Abstract
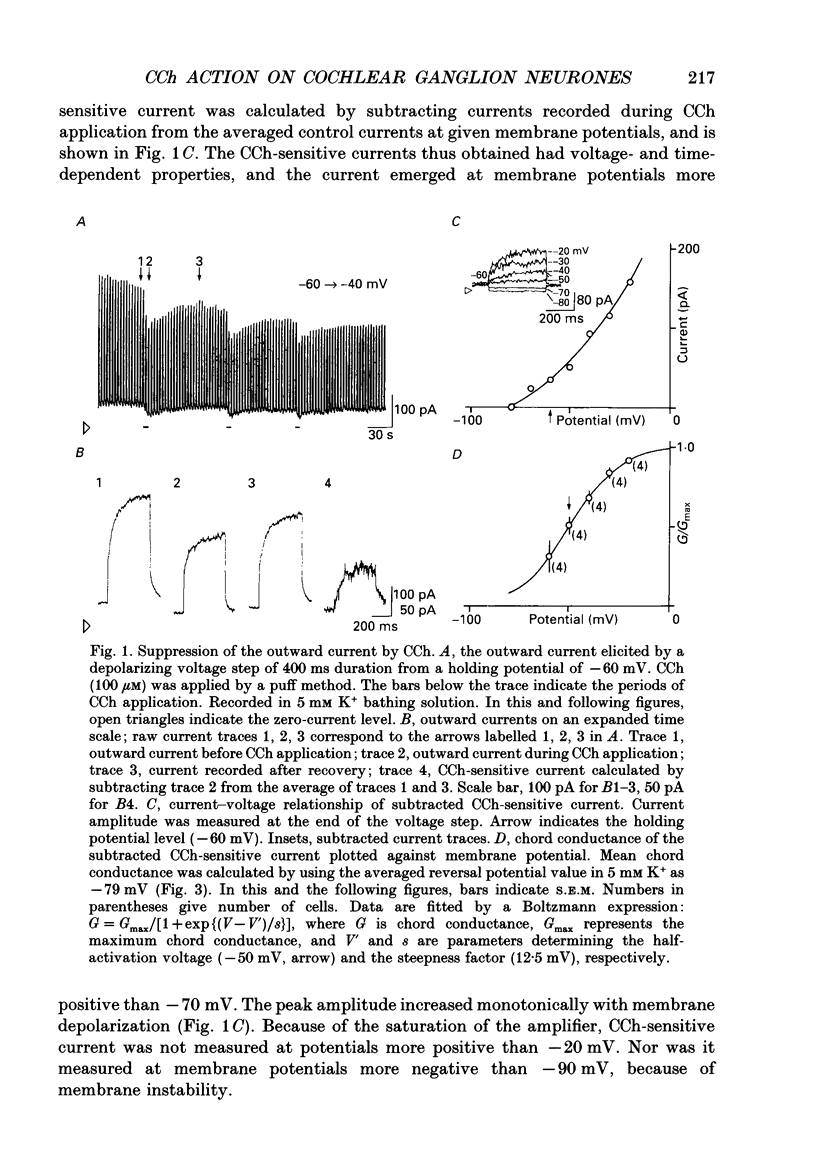
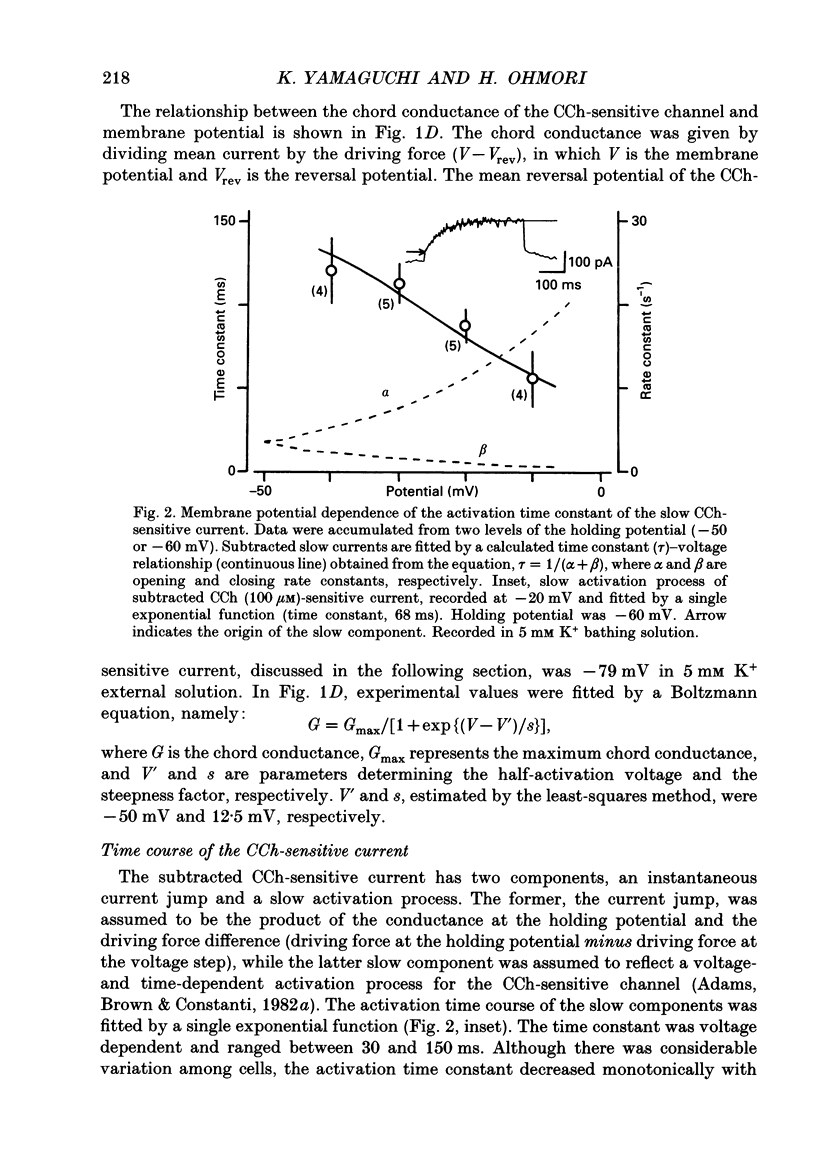
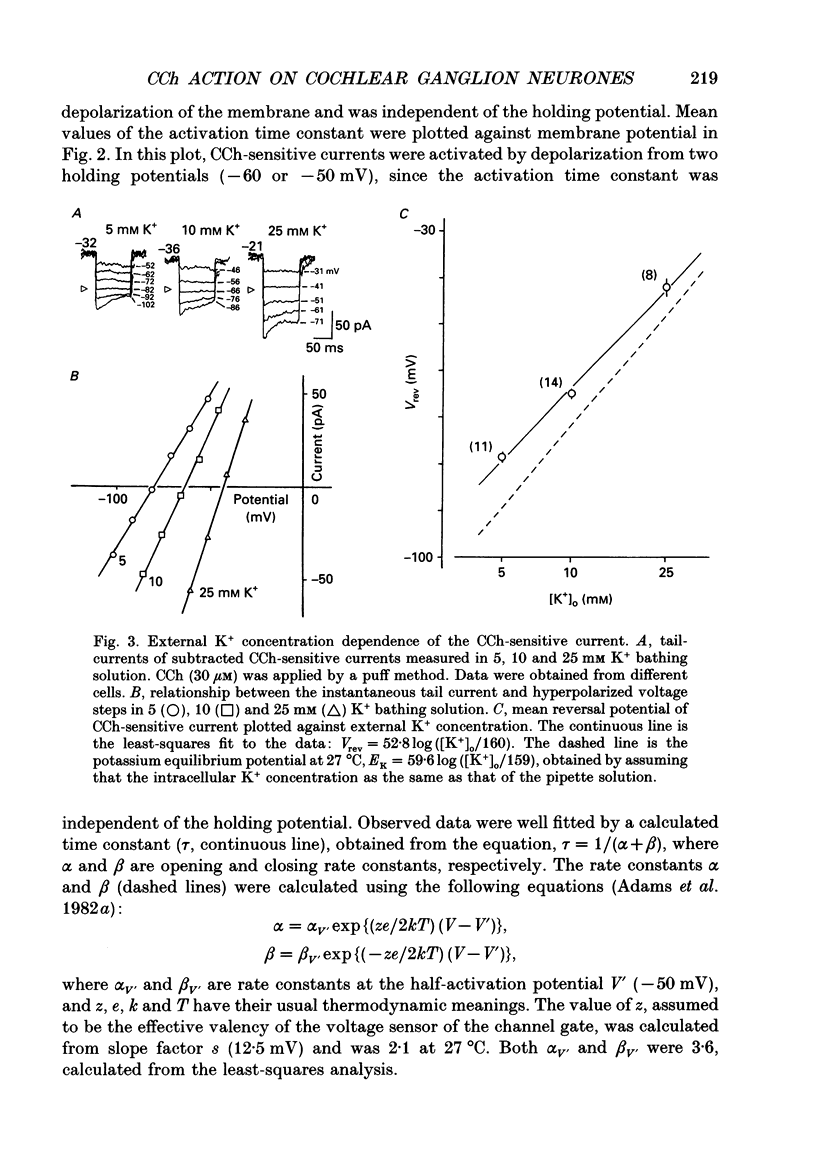
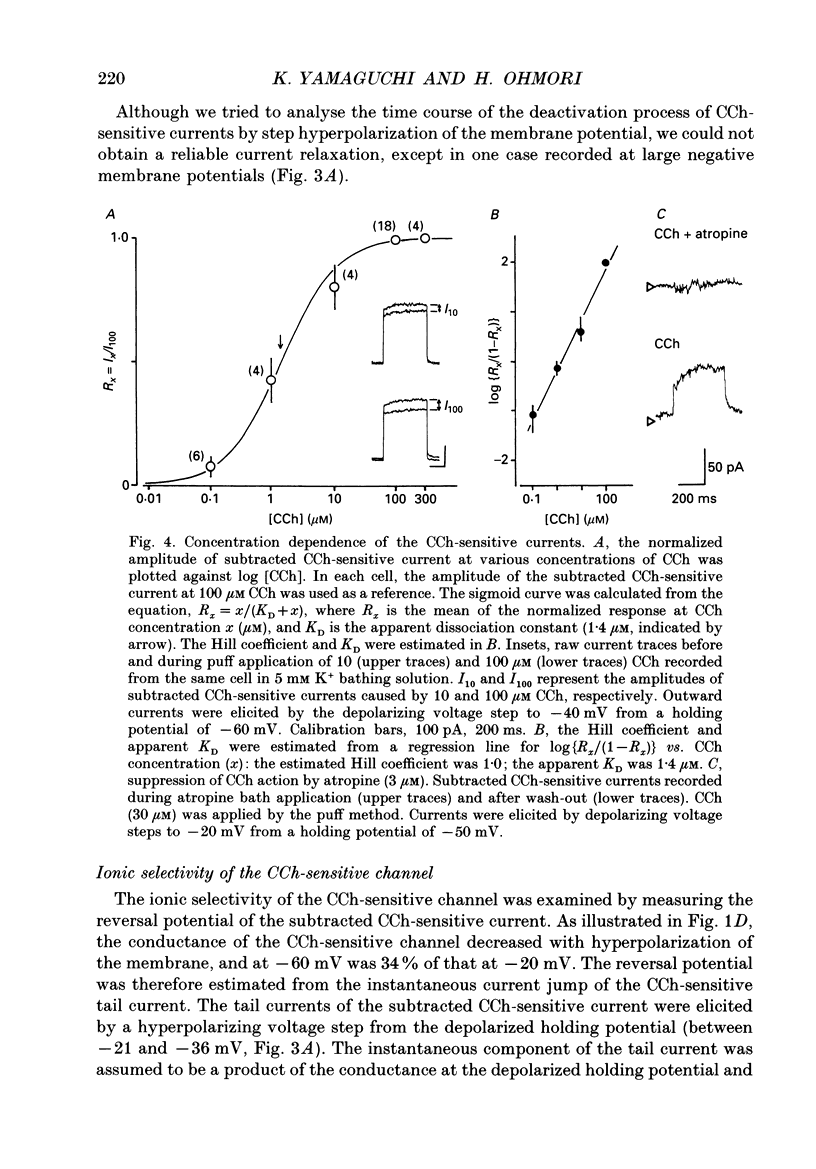
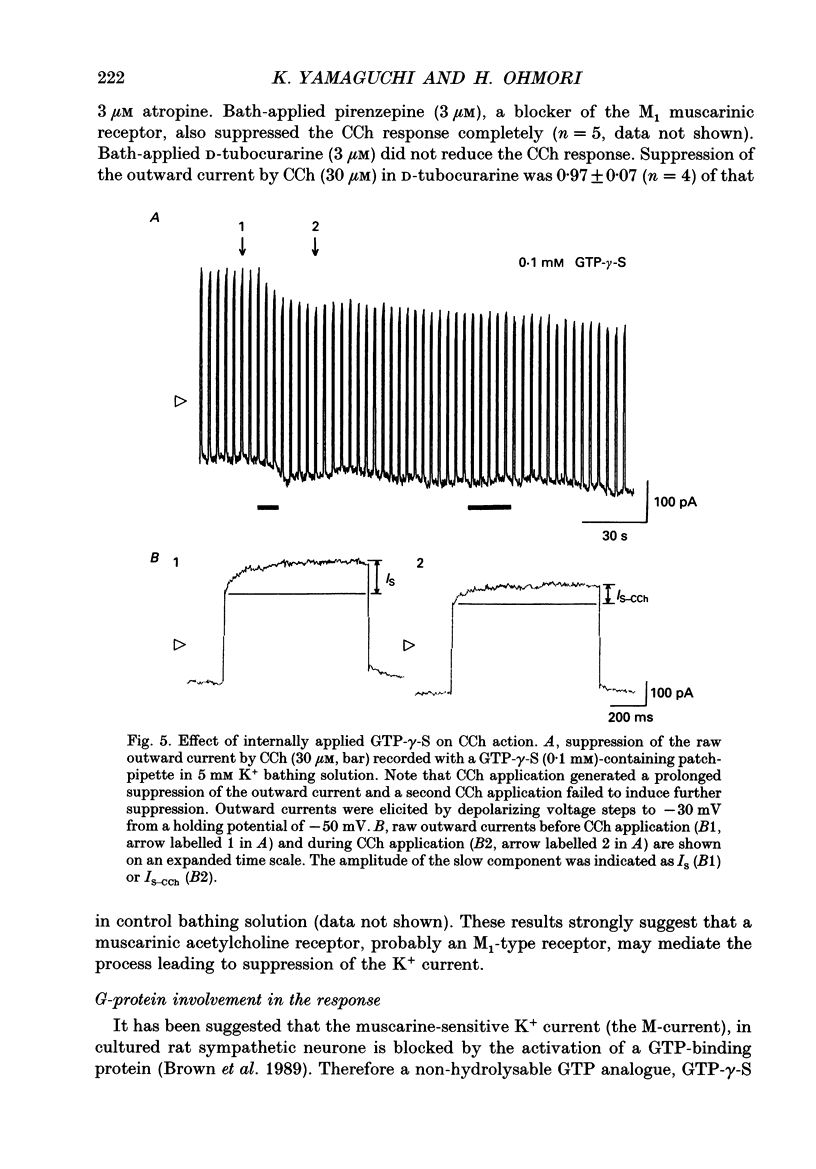
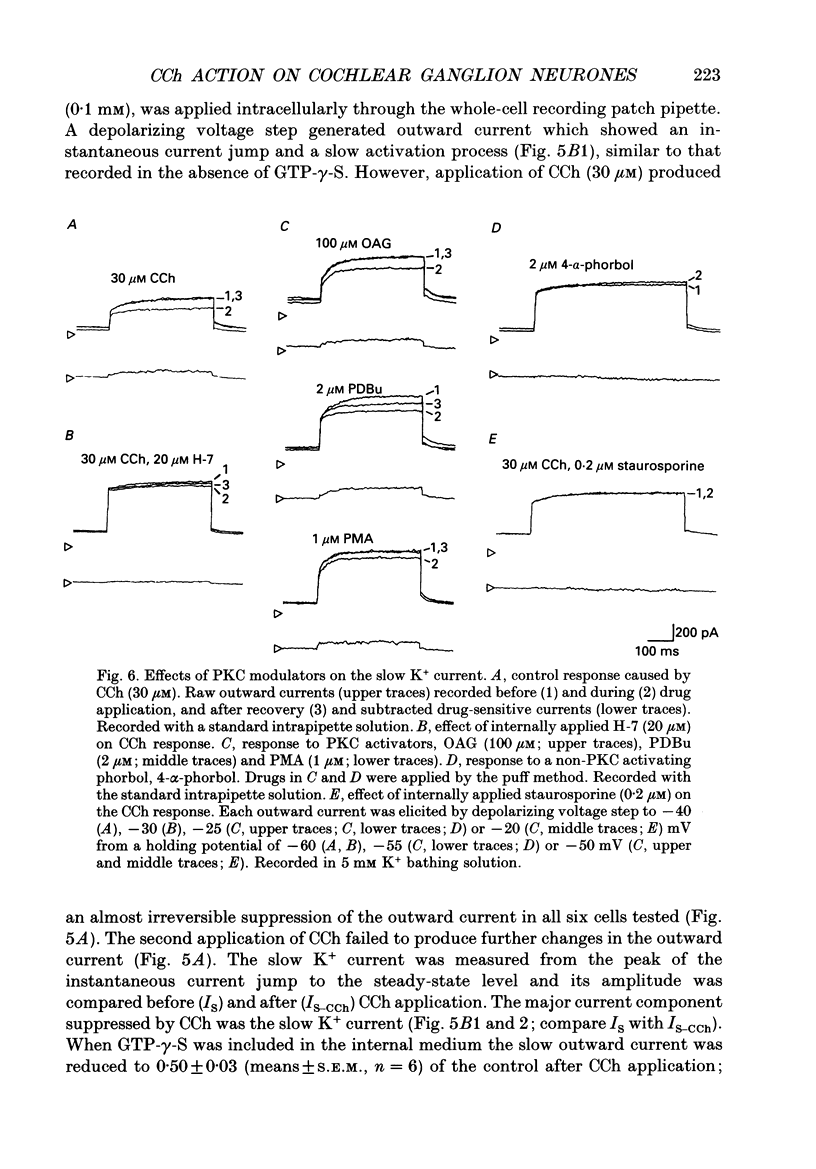
1. Effects of cholinergic agonists on the cultured chick cochlear ganglion (CG) neurone were examined using the whole-cell patch-clamp method. 2. Acetylcholine (ACh, 0.1-100 microM) and its non-hydrolysable form, carbamylcholine (CCh, 0.1-300 microM), suppressed the outward current. The CCh-sensitive current was activated at membrane potentials more positive than -70 mV. 3. The CCh-sensitive current slowly activated after step depolarization with a time constant from 20 to 150 ms. The activation time constant decreased monotonically with depolarization of the membrane. 4. The reversal potential of CCh-sensitive current changed as a function of the external K+ concentration (-79, -65 and -44 mV in 5, 10 and 25 mM, respectively) and was approximately equal to the potassium equilibrium potential (-89, -71 and -48 mV in 5, 10 and 25 mM, respectively). The CCh-sensitive current is concluded to be K+ selective. 