Abstract
Background
Patients have been given magnesium to treat or prevent alcohol withdrawal syndrome (AWS). Evidence to support this practice is limited, and is often based on the controversial link between hypomagnesaemia and AWS.
Objectives
To assess the effects of magnesium for the prevention or treatment of AWS in hospitalised adults.
Search methods
We searched the Cochrane Drugs and Alcohol Group Register of Controlled Trials (August 2012), PubMed (from 1966 to August 2012 ), EMBASE (from 1988 to August 2012), CINAHL (from 1982 to March 2010), Web of Science (1965 to August 2012). We also carried out Internet searches.
Selection criteria
Randomised or quasi‐randomised trials of magnesium for hospitalised adults with, or at risk for, acute alcohol withdrawal.
Data collection and analysis
Two review authors independently extracted data with a standardised data extraction form, contacting the correspondence investigator if the necessary information was not available in the reports. Dichotomous outcomes were analysed by calculating the risk ratio (RR) for each trial, with the uncertainty in each result expressed with a 95% confidence interval (CI). Continuous outcomes were to be analysed by calculating the standardised mean difference (SMD) with 95% CI. For outcomes assessed by scales we compared and pooled the mean score differences from the end of treatment to baseline (post minus pre) in the experimental and control groups.
Main results
Four trials involving 317 people met the inclusion criteria. Three trials studied oral magnesium, with doses ranging from 12.5 mmol/day to 20 mmol/day. One trial studied parenteral magnesium (16.24 mEq q6h for 24 hours). Each trial demonstrated a high risk of bias in at least one domain. There was significant clinical and methodological variation between trials.
We found no study that measured all of the identified primary outcomes and met the objectives of this review. Only one trial measured clinical symptoms of seizure, delirium tremens or components of the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) score. A single outcome (handgrip strength) in three trials (113 people), was amenable to meta‐analysis. There was no significant increase in handgrip strength in the magnesium group (SMD 0.04; 95% CI ‐0.22 to 0.30). No clinically important changes in adverse events were reported.
Authors' conclusions
There is insufficient evidence to determine whether magnesium is beneficial or harmful for the treatment or prevention of alcohol withdrawal syndrome.
Keywords: Adult; Female; Humans; Male; Middle Aged; Administration, Oral; Alcohol Withdrawal Delirium; Alcohol Withdrawal Delirium/drug therapy; Alcohol Withdrawal Seizures; Alcohol Withdrawal Seizures/drug therapy; Alcoholic Beverages; Alcoholic Beverages/adverse effects; Central Nervous System Depressants; Central Nervous System Depressants/adverse effects; Ethanol; Ethanol/adverse effects; Hand Strength; Hospitalization; Magnesium; Magnesium/administration & dosage; Magnesium/blood; Magnesium Sulfate; Magnesium Sulfate/administration & dosage; Randomized Controlled Trials as Topic; Substance Withdrawal Syndrome; Substance Withdrawal Syndrome/blood; Substance Withdrawal Syndrome/drug therapy; Substance Withdrawal Syndrome/prevention & control
Plain language summary
Magnesium for the prevention or treatment of alcohol withdrawal syndrome in adults
Alcohol withdrawal syndrome (AWS) is a set of symptoms experienced when one reduces or stops alcohol consumption after prolonged periods of alcohol intake. Some studies show that AWS coincides with low levels of magnesium in the blood. Since magnesium may play a role in dampening the excitability of the central nervous system, some researchers believe that low levels of magnesium may make the central nervous system 'hyper‐excitable' and may cause AWS symptoms, which include sleeplessness, tremors, anxiety, headache, excessive sweating and reduced appetite. Many AWS treatment protocols therefore recommend magnesium supplementation.
The goal of our review was to determine whether magnesium supplementation prevents or treats AWS in adults. Our review of four trials covering 317 participants determined that there is not enough evidence about the benefits or harms of using magnesium supplements to prevent or treat AWS in adults.
Summary of findings
Summary of findings for the main comparison. Magnesium compared to placebo or standard care for symptoms of alcohol withdrawal syndrome.
| Magnesium compared to placebo or standard care for symptoms of alcohol withdrawal syndrome | ||||||
| Patient or population: people with symptoms of alcohol withdrawal syndrome Settings: hospital Intervention: magnesium Comparison: placebo or standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or standard care | Magnesium | |||||
| muscle strength dynamometer¹ Follow‐up: 6 ‐ 8 weeks | The mean muscle strength in the intervention groups was 0.04 standard deviations higher (0.22 lower to 0.3 higher) | 226 (3 studies) | ⊕⊝⊝⊝ very low2,3,4 | SMD 0.04 (‐0.22 to 0.3) | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 An isokinetic dynamometer was used in Aagaard 2005. A strain‐gauge dynamometer was used in Gullestad 1992 and Poikolainen 2008. 2 All of the included trials demonstrated unclear and high risk of bias in at least one category. 3 Hand grip strength is an indirect measure of muscle weakness‐related quality of life. 4 The total sample size is less than 400 (Cohen 1988) and the 95% confidence interval crosses the no‐effect line.
Background
Description of the condition
In the United States, alcohol dependence has an estimated lifetime and 12‐month prevalence of 12.5% and 3.8% respectively (Hasin 2007). A 2004 survey of six European countries (Belgium, France, Germany, Italy, the Netherlands and Spain) estimated a lifetime and 12‐month prevalence of 5.2% and 1.0% respectively (Alonso 2004). Moreover, 15% to 20% of primary care and hospitalised people have alcohol dependence and 8% have associated withdrawal symptoms (Dissanaike 2006; Foy 1995). The type and severity of symptoms of alcohol withdrawal syndrome (AWS) can vary between people and are positively correlated with the amount and duration of alcohol use. Initial minor withdrawal symptoms include insomnia, tremors, mild anxiety, gastrointestinal (GI) upset, headache, diaphoresis, palpitations, and anorexia, occurring within 6 to 12 hours after alcohol cessation (McKeon 2008). Approximately 7% of people with AWS can develop alcoholic hallucinations (visual, auditory, or tactile) 12 to 24 hours after the last alcohol consumption followed by tonic‐clonic withdrawal seizures in 5% to 10% of them p after 24 to 48 hours. The most life‐threatening complication of alcohol withdrawal is delirium tremens (DT), occurring in approximately 5% of people with AWS, with an associated mortality rate of 1% to 5%. Onset of DT is usually two to four days after withdrawal from alcohol but can also occur up to 14 days after. Symptoms during this time include hallucinations, disorientation, hypertension, tachycardia, low‐grade fever, diaphoresis, increased respiratory rate, and agitation. People have an increased likelihood of developing DT if they have a pre‐existing comorbidity, abnormal liver function, daily heavy alcohol use, older age, and previous history of DT or withdrawal seizures.
The Clinical Institute Withdrawal Assessment for Alcohol (CIWA) score is a validated scale that is the most commonly employed tool to measure withdrawal symptoms and to guide therapy (Sullivan 1989). The categories on this scale include sweating, anxiety, tremor, auditory or visual disturbances, agitation, nausea and vomiting, tactile disturbances, headache, and disorientation. Total symptom scores of more than 15 on this scale or a history of withdrawal seizures indicate that medications should be started at presentation. Unless delirium is present, medication is typically needed for no more than seven days after the last use of alcohol, although some people will report withdrawal symptoms, including sleep problems, for several more weeks. Protracted symptoms may precipitate relapse.
Description of the intervention
Both the standard and prophylactic treatments for people with AWS typically involve benzodiazepines, antipsychotics, folic acid, thiamine, and multivitamins (Mayo‐Smith 1997; Mayo‐Smith 2004; Vincent 2007). In a Cochrane overview (Amato 2011) of sedative benzodiazepines, anticonvulsants, baclofen, gammahydroxybutrate (GHB) and psychotropic analgesic nitrous oxide (PAN), only benzodiazepines performed better than placebo for people with AWS.
In some institutions magnesium sulphate is also given routinely or in response to hypomagnesaemia during the hospital stay where there is a risk of developing AWS or AWS is present (Beroz 1962; Shane 1991; Shulsinger 1977). A retrospective study of people with alcoholism in Seoul (Korea) Hospital showed that magnesium reduces the need for benzodiazepine therapy (Lee 2012). The study also showed that magnesium improves CIWA anxiety and sweating scores (Lee 2012). Nevertheless, current practice guidelines do not recommend routine administration of parenteral magnesium as existing controlled data do not demonstrate improvement in severity of alcohol withdrawal, delirium or seizures with its use (Mayo‐Smith 2004). Different formulations, routes of administration, and doses have been reported in the literature as being used for alcohol withdrawal, from oral magnesium supplements for eight weeks to parenteral magnesium 8 to 16 mEq every four to six hours for 48 to 72 hours (Beroz 1962; Poikolainen 2008; Shane 1991; Shulsinger 1977; Wilson 1984).
How the intervention might work
Current literature suggests the following mechanisms of action for magnesium in people with AWS:
Magnesium may have a moderating effect on elevated liver enzymes in alcoholics, and in theory may cause a decrease in the risk of death from alcoholic liver disease (Gullestad 1992; Poikolainen 2008). Gullestad et al suggest that magnesium is "essential in the maintenance of membrane integrity, which may be of importance in the protection from liver cell damage."
Hyperfunctioning of glutamatergic pathways is posited as an explanation for AWS (Prior 2011). Hyperfunctioning, mainly through N‐methyl‐D‐aspartate (NMDA) receptors, causes an excitotoxic influx of calcium and an increase in oxygen free radicals (Prior 2011). The resulting neural damage manifests as AWS. Magnesium cations may mitigate neural damage by competing with glumate's NMDA receptor binding site (Prior 2011).
