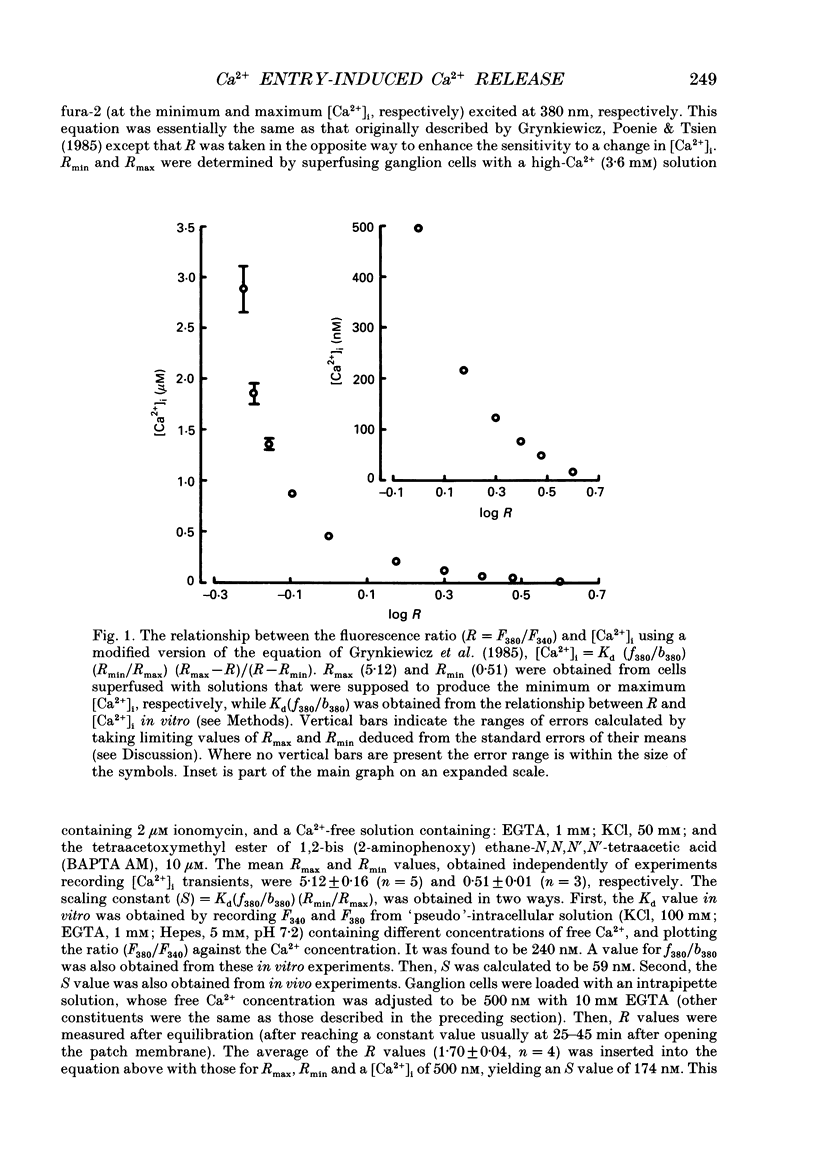
Abstract
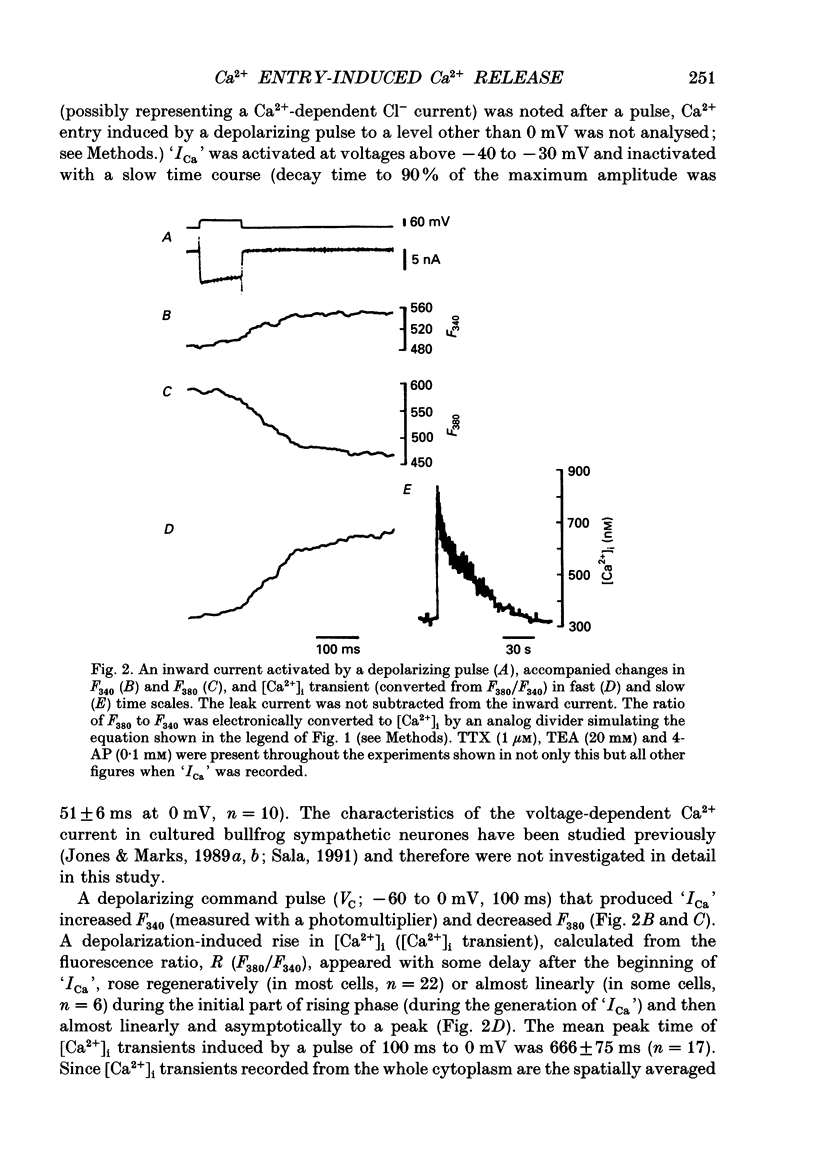
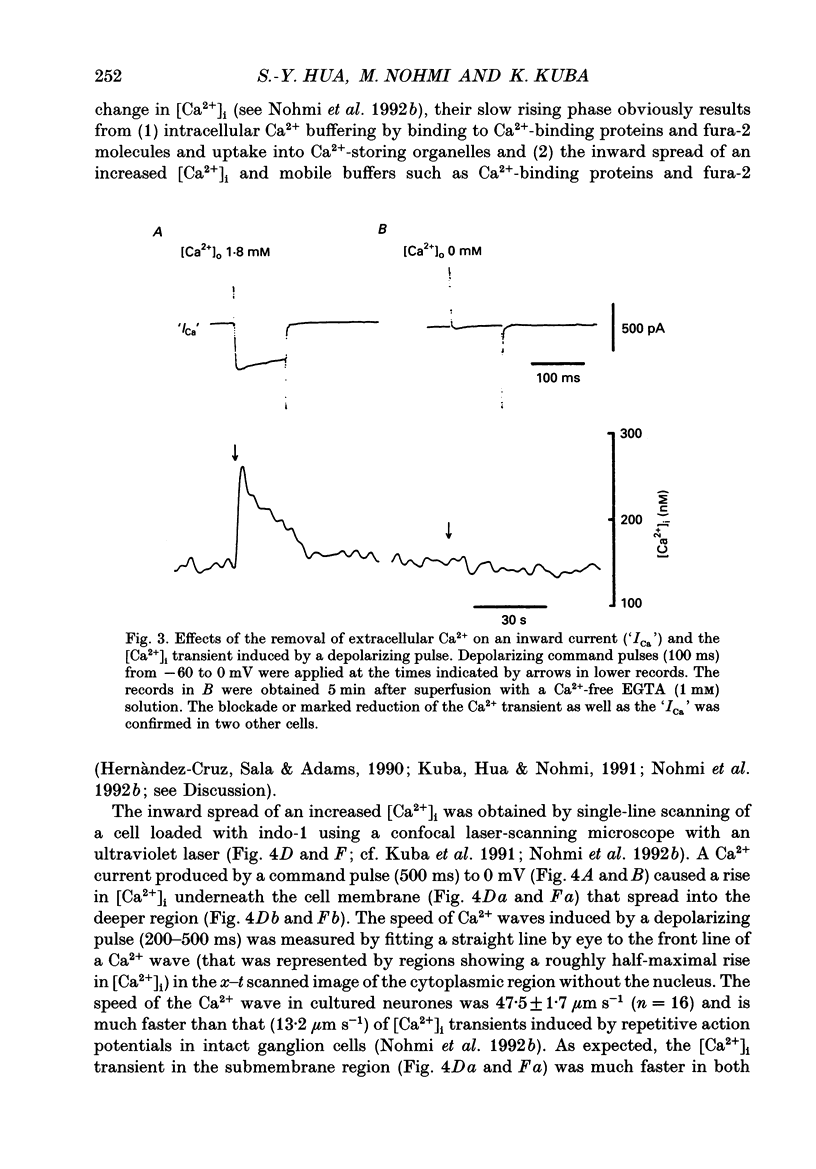
1. A rise in intracellular Ca2+ ([Ca2+]i) and a Ca2+ current (ICa) induced by a depolarizing pulse were simultaneously recorded by fura-2 or indo-1 fluorescence and whole-cell patch clamp techniques in cultured bullfrog sympathetic ganglion cells. 2. [Ca2+]i (calculated from the ratio of fura-2 fluorescences excited at 380 and 340 nm and recorded with a photomultiplier at > 492 nm) rose regeneratively (in most cells) during a command pulse (from -60 to 0 mV, 100 ms), continued to rise thereafter, peaked at 666 ms (on average) and decayed slowly with a half-decay time of 22.8 s. 3. Scanning a single horizontal line across the cytoplasm with an ultraviolet argon ion laser (351 nm) and recording indo-1 fluorescences at two wavelengths (peaked at 410 and 475 nm) with a confocal microscope demonstrated that [Ca2+]i beneath the cell membrane rose much faster than that in the deeper cytoplasm. The time course of the spatial integral of [Ca2+]i, however, corresponded well with that recorded with fura-2 fluorescence using a photomultiplier. 