5. The CCh-sensitive current showed a sigmoid log dose vs. response relationship with an apparent dissociation constant (KD) of 1.4 microM and a Hill coefficient of 1.0. When ACh was applied, an apparent KD of 1.8 microM and a Hill coefficient of 1.0 was measured. 6. The suppression of K+ current by CCh was blocked by atropine (3 microM) and pirenzepine (3 microM), suggesting that the current is mediated by an M1 muscarinic receptor. 7. The CCh suppression of the K+ current was enhanced by GTP-gamma-S (0.1 mM), suggesting that a GTP-binding protein is involved. 8. The CCh suppression of the K+ current was mimicked by protein kinase C activators, 1-oleoyl-2-acetyl-sn-glycerol (OAG, 100 microM), phorbol dibutyrate (PDBu, 2 microM) and phorbol 12-myristate 13-acetate (PMA, 1 microM). The protein kinase inhibitor, staurosporine (0.2 microM) applied internally blocked the CCh suppression of the K+ current which suggests an involvement of protein kinase C.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art J. J., Fettiplace R., Fuchs P. A. Synaptic hyperpolarization and inhibition of turtle cochlear hair cells. J Physiol. 1984 Nov;356:525–550. doi: 10.1113/jphysiol.1984.sp015481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bosma M. M., Hille B. Protein kinase C is not necessary for peptide-induced suppression of M current or for desensitization of the peptide receptors. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2943–2947. doi: 10.1073/pnas.86.8.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Inositol 1,4,5-trisphosphate and diacylglycerol mimic bradykinin effects on mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:185–207. doi: 10.1113/jphysiol.1988.sp016995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Marrion N. V., Smart T. G. On the transduction mechanism for muscarine-induced inhibition of M-current in cultured rat sympathetic neurones. J Physiol. 1989 Jun;413:469–488. doi: 10.1113/jphysiol.1989.sp017664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. M. Acetylcholinesterase activity in the embryonic chick's inner ear. Hear Res. 1987;28(1):57–63. doi: 10.1016/0378-5955(87)90153-5. [DOI] [PubMed] [Google Scholar]
- Fex J., Altschuler R. A. Neurotransmitter-related immunocytochemistry of the organ of Corti. Hear Res. 1986;22:249–263. doi: 10.1016/0378-5955(86)90102-4. [DOI] [PubMed] [Google Scholar]
- Furukawa T. Effects of efferent stimulation on the saccule of goldfish. J Physiol. 1981 Jun;315:203–215. doi: 10.1113/jphysiol.1981.sp013742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahwiler B. H., Brown D. A. Functional innervation of cultured hippocampal neurones by cholinergic afferents from co-cultured septal explants. Nature. 1985 Feb 14;313(6003):577–579. doi: 10.1038/313577a0. [DOI] [PubMed] [Google Scholar]