Since several studies have correlated serum magnesium levels with severity of depression (see Murck 2002 for review), magnesium supplementation may regulate anxiety and depression during alcohol withdrawal. The antidepressant and anxiolytic effects of magnesium are in line with its NMDA antagonistic property; that is, NMDA antagonism at the level of the hypothalamus decreases hypothalamic‐pituitary‐adrenal (HPA) activity (Murck 2002), a known marker for affective disorders (Holsboer 2000).
The relation between magnesium level and alcohol ingestion was first demonstrated in the 1950s (Flink 1954; Flink 1956). In a series of experiments in the 1960s, it was found that in people with alcohol dependence the severity of delirium tremens (DT) symptoms was correlated with the degree of hypomagnesaemia measured (Embry 1987; Suter 1955). This relation was also found when magnesium levels were measured from cerebrospinal fluid (Glickman 1962; Mendelson 1969). The mechanism for this phenomenon was subsequently studied. In some studies, it was demonstrated that alcohol administration led to an acute increase in magnesium excretion in the range of 167% to 260% greater than for control subjects. Furthermore, decreased oral intake secondary to chronic alcoholism would also contribute to decreased magnesium levels (Jermain 1992). The authors also found a correlation between chronic alcoholism and low muscle magnesium levels. An increase in long chain free fatty acids has been described in people experiencing DT (Mays 1970). It is thought that these acids bind magnesium and thus lower the amount of free circulating magnesium in the blood. A correlation has also been found during alcohol withdrawal between hypomagnesaemia and sinus tachycardia (Shane 1991).
While hypomagnesaemia is a common finding in people undergoing AWS, it is unclear whether they are directly related or are two concurrent conditions occurring independently of each other. This distinction is further complicated by the similarity in signs and symptoms of the two conditions. For example, people with magnesium deficiency tetany but without AWS can present with spasms of facial muscles and extremities, convulsions, and laryngeal stridor (Jermain 1992). Other research disputing the correlation between hypomagnesaemia and AWS includes findings where the serum magnesium level returns to normal spontaneously before DT develops (Mendelson 1959; Victor 1973). In Martin 1959, it was found that there was no correlation between serum magnesium concentrations and hallucinations and severe tremors in people with AWS. Some authors (Jermain 1992) have concluded that there is no causal relationship between hypomagnesium and AWS, and that "routine administration of parenteral magnesium sulfate in patients with DT is not recommended."
Why it is important to do this review
Despite the controversial theories behind the correlation between hypomagnesaemia and AWS, magnesium is being used empirically at many institutions for people with AWS. Some institutions include magnesium as part of their alcohol withdrawal protocol, while others do not. Some authors have justified the use of magnesium in all patients as “magnesium is relatively safe”. On the other hand, it is unknown if magnesium treatment leads to harm, which may include serious adverse effects of magnesium such as central nervous system (CNS) depression, arrhythmias, heart block, hypotension, respiratory tract paralysis, coagulopathies, and hyporeflexia.
Objectives
To assess the effects of magnesium treatment/prophylaxis on the severity, duration, and progression of symptoms of alcohol withdrawal syndrome (AWS) in people hospitalised with AWS or those who are at risk of developing AWS.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). If full reports of trials were unavailable, we contacted the authors to obtain the information.
Types of participants
Hospitalised adults with a current history of alcohol dependence, at risk for or already in acute withdrawal.
Types of interventions
Any formulation, route of administration, or dose of magnesium in addition to standard of usual care. The comparator was placebo (usual standard of care).
Types of outcome measures
Primary outcomes
Number of participants with at least one seizure;
Number of participants who developed a first episode of delirium tremens (DT) (resolution and prevention of occurrences);
Number of participants who achieved a Clinical Institute Withdrawal Assessment for Alcohol (CIWA) score of 10 points or less.
Secondary outcomes
Death;
Serious adverse events;
Organ dysfunction (cardiac, renal, respiratory depression);
CIWA score components: nausea/vomiting, hallucinations, disorientation, hypertension, tachycardia, low‐grade fever, diaphoresis, increased respiratory rate, and agitation;
AWS severity;
Muscle weakness;
Bleeding;
Coma;
Paralysis;
Total adverse events;
Use of sedatives/psychotropics/phenytoin;
Hypotension;
Length of hospital stay;
Sleep.
Search methods for identification of studies
The searches incorporated a number of methods to identify completed or ongoing studies.
Electronic searches
We searched the following electronic databases:
The Cochrane Drugs and Alcohol Group Specialised Register (August 2012);
The Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2012, issue 8);
PubMed (from 1966 to August 2012);
EMBASE (from 1988 to August 2012);
CINAHL (from 1982 to March 2010);
Web of Science (1965 to August 2012).
We searched the databases using a strategy developed incorporating the filter for the identification of RCTs (Cochrane Handbook) combined with selected MeSH terms and free‐text terms relating to alcohol withdrawal syndrome. The PubMed search strategy was modified for the other databases using the appropriate controlled vocabulary.
The search strategies for all databases are shown in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5, Appendix 6.
We searched for ongoing clinical trials and unpublished studies with Internet searches on the following sites:
ClinicalTrials.gov (www.clinicaltrials.gov);
WHO International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/);
Current Controlled Trials (http://www.controlled‐trials.com/);
CenterWatch Clinical Trials Listing Service (http://centrewatch.com).
Searching other resources
We also searched:
References of the articles obtained by any means;
Conference proceedings likely to contain trials relevant to the review;
Contacting investigators and relevant trial authors seeking information about unpublished or incomplete trials.
All searches included non‐English language literature, and non‐English studies with English abstracts were assessed for inclusion. We translated studies considered likely to meet the inclusion criteria.
Data collection and analysis
Selection of studies
We assessed reports identified by the electronic searches for relevance based on a screening of titles and abstracts. Two review authors (AC and IK) independently inspected all study citations identified by the initial electronic searches, and obtained full reports of the studies of agreed relevance. Where there was disagreement, we acquired the full reports for more detailed scrutiny and consulted a third author to resolve disputes (AT). The review authors (AC and IK) then independently inspected all full study reports. We contacted the correspondence investigator if the necessary information was not available in the reports. An updated search was conducted in August 2012. All citations identified in the updated search were screened by one author (MS). Two authors (MS and AT) then screened full text articles identified studies that needed further review priot to inclusion.
Data extraction and management
Once all studies had been identified, the two review authors who had performed the original screening examined in detail those which fulfilled the inclusion criteria,using a standardised data extraction form.
Assessment of risk of bias in included studies
The 'risk of bias' assessment for RCTs and controlled clinical trials (CCTs) in this review was performed using the criteria recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook). The recommended approach for assessing risk of bias in studies included in Cochrane reviews is a two‐part tool, addressing seven specific domains, namely sequence generation and allocation concealment (selection bias), blinding of participants and providers (performance bias), blinding of outcome assessor (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other possible sources of bias. The first step is to describe what was reported to have happened in the study, and the second is to assign a judgement about the risk of bias for that domain as being at low, high or unclear risk. To make these judgements we used the criteria set out in the Cochrane Handbook but adapted to the addiction field. See Table 2 for details.
1. Criteria for risk of bias in RCTs and CCTs.
|
Item |
Judgement |
Description |
|
| 1 | random sequence generation (selection bias) | Low | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization |
| High | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention | ||
| Unclear | Insufficient information about the sequence generation process to permit judgement of ‘Yes’ or ‘No’. | ||
| 2 | allocation concealment (selection bias) | Low | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. |
| High | Investigators enrolling participants could possibly foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or non opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | ||
| Unclear | Insufficient information to permit judgement of ‘Yes’ or ‘No’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement | ||
| 3 | blinding of patients, provider, outcome assessor |
Low |
Blinding of participants, providers and outcome assessor and unlikely that the blinding could have been broken; Either participants or providers were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias. No blinding, but the objective outcome measurement are not likely to be influenced by lack of blinding |
| High | No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding; Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken; Either participants or outcome assessor were not blinded, and the non‐blinding of others likely to introduce bias |
||
| Unclear | Insufficient information to permit judgement of ‘Yes’ or ‘No’; | ||
| 5 | incomplete outcome data For all outcomes except retention in treatment or drop‐out |
Low |
No missing outcome data; Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; Missing data have been imputed using appropriate methods All randomised patients are reported/analysed in the group they were allocated to by randomisation irrespective of non‐compliance and co‐interventions (intention to treat) |
| High | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; |
||
| Unclear | Insufficient reporting of attrition/exclusions to permit judgement of ‘Yes’ or ‘No’ (e.g. number randomised not stated, no reasons for missing data provided; number of drop out not reported for each group); |
We addressed the domains of sequence generation and allocation concealment (avoidance of selection bias) by a single entry for each study.
Incomplete outcome data (avoidance of attrition bias) were assessed separately for results at the end of the study period and for results at follow‐up.
Measures of treatment effect
We analysed dichotomous outcomes by calculating the risk ratio (RR) for each trial, with the uncertainty in each result expressed with a 95% confidence interval (CI). Continuous outcomes were to be analysed by calculating the standardised mean difference (SMD) with a 95% CI. For outcomes assessed by scales we compared and pooled the mean score differences from the end of treatment to baseline (post minus pre) in the experimental and control groups.
Unit of analysis issues
Data from all patients individually randomised to each intervention were used in the analyses. We took care to identify situations where data were censored or excluded, and to distinguish between the total number of events and the total number of patients with a first event.
Dealing with missing data
In general if there were missing data, we contacted the authors of the study for clarification.
Assessment of heterogeneity
We conducted assessments for heterogeneity across the studies using the I² statistic (taking a threshold of 30% to 60% as indicating important heterogeneity) and the Chi² statistic (with statistical significance set at P < 0.10) (Cochrane Handbook; Higgins 2003). We explored clinical and methodological possible sources of heterogeneity, considering various study characteristics, including baseline risk factors for the outcomes of interest, duration of studies, age, type of magnesium, and gender distribution of participants across the studies.