4. [Ca2+]i measured by fura-2 fluorescence ratio using a photomultiplier did not increase during a strong depolarizing pulse (-60 to +80 mV), but sometimes rose after the pulse. A depolarization-induced rise in [Ca2+]i ([Ca2+]i transient) was blocked in a Ca(2+)-free, EGTA solution, reduced by lowering the extracellular Ca2+ concentration ([Ca2+]o) to 0.45 or 0.9 mM and enhanced by raising [Ca2+]o to 7.2 or 14.4 nM. 5. The extracellular Ca2+ dependence was non-linear when long depolarizing pulses (up to 500 ms) were applied; the amplitude of [Ca2+]i transient/Ca2+ entry (unit [Ca2+]i transient) increased with an increase in Ca2+ entry. 6. Increasing the duration of depolarization (-50 or -60 to 0 mV) from 20 to 500 ms enhanced asymptotically the integral of ICa (due to inactivation), and progressively the magnitude of [Ca2+]i transients, leading to the apparent non-linear dependence of unit [Ca2+]i transient on Ca2+ entry as well as on the duration of membrane depolarization. The peak time of [Ca2+]i transient was unchanged for pulse durations up to 300 ms, but prolonged with an increase in pulse duration to 500 ms. 7. Inhibitors of Ca2+ release from intracellular Ca2+ reservoirs, dantrolene (10 microM) and ryanodine (50 microM), blocked the [Ca2+]i transient to 56 and 30%, respectively, of the control. 8. The higher the basal [Ca2+]i level, the greater was the magnitude of the [Ca2+]i transients.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
