 mM Mg2+. 6. Mean open and closed times within the burst-like openings of the channel remained unaffected during the change in pHi. 7. We conclude that the cardiac K+ATP channel is modulated by a change in the intracellular pH. The channel modulation consisted of the increase in the channel activity and a decrease in the permeability. The former effect was due to the decrease in the sensitivity of the channel to ATP and the reactivation of the channel which is during the process of run-down in activity.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Kakei M. ATP-sensitive K+ channels in rat pancreatic beta-cells: modulation by ATP and Mg2+ ions. J Physiol. 1989 Sep;416:349–367. doi: 10.1113/jphysiol.1989.sp017765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford M. L., Boden P. R., Treherne J. M. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990 Jan;415(4):479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Ikeuchi M., Fujimoto W. Y. Lowering of pHi inhibits Ca2+-activated K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]
- Davies N. W. Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature. 1990 Jan 25;343(6256):375–377. doi: 10.1038/343375a0. [DOI] [PubMed] [Google Scholar]
- Davies N. W., Standen N. B., Stanfield P. R. The effect of intracellular pH on ATP-dependent potassium channels of frog skeletal muscle. J Physiol. 1992 Jan;445:549–568. doi: 10.1113/jphysiol.1992.sp018939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. Intracellular ADP activates K+ channels that are inhibited by ATP in an insulin-secreting cell line. FEBS Lett. 1986 Nov 10;208(1):59–62. doi: 10.1016/0014-5793(86)81532-0. [DOI] [PubMed] [Google Scholar]
- Elliott A. C., Smith G. L., Allen D. G. Simultaneous measurements of action potential duration and intracellular ATP in isolated ferret hearts exposed to cyanide. Circ Res. 1989 Mar;64(3):583–591. doi: 10.1161/01.res.64.3.583. [DOI] [PubMed] [Google Scholar]
- Faivre J. F., Findlay I. Action potential duration and activation of ATP-sensitive potassium current in isolated guinea-pig ventricular myocytes. Biochim Biophys Acta. 1990 Nov 2;1029(1):167–172. doi: 10.1016/0005-2736(90)90450-3. [DOI] [PubMed] [Google Scholar]
- Findlay I. ATP-sensitive K+ channels in rat ventricular myocytes are blocked and inactivated by internal divalent cations. Pflugers Arch. 1987 Oct;410(3):313–320. doi: 10.1007/BF00580282. [DOI] [PubMed] [Google Scholar]
- Findlay I. ATP4- and ATP.Mg inhibit the ATP-sensitive K+ channel of rat ventricular myocytes. Pflugers Arch. 1988 Jul;412(1-2):37–41. doi: 10.1007/BF00583729. [DOI] [PubMed] [Google Scholar]
- Findlay I. Effects of ADP upon the ATP-sensitive K+ channel in rat ventricular myocytes. J Membr Biol. 1988;101(1):83–92. doi: 10.1007/BF01872823. [DOI] [PubMed] [Google Scholar]
- Fosset M., De Weille J. R., Green R. D., Schmid-Antomarchi H., Lazdunski M. Antidiabetic sulfonylureas control action potential properties in heart cells via high affinity receptors that are linked to ATP-dependent K+ channels. J Biol Chem. 1988 Jun 15;263(17):7933–7936. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoppe D., Lux H. D., Schachner M., Kettenmann H. Activation of K+ currents in cultured Schwann cells is controlled by extracellular pH. Pflugers Arch. 1989 Oct;415(1):22–28. doi: 10.1007/BF00373137. [DOI] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kakei M., Ashcroft F. M. A microflow superfusion system for use with excised membrane patches. Pflugers Arch. 1987 Jul;409(3):337–341. doi: 10.1007/BF00583487. [DOI] [PubMed] [Google Scholar]
- Kakei M., Kelly R. P., Ashcroft S. J., Ashcroft F. M. The ATP-sensitivity of K+ channels in rat pancreatic B-cells is modulated by ADP. FEBS Lett. 1986 Nov 10;208(1):63–66. doi: 10.1016/0014-5793(86)81533-2. [DOI] [PubMed] [Google Scholar]