Assessment of reporting biases
We planned sensitivity analyses to explore the implications of assuming that missing data were associated with a poor outcome or were imputed. The potential impact of missing data is addressed in the Discussion section.
Data synthesis
We used Cochrane Review Manager 5.1 software for all data analyses, basing quantitative analyses of outcomes on intention‐to‐treat results (i.e. including all participants randomised to their original groups). We used risk ratios (RRs) and the random‐effects model to combine outcomes across trials. For all dichotomous outcomes we calculated absolute risk reduction (ARR = risk difference x 100), and numbers needed to treat for benefit (NNTB = 1/risk difference).
Subgroup analysis and investigation of heterogeneity
We had planned to perform the following subgroup analyses:
Participants with low serum magnesium levels versus those with normal/elevated serum magnesium levels.
Previous co‐morbidities, specifically cardiovascular disease and renal dysfunction.
Studies that enrolled participants experiencing AWS versus studies that enrolled those at risk of AWS.
Participants with severe comorbid diseases versus mild to moderate comorbid diseases, as measured with appropriate validated scales.
Sensitivity analysis
We had planned sensitivity analysis to test for the robustness of the results, including the following analyses:
1. Trials with acceptable randomisation or concealment of allocation compared to those without. 2. Trials preformed with intention‐to‐treat analysis compared to those without. 3. Unblinded versus blinded trials. 4. Different doses of magnesium. 5. Different routes of administration. 6. Number of doses of magnesium.
Our meta‐analysis was not amenable to the planned subgroup and sensitivity analyses, as we had too few included studies to explore these variations.
Results
Description of studies
Results of the search
Of the 278 unique records retrieved by the search, we excluded 217 on the basis of title and abstract and a further 57 records on the basis of a full‐text reading. We included the remaining four records for a qualitative analysis.
Included studies
Participants
Four studies, with a total of 317 participants, were included in our review (see Figure 1). Seventy‐two per cent of the participants were men,and mean ages across the studies ranged from 41 to 57.
1.

Study flow diagram.
Interventions
Three trials (Aagaard 2005; Gullestad 1992; Poikolainen 2008) studied oral magnesium, with doses ranging from 12.5 mmol mg/day to 20 mmol mg/day. One trial (Wilson 1984) studied parenteral magnesium (16.24 mEq Mg sulphate IM q6h for 24 hours).
Settings
Two trials (Aagaard 2005; Poikolainen 2008) recruited participants from a hospital setting, one (Wilson 1984) from an outpatient clinic, while the fourth (Gullestad 1992) recruited from both a hospital and an outpatient clinic.
Countries of Origin
Norway (Gullestad 1992), Denmark (Aagaard 2005), Finland (Poikolainen 2008) and Canada (Wilson 1984).
See Characteristics of included studies table for further details.
Excluded studies
Of the 61 studies that we considered for a full‐text review, 57 were excluded for the following reasons:
Many (32) were not performed in people with alcohol dependence.
Many (20) did not trial magnesium.
In three studies, magnesium was not the only intervention.
A few were not randomised controlled trials.
Gisselman 1982 was not for acute withdrawal; all participants had been abstinent for at least three months.
See Characteristics of excluded studies table for reasons for exclusion.
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
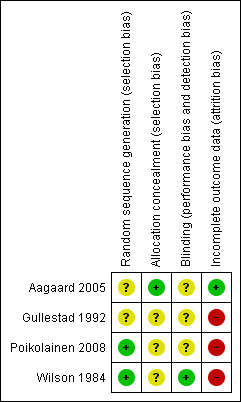
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Random sequence generation
Two studies were assessed as having unclear risk of bias, as it was stated that participants were randomised but without describing the methods (Aagaard 2005; Gullestad 1992). The other two trials were assessed as being at low risk of bias, as the randomisation sequence was acceptable and described in sufficient detail (Poikolainen 2008; Wilson 1984)
Allocation
Three included studies were assessed as being at unclear risk of bias, as only Aagaard 2005 reported concealment procedures in sufficient detail.
Blinding
Three studies were deemed to have an unclear risk of bias, as they either reported a double‐blind protocol but without describing the method (Gullestad 1992; Poikolainen 2008), or they provided no information about blinding (Aagaard 2005). Only one trial described blinding in sufficient detail to be assessed as being at low risk of bias for this domain (Wilson 1984).
Incomplete outcome data
Three trials (Gullestad 1992, Poikolainen 2008, Wilson 1984) were determined to be at high risk of bias for incomplete reporting of outcome data, as participants were lost to follow‐up without explanation. The trial authors did not respond to our requests for the missing data.
Effects of interventions
See: Table 1
Most of our outcomes of interest were not covered by the included studies. Among the secondary outcomes, only muscle strength was reported by three trials and was amenable to meta‐analysis (see Appendix 7).
The primary outcomes studied were muscle mass (no significant difference between groups) and muscle strength.
Muscle mass, measured by a formula that uses 24‐hour urinary creatinine, increased by 11% in both groups (14.7+/‐0.9 kg baseline to 16.3+/‐0.8 kg; P = 0.05) (no statistical comparison between groups).
Muscle strength, measured by an isokinetic dynamometer, increased by 14% in both groups (106+/‐6 Nm baseline to 121+/‐6 Nm; P < 0.001) (no statistical comparison between groups).
Additionally, the study did not find statistically significant differences for the following secondary outcomes: serum bilirubin, serum albumin, and plasma alkaline phosphate.
The study reported prothrombin times but did not report on bleeding rates.
Statistically significant differences between the magnesium group and the placebo group were found at the end of the study for the following outcomes:
Electrolytes
Participants in the magnesium group had higher levels of sodium, potassium, and magnesium.
-
Liver Enzymes
Participants in the magnesium group had lower levels of both aspartate aminotransferase and alanine aminotransferase.
Additionally, in the magnesium group, maximal handgrip strength, measured by a strain‐gauge dynamometer, increased significantly from baseline ((54+/‐29 bar to 62+/‐33 bar; P < 0.05) and (52+/‐31 bar to 57+/‐32 bar; P < 0.01) for right and left hand, respectively) while in the placebo group maximal handgrip strength remained unchanged.
The study reported on blood pressure changes, but not on participants experiencing hypotensive episodes.
An intention‐to‐treat analysis found no difference between groups in any outcomes measured. An analysis of study completers (27 of 64 allocated to magnesium and 31 of 54 allocated to placebo) found significant differences between groups for the following outcomes:
-
Electrolytes
After controlling for baseline serum magnesium levels, coffee consumption and non‐compliance, participants in the treatment group were found to have higher levels of serum magnesium.
-
Liver Enzymes
After controlling for age, body weight, baseline alcohol intake, subsequent change in alcohol intake and baseline serum aspartate aminotransferase (S‐AST), participants in the treatment groups were found to have lower levels of S‐AST.
They measured handgrip strength with a strain‐gauge dynamometer, but found no differences between intervention and placebo groups for either right or left hand comparisons (P = 0.445 and 0.436 for right and left hand respectively). It should be noted that this analysis was based on fewer than half the randomised population (i.e. people that completed the study).
People in the treatment group had higher levels of serum magnesium. This significant difference was transient, however, with no difference between groups 48 hours after intramuscular administration of magnesium. As participants were discharged, they were excluded from analysis of serum magnesium. Sufficient participants were available for analysis up to 72 hours post‐dose, but more than 20% were lost by 96 hours post‐dose.
Three of 50 participants in each group developed delirium tremens. Two of 50 participants in the magnesium group developed grand mal seizures, compared with three of 50 in the placebo group. No statistically significant differences were found, but this trial may have been underpowered for these outcomes.
The investigators reported no difference in alcohol withdrawal symptoms between groups (no statistical analysis provided) based on an investigator‐developed withdrawal rating scale that included several variables (diaphoresis, tremor, vomiting, hallucinations, withdrawal severity, grand mal seizures, delirium tremens) in common with the CIWA rating scale.
The investigators reported no difference in the amounts of first‐24 hour (P > 0.10), post‐24 hour (P > 0.10) and total (P > 0.10) chlordiazepoxide required to control AWS. Again, the trial may have been underpowered for these outcomes.
Muscle Strength
Three studies (Aagaard 2005; Gullestad 1992; Poikolainen 2008) measured differences in muscle strength between participants who received magnesium or placebo. The meta‐analysis detected no statistical heterogeneity or statistically significant difference in muscle strength (as measured by isokinetic and strain‐guage dynamometer) between the magnesium and placebo groups (standardised mean difference (SMD) 0.04; 95% confidence interval (CI) ‐0.22 to 0.30, Analysis 1.1; Figure 4).
1.1. Analysis.

Comparison 1 Magnesium versus placebo, Outcome 1 Muscle strength.
4.

Forest plot of comparison: 1 Magnesium versus placebo, outcome: 1.1 Muscle strength.
Discussion
Magnesium is being used in practice in the prevention and treatment of alcohol withdrawal. The degree of use is varied, with some clinicians routinely treating all people admitted with alcohol withdrawal, some using it only in people with low serum magnesium levels, and some not at all. It is hypothesised that the decrease in magnesium causes symptoms of alcohol withdrawal and possibly death. Possible mechanisms include: decreased magnesium intake, increased magnesium excretion, increased concentration of magnesium‐binding fatty acids, and modification of liver enzyme elevation. However, there are also conflicts within the literature about the role of magnesium and its utility for alcohol withdrawal.
Summary of main results
Only one included study (Wilson 1984) measured at least one of the primary outcomes specified in our protocol. Of the secondary outcomes identified, only muscle weakness (strength) was measured in three of the four included trials that allowed for meta‐analysis. However, the effect did not show a statistically significant difference in muscle strength between magnesium supplementation and placebo.
The included trials did not provide sufficient harms data for meta‐analysis; the risk of serious adverse events for magnesium (such as central nervous system (CNS) depression, arrhythmias, heart block, hypotension, respiratory tract paralysis, coagulopathies, and hyporeflexia) remains uncertain.