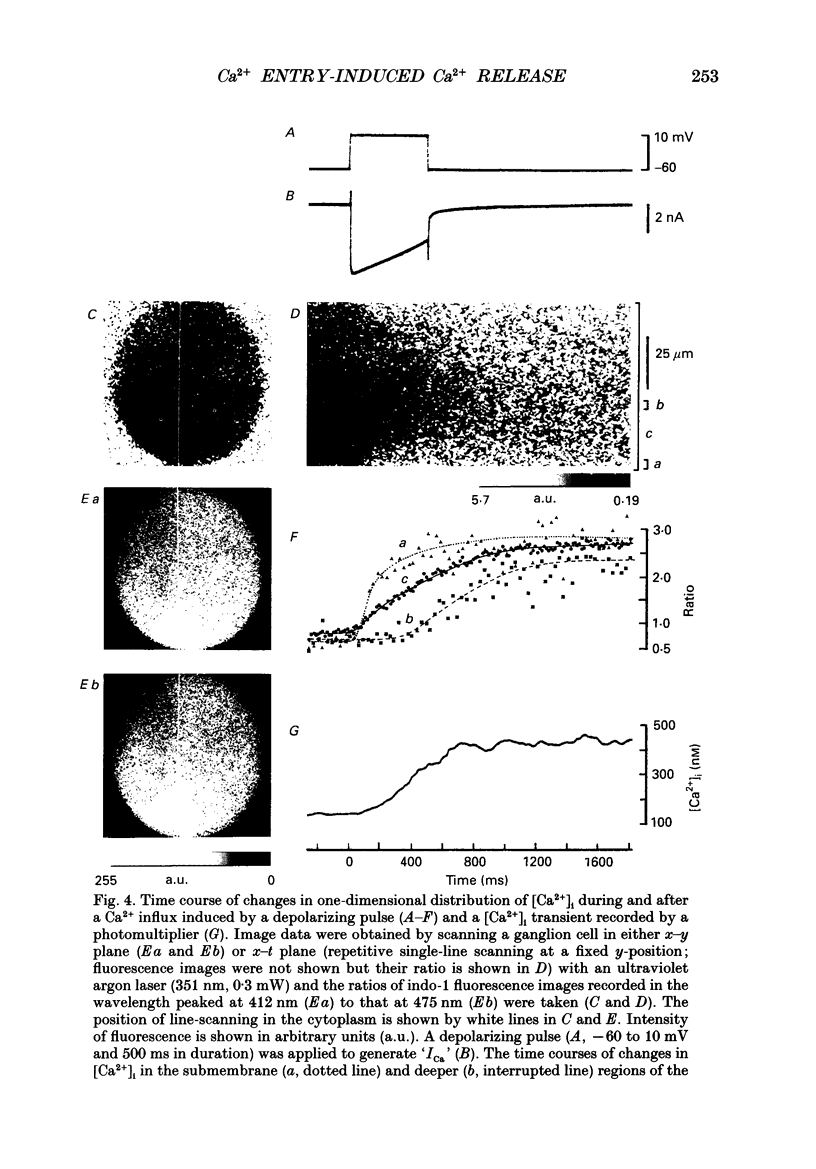
PDF





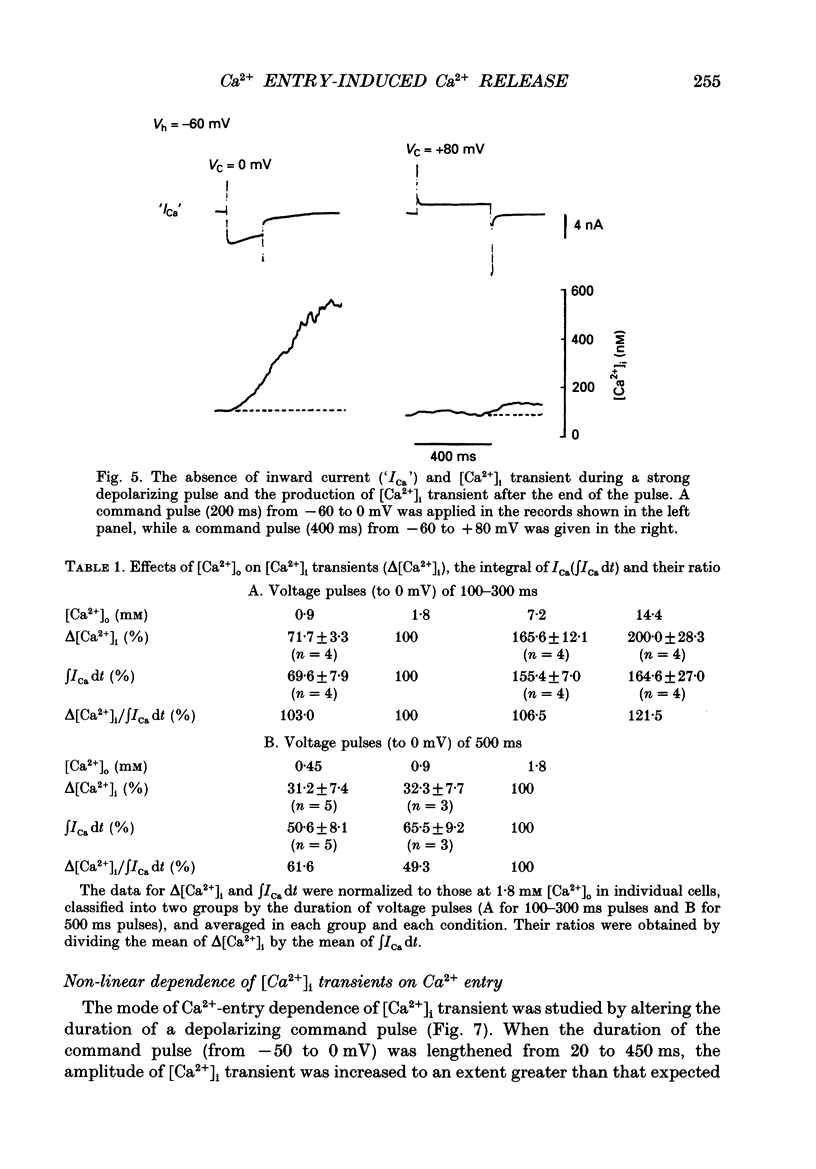
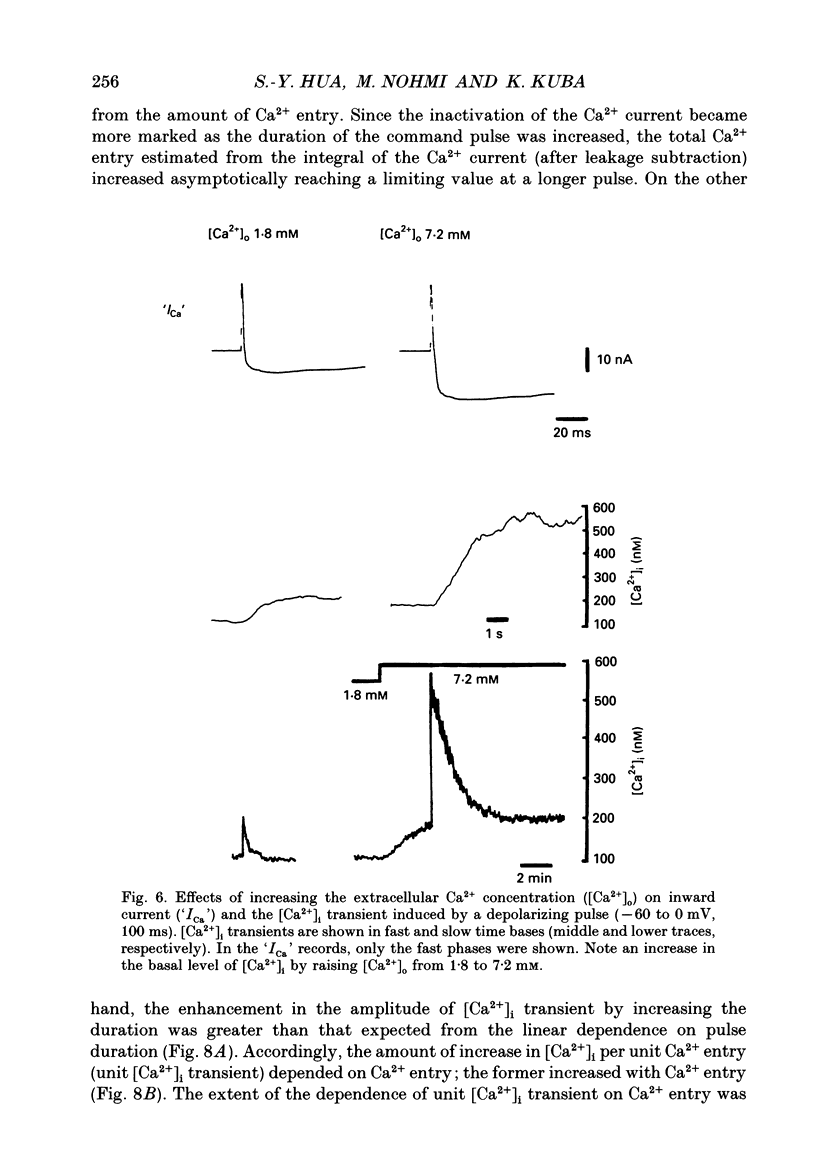
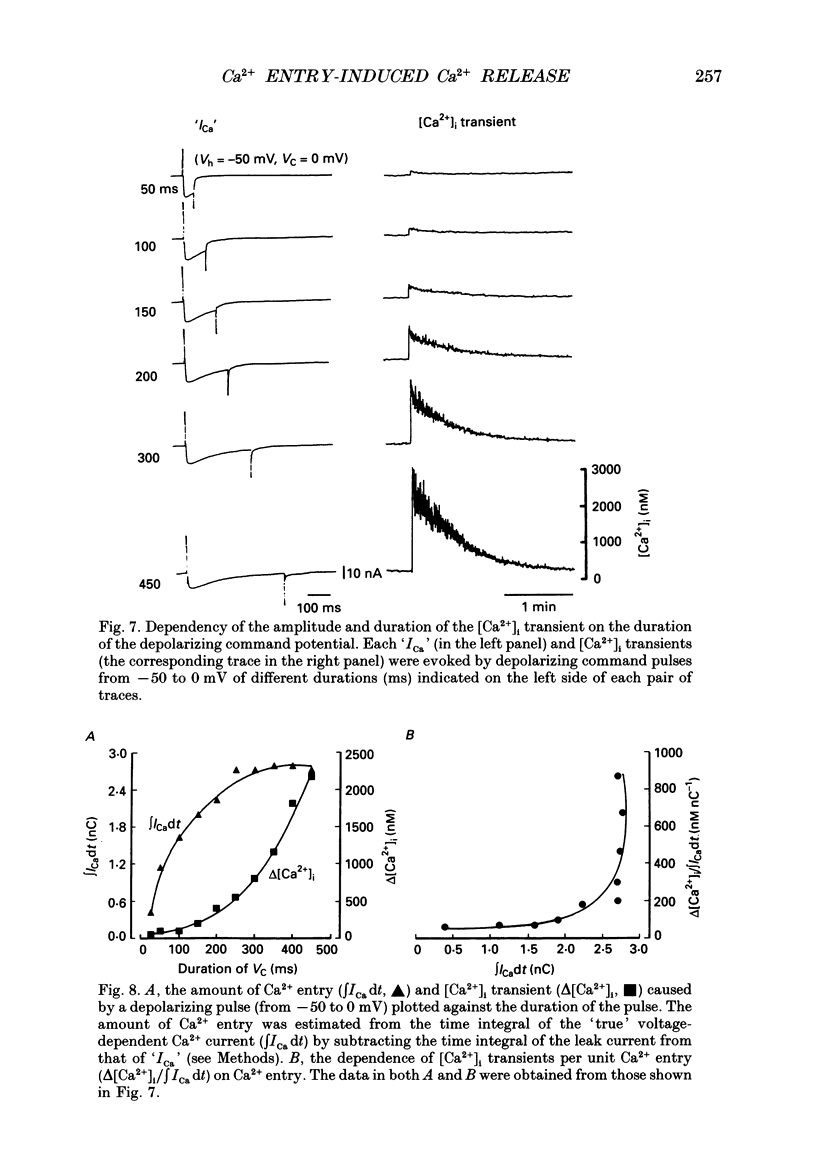









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Akaike N., Sadoshima J. Caffeine affects four different ionic currents in the bull-frog sympathetic neurone. J Physiol. 1989 May;412:221–244. doi: 10.1113/jphysiol.1989.sp017612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Hille B. The inner quaternary ammonium ion receptor in potassium channels of the node of Ranvier. J Gen Physiol. 1972 Apr;59(4):388–400. doi: 10.1085/jgp.59.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt J. E., Hainaut K. Inhibition of the intracellular release of calcium by Dantrolene in barnacle giant muscle fibres. J Physiol. 1977 Feb;265(2):565–585. doi: 10.1113/jphysiol.1977.sp011731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M., Coronado R. Ryanodine receptor channel of sarcoplasmic reticulum. Trends Neurosci. 1988 Oct;11(10):453–457. doi: 10.1016/0166-2236(88)90198-1. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- Fujimoto S., Yamamoto K., Kuba K., Morita K., Kato E. Calcium localization in the sympathetic ganglion of the bullfrog and effects of caffeine. Brain Res. 1980 Nov 24;202(1):21–32. [PubMed] [Google Scholar]