- Kakei M., Noma A. Adenosine-5'-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. J Physiol. 1984 Jul;352:265–284. doi: 10.1113/jphysiol.1984.sp015290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei M., Noma A., Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H., Takagi K., Satake T., Tokuno H., Tomita T. Effects of intracellular pH on calcium-activated potassium channels in rabbit tracheal smooth muscle. J Physiol. 1990 May;424:445–457. doi: 10.1113/jphysiol.1990.sp018076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989 Dec;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misler S., Gillis K., Tabcharani J. Modulation of gating of a metabolically regulated, ATP-dependent K+ channel by intracellular pH in B cells of the pancreatic islet. J Membr Biol. 1989 Jul;109(2):135–143. doi: 10.1007/BF01870852. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Lederer W. J. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J Physiol. 1990 Apr;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Noma A., Shibasaki T. Membrane current through adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:463–480. doi: 10.1113/jphysiol.1985.sp015722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Kubota T., Yamaguchi J., Fujimoto M. Regulation of inwardly rectifying K+ channels by intracellular pH in opossum kidney cells. Pflugers Arch. 1990 Apr;416(1-2):138–143. doi: 10.1007/BF00370235. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Zünkler B. J., Trube G. Dual effects of ATP on K+ currents of mouse pancreatic beta-cells. Pflugers Arch. 1987 Feb;408(2):133–138. doi: 10.1007/BF00581342. [DOI] [PubMed] [Google Scholar]
- Pappone P. A., Barchfeld G. L. Modifications of single acetylcholine-activated channels in BC3H-1 cells. Effects of trimethyloxonium and pH. J Gen Physiol. 1990 Jul;96(1):1–22. doi: 10.1085/jgp.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985 Aug 22;316(6030):736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Ashford M. L., Cook D. L., Hales C. N. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet. 1985 Aug 31;2(8453):474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- Takano M., Qin D. Y., Noma A. ATP-dependent decay and recovery of K+ channels in guinea pig cardiac myocytes. Am J Physiol. 1990 Jan;258(1 Pt 2):H45–H50. doi: 10.1152/ajpheart.1990.258.1.H45. [DOI] [PubMed] [Google Scholar]
- Taniguchi J., Kokubun S., Noma A., Irisawa H. Spontaneously active cells isolated from the sino-atrial and atrio-ventricular nodes of the rabbit heart. Jpn J Physiol. 1981;31(4):547–558. doi: 10.2170/jjphysiol.31.547. [DOI] [PubMed] [Google Scholar]
- Trube G., Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Arch. 1984 Jun;401(2):178–184. doi: 10.1007/BF00583879. [DOI] [PubMed] [Google Scholar]
- Vanheel B., Leybaert L., De Hemptinne A., Leusen I. Simulated ischemia and intracellular pH in isolated ventricular muscle. Am J Physiol. 1989 Aug;257(2 Pt 1):C365–C376. doi: 10.1152/ajpcell.1989.257.2.C365. [DOI] [PubMed] [Google Scholar]
- Weiss J. N., Venkatesh N., Lamp S. T. ATP-sensitive K+ channels and cellular K+ loss in hypoxic and ischaemic mammalian ventricle. J Physiol. 1992 Feb;447:649–673. doi: 10.1113/jphysiol.1992.sp019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A. A., Escande D., Schumacher C. A., Thuringer D., Mestre M., Fiolet J. W., Janse M. J. Potassium accumulation in the globally ischemic mammalian heart. A role for the ATP-sensitive potassium channel. Circ Res. 1990 Oct;67(4):835–843. doi: 10.1161/01.res.67.4.835. [DOI] [PubMed] [Google Scholar]
- Yazawa K., Kaibara M., Ohara M., Kameyama M. An improved method for isolating cardiac myocytes useful for patch-clamp studies. Jpn J Physiol. 1990;40(1):157–163. doi: 10.2170/jjphysiol.40.157. [DOI] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]