Overall completeness and applicability of evidence
Only Wilson 1984 measured clinical symptoms of seizure, delirium tremens or components of the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) score. However, it suffers from many limitations and describes participant populations and practices more than 20 years ago, some of which may not be applicable to present management of alcohol withdrawal. The one secondary outcome, reported in three trials, that could be meta‐analysed was muscle strength. However, this result should be interpreted with caution, as it is a surrogate marker for alcohol withdrawal complications, and confounding factors in measuring this outcome were not addressed or accounted for in any of the trials.
Quality of the evidence
The overall quality of the evidence is poor. All four included trials demonstrated unclear or high risks of bias in at least one domain. There was significant clinical heterogeneity in the studies pooled for meta‐analysis for participant population; diagnosis of alcohol dependence; inclusion criteria; type, dose and duration of magnesium treatment; and type of treatment facility. While the degree of clinical and methodological variation between the studies is high, the I² value (0%) of our meta‐analysis seems to indicate low statistical heterogeneity (Cochrane Handbook). However, given that the meta‐analysis included few studies of small size, our I² value is not a reliable indicator of heterogeneity.
Potential biases in the review process
We could not obtain additional information from authors of trials where risk of bias was unclear. We were unable to evaluate a trial published in Russian (Enmin 1969), as no translation was possible.
Although we specified subgroups and proposed sensitivity analyses a priori, the paucity of retrieved studies and the heterogeneity of outcomes measured meant we could not perform meaningful subgroup or sensitivity meta‐analysis.
Agreements and disagreements with other studies or reviews
Clinical practice guidelines published in 2004 do not recommend treatment of alcohol withdrawal delirium with magnesium, but they do report a suggestion of decreased neuromuscular activity with magnesium administration (Mayo‐Smith 2004). The guideline recommendation to correct electrolyte abnormalities, including magnesium deficiency, stems from expert opinion and biological rationale.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to support the routine use magnesium for prophylaxis or treatment in people experiencing or at risk of alcohol withdrawal. There is also insufficient evidence for magnesium treatment or prophylaxis in people with low serum magnesium experiencing or at risk of alcohol withdrawal .
Nevertheless, current practice guidelines recommend "that fluid status and electrolyte levels be monitored carefully and any abnormalities be corrected" (Mayo‐Smith 2004).
Implications for research.
Further research is needed to determine the role of magnesium in the prevention and treatment of alcohol withdrawal syndrome (AWS). A major limitation of this review was the paucity and heterogeneity of the studies' reported outcomes. The optimal trial would be randomised and placebo‐controlled, with adequate power to detect a statistically significant difference in the important complications of AWS (i.e. death, seizures and delirium tremens). Since the symptomatology of AWS is varied, it would be best captured by validated symptom scales such as the CIWA score. Total adverse events and serious adverse events are probably the appropriate outcomes for safety data.
Acknowledgements
Stephen Adams for assistance in retrieving articles.
Appendices
Appendix 1. CDAG Specialized Register search strategy
Alcohol* AND (magnesium OR MgSO4)
Appendix 2. CENTRAL search strategy
MeSH descriptor Alcohol‐Related Disorders explode all trees
MeSH descriptor Substance Withdrawal Syndrome explode all trees
(alcohol*):ti,ab,kw
(#1 OR #2 OR #3)
MeSH descriptor Magnesium Sulfate explode all trees
MeSH descriptor Magnesium explode all trees
(magnesium):ti,ab,kw
(MgSO4):ti,ab,kw
(( #5 AND or#6 ) OR #7 OR #8)
(#4 AND #9)
Appendix 3. PubMed search strategy
Alcohol‐related disorders[MeSH]
Alcohol‐Induced Disorders, Nervous System [MeSH]
((alcohol*[tiab] ) AND (disorder*[tiab] OR withdr*[tiab] OR abstinen*[tiab] OR abstain*[tiab] OR detox*[tiab] OR neuropathy[tiab] ))
#1 OR #2 OR #3
Magnesium[MeSH]
Magnesium Sulfate[MeSH]
MgSO4[tiab]
Magnesium [tiab]
#5 OR #6 OR #7 OR #8
randomized controlled trial [pt]
controlled clinical trial [pt]
randomized [tiab]
placebo [tiab]
drug therapy [sh]
randomly [tiab]
trial [tiab]
groups [tiab]
#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17
animals [mh] NOT humans [mh]
#18 NOT #19
#4 AND #9 AND #20
Appendix 4. EMBASE search strategy
'alcoholism'/exp
(alcohol*:ab,ti AND (disorder*:ab,ti OR withdr*:ab,ti OR abstinen*:ab,ti OR abstain*:ab,ti OR detox*:ab,ti OR neuropathy:ab,ti))
#1 OR #2
'magnesium'/exp
'magnesium sulfate'/exp
magnesium:ab,ti OR mgso4:ab,ti
#4 or #5 or #6
'crossover procedure'/exp
double blind procedure'/exp
'single blind procedure'/exp
'controlled clinical trial'/exp
'clinical trial'/exp OR
placebo:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti
random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti)
'randomized controlled trial'/exp
#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
#3 AND #7 AND #16 AND [humans]/lim AND [embase]/lim
Appendix 5. CINAHL search strategy
MH Alcohol‐related disorders
AB ((alcohol) and (disorder* or withdr* or abstinen* or abstain* or detox* or neuropathy))
TI ((alcohol) and (disorder* or withdr* or abstinen* or abstain* or detox* or neuropathy))
S1 OR S2 OR S3
MH 'Dietary Supplements'
MH Magnesium Sulfate
TX Magnesium
TX MgSO*
S5 or S6 or S7 or S
MH 'Animals' not (MH 'Animals' and MH 'Humans')
S4 and S9
S11 not S10
Appendix 6. ISI Web of Science search strategy
Topic=(alcohol*)
Topic=((disorder* OR withdr* OR abstinen* OR abstain* OR detox* OR neuropathy))
Topic=((magnesium OR MgSO4))
#1 AND #2 AND #3
Timespan=All Years. Databases=SCI‐EXPANDED, SSCI, A&HCI.
Appendix 7. Effects of interventions
| Outcomes Assessed by Included Studies | ||||
| Primary Outcome | Aagaard 2005 | Gullestad 1992 | Poikolainen 2008 | Wilson 1984 |
| Seizures | ✓ | |||
| Delerium Tremens | ✓ | |||
| CIWA Score | ||||
| Secondary Outcomes | ||||
| Death | ||||
| Serious Adverse Events | ||||
| Organ Dysfunction | ||||
| CIWA Score | ||||
| AWS Severity | ✓ | |||
| Muscle strength | ✓ | ✓ | ✓ | |
| Bleeding | ✓ | |||
| Coma | ||||
| Paralysis | ||||
| Total Adverse Events | ||||
| Use of sedatives/psychotropics/phenytoin | ✓ | |||
| Hypotension | ✓ | |||
| Length of hospital stay | ||||
| Sleep | ||||
Data and analyses
Comparison 1. Magnesium versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Muscle strength | 3 | 226 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.22, 0.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aagaard 2005.
| Methods | Randomisation: placebo‐controlled, parallel‐group Drop‐outs in placebo group: 10/34 Drop‐outs in treatment group: 7/25 |
|
| Participants | N = 59 randomised (49 men); mean age = 49 (range:34 ‐ 61) Recruitment Setting: hospital department of hepatology and gastroenterology in Denmark (1) Diagnosed with alcoholic liver disease (diagnosis based on liver biopsy or clinical, biochemical and ultrasonographic criteria) (2) Mean alcohol consumption of 187g/day (range: 60 ‐ 480 g/day); all participants had mean daily alcohol consumption of at least 60 g/day for minimum of 5 years (3) Median time of abstinence was 1 week; 30 participants had been abstinent for less than 3 weeks before baseline examinations; 15 were consuming alcohol during the study, but had reduced their daily alcohol consumption (from 140 g/day to 20 g/day) (4) 41% of the Mg‐treated participants had taken spironolactone for two weeks or more prior to study entry; 22% of the placebo‐treated had done so. |
|
| Interventions | Treatment: 2 X 8‐hr infusion of Mg (30 mmol MgSO4 dispensed in 11 of glucose solution 55 g/L) and six weeks of Mg (6.25 mmol Mg BID) supplementation Control: 2 X 8‐hr infusion of glucose solution and six weeks of twice‐daily placebo tablet. |
|
| Outcomes | Skeletal muscle content of Mg Maximum isokinetic muscle strength Skeletal muscle mass Skeletal muscle content of Na/K pumps Serum bilirubin Plasma alkaline phosphatase Prothrombin index Serum albumin. |
|
| Notes | Exclusion criteria: Non‐alcoholic liver disease, encephalopathy, neurological, psychiatric or severe cardiopulmonary disturbances, malignancies, insulin‐dependent diabetes mellitus, chronic diarrhoea, renal failure, thyroid disease, skeletal muscle disease Muscle strength data: used muscle strength data for non‐dominant knee which was measured using an isokinetic dynamometer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "patients were randomised to receive Mg or placebo treatment"; "The randomisation code was unknown to the investigators until the end of the data analysis"; "41 % of the Mg‐treated patients had taken spironolactone for 2 weeks or more prior to study entry, whereas only 22% of the placebo‐treated patients had done so" Comment: Although the study mentions a randomisation "code," the study fails to describe the process of randomisation adequately. |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation code was unknown to the investigators until the end of the data analysis" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: While the randomisation code was concealed, information about blinding is not reported. Blinding was probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotes: "One randomised, placebo‐treated patient was withdrawn from the study because of cancer, leaving only 33 patients in the placebo‐treated group. Sixteen patients {7/25 Mg group+9/34 placebo group dropouts) were not examined at the end of the trial, which in 15 patients was due to lack of compliance. In one patient the biopsy material was unusable for technical reasons. Forty‐two patients (18 Mg group+24 placebo group) completed the study"; "Two of the Mg‐treated patients received the drug for only 5/6 weeks. One patient was readmitted to the hospital after 5 weeks without the study medicine, and we chose to do the
examinations at that point. The other patient wanted to abandon the medication but agreed to have the study exit examinations done" Comment: Number of drop‐outs is comparable across groups; reasons for drop‐outs are also comparable (i.e. non‐compliance); analysis was done on participants who failed to complete the entire trial but it is not clear if the outcomes of the 16 patients who were not examined were accounted for. The author did not respond to our request for drop‐outs' missing data. |
Gullestad 1992.