- Furuya S., Ohmori H., Shigemoto T., Sugiyama H. Intracellular calcium mobilization triggered by a glutamate receptor in rat cultured hippocampal cells. J Physiol. 1989 Jul;414:539–548. doi: 10.1113/jphysiol.1989.sp017702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henkart M. P., Reese T. S., Brinley F. J., Jr Endoplasmic reticulum sequesters calcium in the squid giant axon. Science. 1978 Dec 22;202(4374):1300–1303. doi: 10.1126/science.725607. [DOI] [PubMed] [Google Scholar]
- Hernández-Cruz A., Sala F., Adams P. R. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990 Feb 16;247(4944):858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. I. Activation kinetics and pharmacology. J Gen Physiol. 1989 Jul;94(1):151–167. doi: 10.1085/jgp.94.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. II. Inactivation. J Gen Physiol. 1989 Jul;94(1):169–182. doi: 10.1085/jgp.94.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Hua S. Y., Nohmi M. Spatial and dynamic changes in intracellular Ca2+ measured by confocal laser-scanning microscopy in bullfrog sympathetic ganglion cells. Neurosci Res. 1991 May;10(4):245–259. doi: 10.1016/0168-0102(91)90082-a. [DOI] [PubMed] [Google Scholar]
- Kuba K., Morita K., Nohmi M. Origin of calcium ions involved in the generation of a slow afterhyperpolarization in bullfrog sympathetic neurones. Pflugers Arch. 1983 Nov;399(3):194–202. doi: 10.1007/BF00656714. [DOI] [PubMed] [Google Scholar]