- Gifford M. L., Guinan J. J., Jr Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses. Hear Res. 1987;29(2-3):179–194. doi: 10.1016/0378-5955(87)90166-3. [DOI] [PubMed] [Google Scholar]
- Goldberg J. M., Fernández C. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J Neurophysiol. 1980 Apr;43(4):986–1025. doi: 10.1152/jn.1980.43.4.986. [DOI] [PubMed] [Google Scholar]
- Guinan J. J., Jr, Warr W. B., Norris B. E. Differential olivocochlear projections from lateral versus medial zones of the superior olivary complex. J Comp Neurol. 1983 Dec 10;221(3):358–370. doi: 10.1002/cne.902210310. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Highstein S. M., Baker R. Action of the efferent vestibular system on primary afferents in the toadfish, Opsanus tau. J Neurophysiol. 1985 Aug;54(2):370–384. doi: 10.1152/jn.1985.54.2.370. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley G. D., Ashmore J. F. Direct measurement of the action of acetylcholine on isolated outer hair cells of the guinea pig cochlea. Proc Biol Sci. 1991 May 22;244(1310):161–167. doi: 10.1098/rspb.1991.0065. [DOI] [PubMed] [Google Scholar]
- Klinke R., Galley N. Efferent innervation of vestibular and auditory receptors. Physiol Rev. 1974 Apr;54(2):316–357. doi: 10.1152/physrev.1974.54.2.316. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Norris C. H., Housley G. D., Williams W. H., Guth S. L., Guth P. S. The acetylcholine receptors of the semicircular canal in the frog (Rana pipiens). Hear Res. 1988 Feb-Mar;32(2-3):197–206. doi: 10.1016/0378-5955(88)90092-5. [DOI] [PubMed] [Google Scholar]
- Northup J. K., Smigel M. D., Sternweis P. C., Gilman A. G. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution of the activated 45,000-dalton (alpha) subunit. J Biol Chem. 1983 Sep 25;258(18):11369–11376. [PubMed] [Google Scholar]
- Pfaffinger P. J., Leibowitz M. D., Subers E. M., Nathanson N. M., Almers W., Hille B. Agonists that suppress M-current elicit phosphoinositide turnover and Ca2+ transients, but these events do not explain M-current suppression. Neuron. 1988 Aug;1(6):477–484. doi: 10.1016/0896-6273(88)90178-x. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. Muscarine and t-LHRH suppress M-current by activating an IAP-insensitive G-protein. J Neurosci. 1988 Sep;8(9):3343–3353. doi: 10.1523/JNEUROSCI.08-09-03343.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto T., Ohmori H. Muscarinic agonists and ATP increase the intracellular Ca2+ concentration in chick cochlear hair cells. J Physiol. 1990 Jan;420:127–148. doi: 10.1113/jphysiol.1990.sp017904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto T., Ohmori H. Muscarinic receptor hyperpolarizes cochlear hair cells of chick by activating Ca(2+)-activated K+ channels. J Physiol. 1991 Oct;442:669–690. doi: 10.1113/jphysiol.1991.sp018814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Tokimasa T., Akasu T. ATP regulates muscarine-sensitive potassium current in dissociated bull-frog primary afferent neurones. J Physiol. 1990 Jul;426:241–264. doi: 10.1113/jphysiol.1990.sp018136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead M. C., Morest D. K. The growth of cochlear fibers and the formation of their synaptic endings in the avian inner ear: a study with the electron microscope. Neuroscience. 1985 Jan;14(1):277–300. doi: 10.1016/0306-4522(85)90178-2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Ohmori H. Voltage-gated and chemically gated ionic channels in the cultured cochlear ganglion neurone of the chick. J Physiol. 1990 Jan;420:185–206. doi: 10.1113/jphysiol.1990.sp017907. [DOI] [PMC free article] [PubMed] [Google Scholar]