| Methods | Randomisation: placebo‐controlled, double‐blinded, block of 8 Drop‐outs in placebo group: 3/25 Drop‐outs in treatment group: 0/27 |
|
| Participants | N = 52 randomised; mean age = 57 (range: 28 ‐ 84) Recruitment Setting: outpatient clinics and hospital in Norway (1) participants had history of continuous or periodic heavy alcohol abuse for at least 10 years (2) recruits from hospital were admitted for alcohol withdrawal syndrome, pancreatitis or alcohol intoxication. |
|
| Interventions | Treatment: six weeks of 5 mmol Mg‐citrate‐lactate tablets TID Control: matching placebo |
|
| Outcomes | Liver function tests (GGT, ASAT, ALAT, bilirubin, ALP) Haematological parameters (Hgb, white blood cells, MCV, platelets) Electrolytes (Na, K, Cl, Ca) Muscle strength (handgrip strength, as measured by a strain‐grip dynamometer) Blood pressure and heart rate Side effects (general wellbeing, fatigue) |
|
| Notes | Exclusion criteria: serum creatinine > 150 pmol/litre, cardiac AV conduction disturbances, and prior magnesium supplementation Muscle strength data: used data from left hand grip assuming that for most, this was the non‐dominant hand. It was measured using a Martin strain‐gauge dynamometer which measures hand grip strength. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "The patients were randomly assigned to receive either magnesium‐lactate‐citrate tablets, 15 mmol daily (5 mmol 3 times daily) or matching placebo"; "Block randomization of eight was used" Comment: The process of randomization is not described adequately. There was a statistically significant difference in age between participants in the treatment group and participants in the placebo group; the placebo group was younger. |
| Allocation concealment (selection bias) | Unclear risk | Authors do not describe methods for allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "We randomized 49 chronic alcoholics, moderate to heavy drinkers for at least 10 years to receive oral magnesium or placebo treatment for 6 weeks according to a double‐blind protocol" Comment: The study doesn't describe a blinding procedure adequately. It is therefore unclear whether the participants, providers or outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "There were three drop‐outs, all from the placebo group. One died suddenly, and two were lost from follow‐up. These subjects have been excluded in the statistical calculations" Comment: None of the three drop‐outs were accounted for and were all from the placebo group. This may have an impact on the results due to the small sample size. |
Poikolainen 2008.
| Methods | Randomisation: placebo‐controlled, parallel‐group Drop‐outs in placebo group: 23/54 Drop‐outs in treatment group: 37/64 |
|
| Participants | N = 118 randomised Recruitment Setting: outpatient clinic in Finland (1) recruits had mild to moderate alcohol withdrawal symptoms or elevated GGT (men > 80 UI, women > 50 UI) |
|
| Interventions | Treatment: 10 mmol Mg carbonate‐Mg acetate‐Mg hydroxide tablet mixture BID for eight weeks Control: matching placebo |
|
| Outcomes | Primary: (1) GGT Secondary: (1) AST (2) ALT (3) Muscle strength, as measured by handgrip dynamometer (4) Depressive symptoms, as assessed by 21‐item Beck Depression Inventory |
|
| Notes | Exclusion criteria: participants with symptoms (i.e. alcohol‐related psychosis) that required inpatient care; history of heart rhythm disturbances; contraindications against Mg treatment (i.e. heart failure, renal failure); abnormal serum creatinine Muscle strength data: used data from left hand grip assuming that for most, this was the non‐dominant hand. It was measured using a Martin strain‐gauge dynamometer which measures hand grip strength. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized as individuals. Codes determining groups were derived from computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Quotes: "codes [were] applied with the help of sealed opaque envelopes"; "Code was kept secret by one of us (HA) and broken after the last patient had had his after‐treatment examination" Comment: It's unclear whether HA, who apparently knew the randomization code prior to assignment, was involved in assignment and enrolment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Mg and placebo tablets were identical. Both groups were told of the possibility of mild diarrhoea as a side‐effect"; "The effect of Mg was studied in a randomized, parallel group, double‐blind trial" Comment: The blinding procedure is not described adequately. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "in spite of their promise to attend, many subjects failed to keep their follow‐up appointments. The follow‐up examination was completed by 58 patients (49%)" Comment: Data from authors' intention‐to‐treat analysis were not published. Authors were emailed for data in July 2012 but did not respond to our requests. |
Wilson 1984.
| Methods | Randomisation: placebo‐controlled, double‐blinded, parallel‐group Drop‐outs in placebo group: 4 participants not analysed Drop‐outs in treatment group: 5 participants not analysed Note: Number of participants randomised to each treatment group not reported |
|
| Participants | N = 87 randomised; age range:16 ‐ 65 Recruitment Setting: hospital‐based chemical withdrawal unit in Canada |
|
| Interventions | Treatment: 16.24 mEq Mg sulphate IM q6h for 24 hours Control: 0.9% NaCl IM solution All participants received alcohol withdrawal protocol, consisting of routine orders for chlordiazepoxide (50 ‐ 100 rng orally every 6 hours as required) and chlordiazepoxide (100 mg orally every 6 hours) for persistent withdrawal. |
|
| Outcomes | Mean of 3 x 7‐point rating scales used to assess: Diaphoresis Tremor Vomiting Hallucinations Overall severity of withdrawal. Chart review used to assess: Grand mal seizures Delinum tremens Amount of chlordiazepoxide Change in systolic blood pressure Change in diastolic blood pressure Change in heart rate. |
|
| Notes | Exclusion criteria: history of renal insufficiency or cardiac arrhythmia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patient ... was then assigned to treatment with either magnesium sulfate or normal saline according to a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the code was not broken until the study was completed" Comment: Risk of allocation concealment is unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "according to a double‐blind protocol"; "Staff did not have access to the serum magnesium levels until the ratings were completed and the patient had been discharged." "The solutions were supplied in identical ampules" Comment: Patients, intervention providers and outcome assessors probably blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "The small incidence of grand ma1 seizures and delirium tremens encountered in the present study did not lend itself to statistical analysis. Six of 100 patients in the study developed delirium tremens and three such patients were in each treatment group. Similarly, of five patients experiencing a total of six grand mal seizures (one patient in the magnesium sulfate group had two seizures), two were treated with magnesium sulfate and three with placebo" Comment: The above participants were excluded from statistical analysis As people were discharged, they were excluded from any analyses of serum magnesium. Sufficient participants were available for analysis up to 72 hours post‐dose, however more than 20% were lost by 96 hours post‐dose. Data from their intention‐to‐treat analysis were not published; authors did not respond to our request for missing data (email sent July 2012). |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aagaard 2003 | Study participants were not randomised to a magnesium treatment group |
| Alexander 1978 | Study was not conducted in people with a current history of alcohol dependence |
| Avsaroglu 2005 | Study participants were not randomised to a magnesium treatment group |
| Bardhan 1988 | Study was not conducted in participants with a current history of alcohol dependence |
| Bjorneboe 1988 | Study participants were not randomised to a magnesium treatment group. Not a randomised controlled trial. |
| Bonkovsky 1991 | Magnesium was given to study participants in combination with other interventions |
| Coetzee 1998 | Study was not conducted in participants with a current history of alcohol dependence |
| Colson 1964 | Study was not conducted in participants with a current history of alcohol dependence |
| Crews 2001 | Study participants were not randomised to a magnesium treatment group |
| Deynet 1979 | Study participants were not randomised to a magnesium treatment group |
| Dols 1985 | Study was not conducted in people with a current history of alcohol dependence |
| Enmin 1969 | Study participants were not randomised to a magnesium treatment group |
| Fogarty 2006 | Study was not conducted in people with a current history of alcohol dependence |
| Gilleran 1996 | Study was not conducted in people with a current history of alcohol dependence |
| Gisselman 1982 | Patients were not at risk for acute withdrawal; all participants were abstinent for at least three months |
| Gisselman 1983 | Study was a duplicate of Gisselman 1982 |
| Grove 1985 | Study was not conducted in people with a current history of alcohol dependence |
| Hantouche 1998 | Study was not conducted in people with a current history of alcohol dependence |
| Herpin 1996 | Study participants were not randomised to a magnesium treatment group |
| Herr 2000 | Study was not conducted in people with a current history of alcohol dependence |
| Hoes 1979 | Study participants were not randomised to a magnesium treatment group |
| Hoffmann 2004 | Study was not conducted in people with a current history of alcohol dependence |
| Hornyak 2004 | Not a randomised controlled trial |
| Hristova 1997 | Study participants were not randomised to a magnesium treatment group |
| Iber 1987 | Magnesium was given to study participants in combination with other interventions |
| Klag 1993 | Study participants were not randomised to a magnesium treatment group |
| Lee 2012 | Study was a retrospective chart review |
| Meyer 1977 | Study participants were not randomised to a magnesium treatment group |
| Monteiro 1997 | Study was not conducted in people with a current history of alcohol dependence |
| Mooney 1985 | Not a randomised controlled trial |
| Murck 1998 | Study was not conducted in people with a current history of alcohol dependence |
| Mussalo‐Rauhamaa 1987 | Study participants were not randomised to a magnesium treatment group |
| Mutzell 1988 | Study participants were not randomised to a magnesium treatment group |
| Naderi‐Heiden 2005 | Study was not conducted in people with a current history of alcohol dependence |
| Nowson 1989 | Study participants were not randomised to a magnesium treatment group |
| Paolisso 1989 | Study was not conducted in people with a current history of alcohol dependence |
| Pasternak 2005 | Study was not conducted in people with a current history of alcohol dependence |
| Pasternak 2006 | Study was not conducted in people with a current history of alcohol dependence |
| Pienaar 1995 | Study participants were not randomised to a magnesium treatment group |
| Princi 1997 | Study participants were not randomised to a magnesium treatment group |
| Prokop 1970 | Study was not conducted in people with a current history of alcohol dependence |
| Prokop 1971 | Not a randomised controlled trial |
| Pumarino 1996 | Study was not conducted in patients with a current history of alcohol dependence |
| Rissanen 1987 | Study participants were not randomised to a magnesium treatment group |
| Ritschel 1970 | Study was not conducted in people with a current history of alcohol dependence |
| Rouse 1983 | Study was not conducted in people with a current history of alcohol dependence |
| Schmidt 1994 | Study was not conducted in people with a current history of alcohol dependence |
| Schroder 2004 | Study was not conducted in people with a current history of alcohol dependence |
| Sherbaniuk 1985 | Study was not conducted in people with a current history of alcohol dependence |
| Simon 1988 | Study participants were not randomised to a magnesium treatment group |
| Singh 1996 | Study was not conducted in people with a current history of alcohol dependence |
| Sos 2004 | Study was not conducted in people with a current history of alcohol dependence |
| Stamler 1997 | Study participants were not randomised to a magnesium treatment group |
| Steyn 1986 | Study participants were not randomised to a magnesium treatment group |
| Ueshima 2004 | Study was not conducted in people with a current history of alcohol dependence |
| Wheeler 1983 | Study participants were not randomised to a magnesium treatment group |
| Zygmunt 2003 | Study was not conducted in people with a current history of alcohol dependence |
Differences between protocol and review
Minor changes in Objectives section to reflect recommended format.