- Kuba K., Nishi S. Rhythmic hyperpolarizations and depolarization of sympathetic ganglion cells induced by caffeine. J Neurophysiol. 1976 May;39(3):547–563. doi: 10.1152/jn.1976.39.3.547. [DOI] [PubMed] [Google Scholar]
- Kuba K. Release of calcium ions linked to the activation of potassium conductance in a caffeine-treated sympathetic neurone. J Physiol. 1980 Jan;298:251–269. doi: 10.1113/jphysiol.1980.sp013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Takeshita S. Simulation of intracellular Ca2+ oscillation in a sympathetic neurone. J Theor Biol. 1981 Dec 21;93(4):1009–1031. doi: 10.1016/0022-5193(81)90352-0. [DOI] [PubMed] [Google Scholar]
- Kuba K., Tanaka E., Kumamoto E., Minota S. Patch clamp experiments on nicotinic acetylcholine receptor-ion channels in bullfrog sympathetic ganglion cells. Pflugers Arch. 1989 Jun;414(2):105–112. doi: 10.1007/BF00580950. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Sejnowski T. J. Peptidergic and muscarinic excitation at amphibian sympathetic synapses. J Physiol. 1983 Aug;341:257–278. doi: 10.1113/jphysiol.1983.sp014805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. W., Tsien R. Y. Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron. 1988 Jul;1(5):355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Morita K., Koketsu K., Kuba K. Oscillation of [Ca2+]i-linked K+ conductance in bullfrog sympathetic ganglion cell is sensitive to intracellular anions. Nature. 1980 Jan 10;283(5743):204–205. doi: 10.1038/283204a0. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T. E., Nelson K. E. Intra- and extraluminal sarcoplasmic reticulum membrane regulatory sites for Ca2(+)-induced Ca2+ release. FEBS Lett. 1990 Apr 24;263(2):292–294. doi: 10.1016/0014-5793(90)81396-6. [DOI] [PubMed] [Google Scholar]
- Nishi S., Koketsu K. Early and late after discharges of amphibian sympathetic ganglion cells. J Neurophysiol. 1968 Jan;31(1):109–121. doi: 10.1152/jn.1968.31.1.109. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Tokimasa T., Akasu T. Calcium-dependent potassium conductance in neurons of rabbit vesical pelvic ganglia. J Auton Nerv Syst. 1988 Sep;24(1-2):133–145. doi: 10.1016/0165-1838(88)90142-7. [DOI] [PubMed] [Google Scholar]
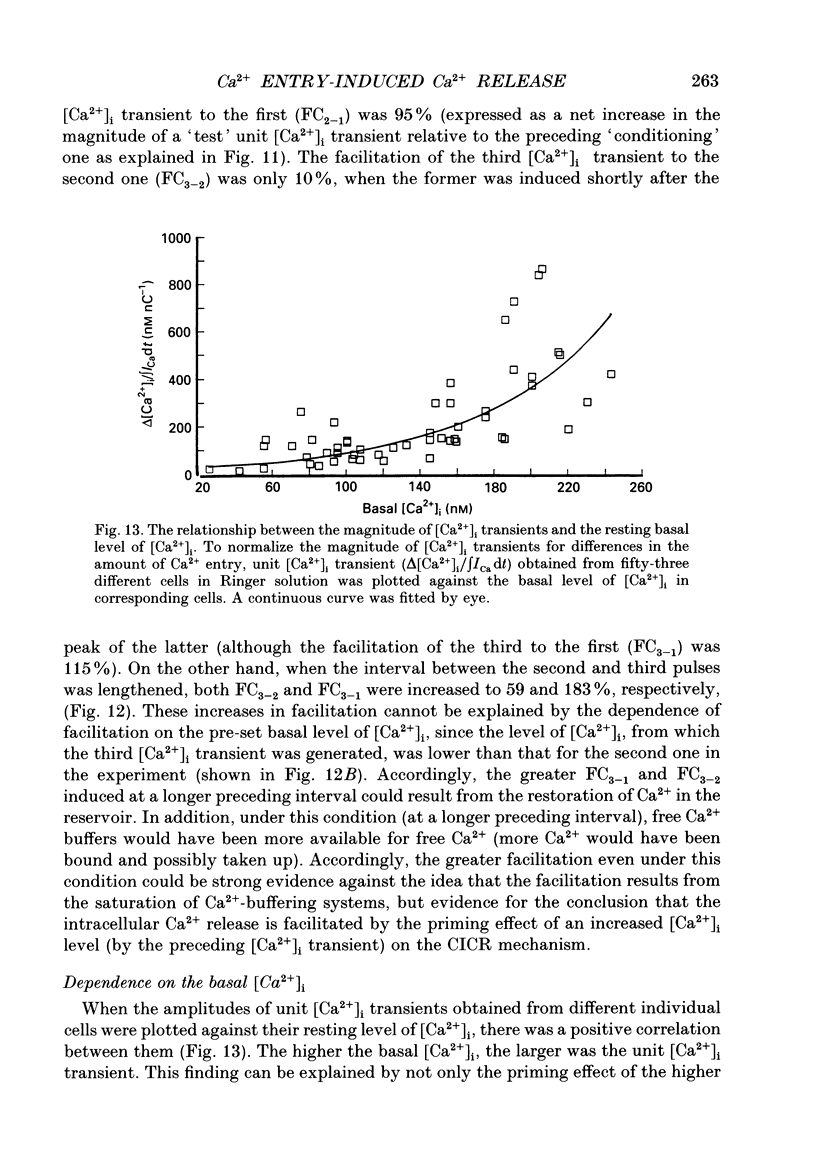
- Nohmi M., Hua S. Y., Kuba K. Basal Ca2+ and the oscillation of Ca2+ in caffeine-treated bullfrog sympathetic neurones. J Physiol. 1992 May;450:513–528. doi: 10.1113/jphysiol.1992.sp019140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohmi M., Hua S. Y., Kuba K. Intracellular calcium dynamics in response to action potentials in bullfrog sympathetic ganglion cells. J Physiol. 1992 Dec;458:171–190. doi: 10.1113/jphysiol.1992.sp019412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohmi M., Kuba K., Hua S. Y. Ultraviolet light activates blocking actions of dantrolene on intracellular Ca2+ release in bullfrog sympathetic neurones. J Biol Chem. 1991 Nov 25;266(33):22254–22259. [PubMed] [Google Scholar]
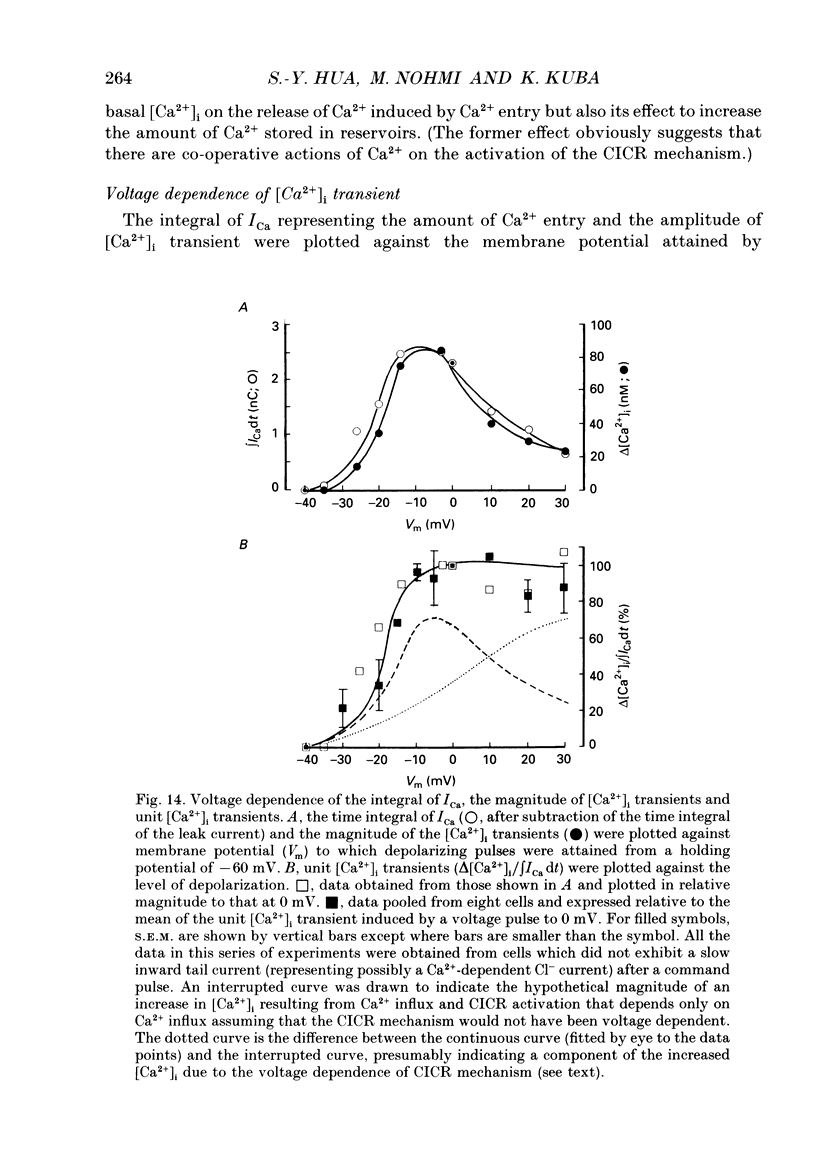
- Näbauer M., Callewaert G., Cleemann L., Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989 May 19;244(4906):800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- Sala F. Activation kinetics of calcium currents in bull-frog sympathetic neurones. J Physiol. 1991 Jun;437:221–238. doi: 10.1113/jphysiol.1991.sp018592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Simon B. J. Inactivation of calcium release from the sarcoplasmic reticulum in frog skeletal muscle. J Physiol. 1988 Nov;405:727–745. doi: 10.1113/jphysiol.1988.sp017358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Willerson J. T., Templeton G. H., Jones L. R., Besch H. R., Jr Ryanodine: its alterations of cat papillary muscle contractile state and responsiveness to inotropic interventions and a suggested mechanism of action. J Pharmacol Exp Ther. 1979 Apr;209(1):37–47. [PubMed] [Google Scholar]