Contributions of authors
I Fan Kuo: protocol development, search screening, study selection, interpretation of the data. Juliana Li: protocol development, search screening, study selection, interpretation of the data. Alice Chan: protocol development, search screening, study selection, interpretation of the data, writing/revising final review. Michael Sarai: Search screening, study selection, interpretation of the data, writing/revising final review. Aaron M Tejani: protocol development, search screening, study selection, interpretation of the data, writing/revising final review.
Sources of support
Internal sources
Office support provided by the Therapeutics Initiative at the University of British Columbia, Canada.
External sources
No sources of support supplied
Declarations of interest
None to declare.
New
References
References to studies included in this review
Aagaard 2005 {published data only}
- Aagaard NK, Andersen H, Vilstrup H, Clausen T, Jakobsen J, Dorup I. Magnesium supplementation and muscle function in patients with alcoholic liver disease: a randomized, placebo‐controlled trial. Scandinavian Journal of Gastroenterology 2005;40(8):972‐9. [DOI] [PubMed] [Google Scholar]
Gullestad 1992 {published data only}
- Gullestad L, Dolva LO, Soyland E, Manger AT, Falch D, Kjekshus J. Oral magnesium supplementation improves metabolic variables and muscle strength in alcoholics. Alcoholism, Clinical and Experimental Research. UNITED STATES: Department of Internal Medicine, Baerum Hospital, Sandvika, Norway., 1992; Vol. 16, issue 5:986‐90. [DOI] [PubMed]
Poikolainen 2008 {published data only}
- Poikolainen K, Alho H. Magnesium treatment in alcoholics: a randomized clinical trial. Substance Abuse Treatment, Prevention and Policy. England: Finnish Foundation for Alcohol Studies, Helsinki, Finland. kari.poikolainen@stakes.fi., 2008; Vol. 3:1. [DOI] [PMC free article] [PubMed]
Wilson 1984 {published data only}
- Wilson A, Vulcano B. A double‐blind, placebo‐controlled trial of magnesium sulfate in the ethanol withdrawal syndrome. Alcoholism, Clinical and Experimental Research 1984;8(6):542‐5. [PUBMED: 6393805] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aagaard 2003 {published data only}
- Aagaard NK, Andersen H, Vilstrup H, Clausen T, Jakobsen J, Dorup I. Decreased muscle strength and contents of Mg and Na,K‐pumps in chronic alcoholics occur independently of liver cirrhosis. Journal of Internal Medicine 2003;253(3):359‐66. [PUBMED: 12603504] [DOI] [PubMed] [Google Scholar]
Alexander 1978 {published data only}
- Alexander PE, Kammen DP, Bunney WE Jr. Serum calcium and magnesium in schizophrenia: relationship to clinical phenomena and neuroleptic treatment. The British Journal of Psychiatry : the Journal of Mental Science 1978;133:143‐9. [PUBMED: 354732] [DOI] [PubMed] [Google Scholar]
Avsaroglu 2005 {published data only}
- Avsaroglu D, Inal TC, Demir M, Attila G, Acarturk E, Emre Evlice Y, et al. Biochemical indicators and cardiac function tests in chronic alcohol abusers. Croatian Medical Journal 2005;46(2):233‐7. [PUBMED: 15849844] [PubMed] [Google Scholar]
Bardhan 1988 {published data only}
- Bardhan KD, Hunter JO, Miller JP, Thomson AB, Graham DY, Russell RI, et al. Antacid maintenance therapy in the prevention of duodenal ulcer relapse. Gut 1988;29(12):1748‐54. [PUBMED: 3065157] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjorneboe 1988 {published data only}
- Bjorneboe GE, Bjorneboe A, Johnsen J, Skylv N, Oftebro H, Gautvik KM, et al. Calcium status and calcium‐regulating hormones in alcoholics. Alcoholism, Clinical and Experimental Research 1988;12(2):229‐32. [PUBMED: 2837104] [DOI] [PubMed] [Google Scholar]
Bonkovsky 1991 {published data only}
- Bonkovsky HL, Singh RH, Jafri IH, Fiellin DA, Smith GS, Simon D, et al. A randomized, controlled trial of treatment of alcoholic hepatitis with parenteral nutrition and oxandrolone. II. Short‐term effects on nitrogen metabolism, metabolic balance, and nutrition. The American Journal of Gastroenterology 1991;86(9):1209‐18. [PUBMED: 1909086] [PubMed] [Google Scholar]
Coetzee 1998 {published data only}
- Coetzee EJ, Dommisse J, Anthony J. A randomised controlled trial of intravenous magnesium sulphate versus placebo in the management of women with severe pre‐eclampsia. British Journal of Obstetrics and Gynaecology 1998;105(3):300‐3. [PUBMED: 9532990] [DOI] [PubMed] [Google Scholar]
Colson 1964 {published data only}
- Colson JA, Gallay C. Trial treatment of hepatic metabolic disorders by an original formula containing mainly ornithine combined with various classical lipotropic substances [Essai de traitement des dysmetabolies hepatiques par une formule originale comportant pour la premiere fois de l'ornithine associé a diverses substances lipotropes classiques]. Toulouse Medical 1964;65:207‐29. [PUBMED: 14143540] [PubMed] [Google Scholar]
Crews 2001 {published data only}
- Crews FT, Braun CJ, Ali R, Knapp DJ. Interaction of nutrition and binge ethanol treatment on brain damage and withdrawal. Journal of Biomedical Science 2001;8(1):134‐42. [PUBMED: 11173987] [DOI] [PubMed] [Google Scholar]
Deynet 1979 {published data only}
- Deynet G, Reimer F. Treatment of alcoholic toxic fatty liver. A placebo‐controlled trial with hepavis (author's transl) [Therapie der alkohol‐toxischen Fettleber. Plazebo‐kontrollierte Doppelblindstudie mit Hepavis.]. MMW, Munchener Medizinische Wochenschrift 1979;121(24):831‐2. [PUBMED: 111085] [PubMed] [Google Scholar]
Dols 1985 {published data only}
- Dols W, Verho M, Rangoonwala B, Jaeger S. Piretanide, a potassium stable diuretic, in the treatment of essential hypertension: a double‐blind comparison of two formulations. The Journal of International Medical Research 1985;13(1):31‐9. [PUBMED: 3884409] [DOI] [PubMed] [Google Scholar]
Enmin 1969 {published data only}
- Enmin GM. Features of active anti‐alcohol therapy of patients who have suffered alcoholic psychoses [Osobennosti aktivnogo protivoalkogol'nogo lecheniia bol'nykh perenesshikh ostrye alkogol'nye psikhozy.]. Zhurnal Nevropatologii i Psikhiatrii Imeni S.S. Korsakova 1969;69(6):899‐905. [PUBMED: 5364243] [PubMed] [Google Scholar]
Fogarty 2006 {published data only}
- Fogarty A, Lewis SA, Scrivener SL, Antoniak M, Pacey S, Pringle M, et al. Corticosteroid sparing effects of vitamin C and magnesium in asthma: a randomised trial. Respiratory Medicine 2006;100(1):174‐9. [PUBMED: 16338599] [DOI] [PubMed] [Google Scholar]
Gilleran 1996 {published data only}
- Gilleran G, O'Leary M, Bartlett WA, Vinall H, Jones AF, Dodson PM. Effects of dietary sodium substitution with potassium and magnesium in hypertensive type II diabetics: a randomised blind controlled parallel study. Journal of Human Hypertension 1996;10(8):517‐21. [PUBMED: 8895035] [PubMed] [Google Scholar]
Gisselman 1982 {published data only}
- Gisselmann A, Moyal R, Mollard MA, Marin A. Placebo‐controlled study of a magnesium salt in 48 persons in a postalcoholic treatment center. Revue Alcool 1982;28:63‐74. [Google Scholar]
Gisselman 1983 {published data only}
- Gisselman A, Moyal R, Moard MA, Marin A. Clinical trial of a magnesium compound against placebo in 48 subjects in a post‐cure center for alcoholism. Gazette Medical Francais 1983;90:57‐61. [Google Scholar]
Grove 1985 {published data only}
- Grove O, Bekker C, Jeppe‐Hansen MG, Karstoft E, Sanchez G, Axelsson CK, et al. Ranitidine and high‐dose antacid in reflux oesophagitis. A randomized, placebo‐controlled trial. Scandinavian Journal of Gastroenterology 1985;20(4):457‐61. [PUBMED: 3895381] [DOI] [PubMed] [Google Scholar]
Hantouche 1998 {published data only}
- Hantouche EG, Guelfi JD, Comet D. alpha‐beta L‐aspartate magnesium in treatment of chronic benzodiazepine abuse: controlled and double‐blind study versus placebo [Alpha‐beta L‐aspartate de magnesium dans l'arret de la consommation chronique des benzodiazepines: etude controlee en double aveugle versus placebo.]. L'Encephale 1998;24(5):469‐79. [PUBMED: 9850822] [PubMed] [Google Scholar]
Herpin 1996 {published data only}
- Herpin D, Ragot S. Ambulatory blood pressure monitoring: non‐pharmacological and pharmacological treatment. Blood Pressure Monitoring 1996;1(3):241‐5. [PUBMED: 10226236] [PubMed] [Google Scholar]
Herr 2000 {published data only}
- Herr DL, Kelly K, Hall JB, Ulatowski J, Fulda GJ, Cason B, et al. Safety and efficacy of propofol with EDTA when used for sedation of surgical intensive care unit patients. Intensive Care Medicine 2000;26 Suppl 4:S452‐62. [PUBMED: 11310908] [DOI] [PubMed] [Google Scholar]
Hoes 1979 {published data only}
- Hoes MJ. The significance of the serum levels of vitamin B‐1 and magnesium in delirium tremens and alcoholism. The Journal of Clinical Psychiatry 1979;40(11):476‐9. [PUBMED: 489530] [PubMed] [Google Scholar]
Hoffmann 2004 {published data only}
- Hoffmann K, Schulze MB, Schienkiewitz A, Nothlings U, Boeing H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. American Journal of Epidemiology 2004;159(10):935‐44. [PUBMED: 15128605] [DOI] [PubMed] [Google Scholar]
Hornyak 2004 {published data only}
- Hornyak M, Haas P, Veit J, Gann H, Riemann D. Magnesium treatment of primary alcohol‐dependent patients during subacute withdrawal: an open pilot study with polysomnography. Alcoholism, Clinical and Experimental Research 2004;28(11):1702‐9. [PUBMED: 15547457] [DOI] [PubMed] [Google Scholar]
Hristova 1997 {published data only}
- Hristova EN, Rehak NN, Cecco S, Ruddel M, Herion D, Eckardt M, et al. Serum ionized magnesium in chronic alcoholism: is it really decreased?. Clinical Chemistry 1997;43(2):394‐9. [PUBMED: 9023146] [PubMed] [Google Scholar]
Iber 1987 {published data only}
- Iber FL. Evaluation of an oral solution to accelerate alcoholism detoxification. Alcoholism, Clinical and Experimental Research 1987;11(3):305‐8. [PUBMED: 3307499] [DOI] [PubMed] [Google Scholar]
Klag 1993 {published data only}
- Klag MJ, He J, Whelton PK, Chen JY, Qian MC, He GQ. Alcohol use and blood pressure in an unacculturated society. Hypertension 1993;22(3):365‐70. [PUBMED: 8349329] [DOI] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee KS, Joe KH. Magnesium & acamprosate for the treatment of alcohol withdrawal. Alcoholism, Clinical and Experimental Research 2012;36:296A. [Google Scholar]
Meyer 1977 {published data only}
- Meyer JG, Urban K. Electrolyte changes and acid base balance after alcohol withdrawal, with special reference to rum fits and magnesium depletion. Journal of Neurology 1977;215(2):135‐40. [PUBMED: 68105] [PubMed] [Google Scholar]
Monteiro 1997 {published data only}
- Monteiro CP, Varela A, Pinto M, Neves J, Felisberto GM, Vaz C, et al. Effect of an aerobic training on magnesium, trace elements and antioxidant systems in a Down syndrome population. Magnesium Research 1997;10(1):65‐71. [PUBMED: 9339840] [PubMed] [Google Scholar]
Mooney 1985 {published data only}
Murck 1998 {published data only}
- Murck H, Steiger A. Mg2+ reduces ACTH secretion and enhances spindle power without changing delta power during sleep in men ‐‐ possible therapeutic implications. Psychopharmacology 1998;137(3):247‐52. [PUBMED: 9683002] [DOI] [PubMed] [Google Scholar]
Mussalo‐Rauhamaa 1987 {published data only}
- Mussalo‐Rauhamaa H, Poikolainen K, Karkkainen P, Lehto J. Decreased serum selenium and magnesium levels in drunkenness arrestees. Drug and Alcohol Dependence 1987;20(2):95‐103. [PUBMED: 3678053] [DOI] [PubMed] [Google Scholar]
Mutzell 1988 {published data only}
- Mutzell S. Cardiovascular and some biochemical effects of high alcohol consumption. Upsala Journal of Medical Sciences 1988;93(3):277‐88. [PUBMED: 3238823] [DOI] [PubMed] [Google Scholar]
Naderi‐Heiden 2005 {published data only}
- Naderi‐Heiden A, Frey R, Presslich O, Frottier P, Willinger U, Blasbichler T, et al. Effect of intravenous magnesium sulphate in reducing irritability and restlessness in pure and polysubstance opiate detoxification. Psychiatry Research 2005;135(1):53‐63. [PUBMED: 15893381] [DOI] [PubMed] [Google Scholar]
Nowson 1989 {published data only}
- Nowson CA, Morgan TO. Magnesium supplementation in mild hypertensive patients on a moderately low sodium diet. Clinical and Experimental Pharmacology and Physiology 1989;16(4):299‐302. [PUBMED: 2663263] [DOI] [PubMed] [Google Scholar]
Paolisso 1989 {published data only}
Pasternak 2005 {published data only}
- Pasternak K, Dabrowski W, Wyciszczok T, Korycinska A, Dobija J, Biernacka J, et al. The relationship between magnesium, epinephrine and norepinephrine blood concentrations during CABG with normovolemic hemodilution. Magnesium Research 2005;18(4):245‐52. [PUBMED: 16548139] [PubMed] [Google Scholar]
Pasternak 2006 {published data only}
- Pasternak K, Dabrowski W, Dobija J, Wronskal J, Rzecki Z, Biernacka J. The effect of preoperative magnesium supplementation on blood catecholamine concentrations in patients undergoing CABG. Magnesium Research 2006;19(2):113‐22. [PUBMED: 16955723] [PubMed] [Google Scholar]
Pienaar 1995 {published data only}
- Pienaar WP, Roberts MC, Emsley RA, Aalbers C, Taljaard FJ. The thyrotropin releasing hormone stimulation test in alcoholism. Alcohol and Alcoholism 1995;30(5):661‐7. [PUBMED: 8554651] [PubMed] [Google Scholar]
Princi 1997 {published data only}
- Princi T, Artero M, Malusa N, Uxa L, Livia V, Reina G. Serum and intracellular magnesium concentrations in intoxicated chronic alcoholic and control subjects. Drug and A lcohol Dependence 1997;46(1‐2):119‐22. [PUBMED: 9246560] [DOI] [PubMed] [Google Scholar]
Prokop 1970 {published data only}
- Prokop L. Studies of the vegetative action mechanism of kavain and magnesium orotate [Untersuchungen zum vegetativen Wirkungsmechaismus von Kavain und Magnesium‐Orotat.]. HNO 1970;18(11):399‐402. [PUBMED: 4933704] [PubMed] [Google Scholar]
Prokop 1971 {published data only}
- Prokop L. Studies of the vegetative action mechanism of kavain and magnesium orotate [Untersuchungen zum vegetativen Wirkungsmechanismus von Kavain und Magnesium‐Orotat.]. Wiener Medizinische Wochenschrift (1946) 1971;121(19):399‐402. [PUBMED: 4934590] [PubMed] [Google Scholar]
Pumarino 1996 {published data only}
- Pumarino H, Gonzalez P, Oviedo S, Lillo R, Bustamante E. Assessment of bone status in intermittent and continuous alcoholics, without evidence of liver damage [Evaluacion del compromiso oseo en alcoholicos intermitentes y continuos, sin dano hepatico evidente.]. Revista Medica de Chile 1996;124(4):423‐30. [PUBMED: 9110481] [PubMed] [Google Scholar]
Rissanen 1987 {published data only}
- Rissanen A, Sarlio‐Lahteenkorva S, Alfthan G, Gref CG, Keso L, Salaspuro M. Employed problem drinkers: a nutritional risk group?. The American Journal of Clinical Nutrition 1987;45(2):456‐61. [PUBMED: 3812344] [DOI] [PubMed] [Google Scholar]
Ritschel 1970 {published data only}
- Ritschel WA, Wagensonner D, Heringer EA. Statistical evaluation of the effect of 3 stimulants of cellular metabolism [Statistische Auswertung uber die Wirksamkeit dreier Zell‐Stoffwechsel‐Stimulantia.]. Schweizerische Rundschau fur Medizin Praxis 1970;59(36):1278‐83. [PUBMED: 5283789] [PubMed] [Google Scholar]
Rouse 1983 {published data only}
- Rouse IL, Armstrong BK, Beilin LJ. The relationship of blood pressure to diet and lifestyle in two religious populations. Journal of Hypertension 1983;1(1):65‐71. [PUBMED: 6681026] [PubMed] [Google Scholar]
Schmidt 1994 {published data only}
- Schmidt ME, Kruesi MJ, Elia J, Borcherding BG, Elin RJ, Hosseini JM, et al. Effect of dextroamphetamine and methylphenidate on calcium and magnesium concentration in hyperactive boys. Psychiatry Research 1994;54(2):199‐210. [PUBMED: 7761553] [DOI] [PubMed] [Google Scholar]
Schroder 2004 {published data only}
- Schroder H, Marrugat J, Covas M, Elosua R, Pena A, Weinbrenner T, et al. Population dietary habits and physical activity modification with age. European J ournal of Clinical Nutrition 2004;58(2):302‐11. [PUBMED: 14749751] [DOI] [PubMed] [Google Scholar]
Sherbaniuk 1985 {published data only}
- Sherbaniuk RW, Wensel RH, Bailey RJ, Kirdeikis P, Fisher D, Thomson AB. Comparative study of cimetidine and Mylanta II in the 6‐week treatment of gastric ulcer. Journal of Clinical Gastroenterology 1985;7(3):211‐5. [PUBMED: 3894494] [DOI] [PubMed] [Google Scholar]
Simon 1988 {published data only}
- Simon J, Filipovsky J, Rosolova H, Topolcan O, Karlicek V. Cross‐sectional study of beer consumption and blood pressure in middle‐aged men. Journal of Human Hypertension 1988;2(1):1‐6. [PUBMED: 3266252] [PubMed] [Google Scholar]
Singh 1996 {published data only}
- Singh RB, Rastogi V, Singh R, Niaz MA, Srivastav S, Aslam M, et al. Magnesium and antioxidant vitamin status and risk of complications of ageing in an elderly urban population. Magnesium Research 1996;9(4):299‐306. [PUBMED: 9247878] [PubMed] [Google Scholar]
Sos 2004 {published data only}
- Sos C. Reduction of plasma lactate elevation and proteinuria by a complex dietary supplement in swimmers during over‐loading training. Acta Physiologica Hungarica 2004;91(3‐4):211‐9. [PUBMED: 16438115] [DOI] [PubMed] [Google Scholar]
Stamler 1997 {published data only}
- Stamler J, Caggiula AW, Grandits GA. Relation of body mass and alcohol, nutrient, fiber, and caffeine intakes to blood pressure in the special intervention and usual care groups in the Multiple Risk Factor Intervention Trial. American Journal of Clinical Nutrition 1997;65(1 Suppl):338S‐65S. [PUBMED: 8988947] [DOI] [PubMed] [Google Scholar]
Steyn 1986 {published data only}
- Steyn K, Jooste PL, Fourie JM, Parry CD, Rossouw JE. Hypertension in the coloured population of the Cape Peninsula. South African Medical Journal 1986;69(3):165‐9. [PUBMED: 3945870] [PubMed] [Google Scholar]
Ueshima 2004 {published data only}
- Ueshima K, Tachibana H, Suzuki T, Hiramori K. Factors affecting the blood concentration of ionized magnesium in patients in the acute phase of myocardial infarction. Heart and Vessels 2004;19(6):267‐70. [PUBMED: 15799172] [DOI] [PubMed] [Google Scholar]
Wheeler 1983 {published data only}
- Wheeler MS, Butts JD, Hudson P. Vitreous humor magnesium in alcoholics. The American Journal of Forensic Medicine and Pathology 1983;4(2):105‐10. [PUBMED: 6858995] [DOI] [PubMed] [Google Scholar]
Zygmunt 2003 {published data only}
- Zygmunt M, Heilmann L, Berg C, Wallwiener D, Grischke E, Munstedt K, et al. Local and systemic tolerability of magnesium sulphate for tocolysis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2003;107(2):168‐75. [PUBMED: 12648863] [DOI] [PubMed] [Google Scholar]
Additional references
Alonso 2004
- ESEMeD/MHEDEA 2000 Investigators. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatrica Scandinavica. Supplementum 2004;21(7):420. [DOI] [PubMed] [Google Scholar]
Amato 2011
- Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD008537] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beroz 1962
- Beroz E, Conran P, Blanchard RW. Parenteral magnesium in the prophylaxis and treatment of delirium tremens. American Journal of Psychiatry 1962;118:1042‐43. [DOI] [PubMed] [Google Scholar]
Cochrane Handbook
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Cohen 1988
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Edition. New Jersey: Lawrence Erlbaum Associates, 1988. [Google Scholar]
Dissanaike 2006
- Dissanaike S, Halldorsson A, Frezza EE, Griswold J. An ethanol protocol to prevent alcohol withdrawal syndrome. Journal of the American College of Surgeons 2006;203:186‐91. [DOI] [PubMed] [Google Scholar]
Embry 1987
- Embry CK, Lippmann. Use of magnesium sulphate in alcohol withdrawal. American Family Physician 1987;35:167‐70. [PubMed] [Google Scholar]
Flink 1954
- Flink EB, Stutzman FL, Anderson AR, Konig T, Fraser R. Magnesium deficiency after prolonged parenteral fluid administration and after chronic alcoholism complicated by delirium tremens. Journal of Laboratory and Clinical Medicine 1954;43(2):169‐83. [PubMed] [Google Scholar]
Flink 1956
- Flink EB. Magnesium deficiency syndrome in man. Journal of the American Medical Association 1956;160:1406‐9. [PubMed] [Google Scholar]
Foy 1995
- Foy A, Kay J. The incidence of alcohol‐related problems and the risk of alcohol withdrawal in a general hospital population. Drug and Alcohol Review 1995;14:49‐54. [DOI] [PubMed] [Google Scholar]
Glickman 1962
- Glickman LS, Schenker V, Grolnick S, Green A, Schenker A. Cerebrospinal fluid cation levels in delirium tremens with special reference to magnesium. Journal of Nervous and Mental Disease 1962;134:410‐4. [PUBMED: 13899236] [DOI] [PubMed] [Google Scholar]
Hasin 2007
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM‐IV alcohol abuse and dependence in the United States. Archives of General Psychiatry 2007;64:830‐42. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holsboer 2000
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000;23:477‐501. [DOI] [PubMed] [Google Scholar]
Jermain 1992
- Jermain DM, Drismon ML, Nisbet RB. Controversies over the use of magnesium sulphate in delirium tremens. Annals of Pharmacotherapy 1992;26:650‐2. [DOI] [PubMed] [Google Scholar]
Martin 1959
- Martin HE, McCuskey C, Tupikova N. Electrolyte disturbance in acute alcoholism with particular reference to magnesium. American Journal of Clinical Nutrition 1959;7:191‐6. [DOI] [PubMed] [Google Scholar]
Mayo‐Smith 1997
- Mayo‐Smith MF. Pharmacological management of alcohol withdrawal: a meta‐analysis and evidence‐based practice guideline. JAMA 1997;278:144‐51. [DOI] [PubMed] [Google Scholar]
Mayo‐Smith 2004
- Mayo‐Smith MF, Beecher LH, Fischer TL, Gorelick DA, Guillaume JL, Hill A, et al. Management of alcohol withdrawal delirium: an evidence‐based practice guideline. Archives of Internal Medicine 2004;164:1405‐12. [DOI] [PubMed] [Google Scholar]
Mays 1970
- Mays ET, Ransdell HT, DeWeese BM. Metabolic changes in surgical delirium tremens. Surgery 1970;67(5):780‐8. [PUBMED: 5438679] [PubMed] [Google Scholar]
McKeon 2008
- McKeon A, Frye MA, Delanty N. The alcohol withdrawal syndrome. Journal of Neurology, Neurosurgery and Psychiatry 2008;79:854‐862. [DOI] [PubMed] [Google Scholar]
Mendelson 1959
- Mendelson J, Wexler D, Kubzansky P, Leiderman H, Solomon P. Serum magnesium in delirium tremens and alcoholic hallucinosis. Journal of Nervous and Mental Disease 1959;128:352‐7. [PubMed] [Google Scholar]
Mendelson 1969
- Mendelson JH, Ogata M, Mello NK. Effects of alcohol ingestion and withdrawal on magnesium status of alcoholics: clinical and experimental findings. Annals of the New York Academy of Sciences 1969;162:918‐33. [DOI] [PubMed] [Google Scholar]
Murck 2002
- Murck H. Magnesium and affective disorders. Nutritional Neuroscience 2002;5:375‐389. [DOI] [PubMed] [Google Scholar]
Prior 2011
- Prior PL, Galduroz JC. Glutamatergic hyperfunctioning during alcohol withdrawal syndrome: therapeutic perspective with zinc and magnesium.. Medical Hypotheses 2011 Sep;77(3):368‐70. [DOI] [PubMed] [Google Scholar]
Shane 1991
- Shane SR, Flink EB. Magnesium deficiency in alcohol addiction and withdrawal. Magnesium and Trace Elements 1991‐1992;10:263‐68. [PubMed] [Google Scholar]
Shulsinger 1977
- Shulsinger OZ, Forni PJ, Clyman BB. Magnesium sulfate in the treatment of alcohol withdrawal: a comparison with diazepam. Currents in alcoholism. New York: Grune and Stratton, 1977:319‐27. [Google Scholar]
Sullivan 1989
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA‐Ar). British Journal of Addiction 1989;84(11):1353‐7. [PUBMED: 2597811] [DOI] [PubMed] [Google Scholar]
Suter 1955
- Suter C, Klingman WO. Neurologic manifestations of magnesium depletion states. Neurology 1955;4:691‐9. [DOI] [PubMed] [Google Scholar]
Victor 1973
- Victor M. The role of hypomagnesaemia and alkalosis to alcohol withdrawal symptoms. Annals of the New York Academy of Sciences 1973;215:235‐48. [DOI] [PubMed] [Google Scholar]
Vincent 2007
- Vincent WR, Smith KM, Winstead PS, Lewis DA. Review of alcohol withdrawal in the hospitalised patient: management. Orthopedics 2007;30:446‐49. [DOI] [PubMed] [Google Scholar]