- Talo A., Stern M. D., Spurgeon H. A., Isenberg G., Lakatta E. G. Sustained subthreshold-for-twitch depolarization in rat single ventricular myocytes causes sustained calcium channel activation and sarcoplasmic reticulum calcium release. J Gen Physiol. 1990 Nov;96(5):1085–1103. doi: 10.1085/jgp.96.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. A., Hirning L. D., Miller R. J. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol Pharmacol. 1988 Nov;34(5):664–673. [PubMed] [Google Scholar]
- Thayer S. A., Miller R. J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990 Jun;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosaka T., Chichibu S., Libet B. Intracellular analysis of slow inhibitors and excitatory postsynaptic potentials in sympathetic ganglia of the frog. J Neurophysiol. 1968 May;31(3):396–409. doi: 10.1152/jn.1968.31.3.396. [DOI] [PubMed] [Google Scholar]
- Walton P. D., Airey J. A., Sutko J. L., Beck C. F., Mignery G. A., Südhof T. C., Deerinck T. J., Ellisman M. H. Ryanodine and inositol trisphosphate receptors coexist in avian cerebellar Purkinje neurons. J Cell Biol. 1991 Jun;113(5):1145–1157. doi: 10.1083/jcb.113.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G. Cytoplasmic [Ca2+] in mammalian ventricle: dynamic control by cellular processes. Annu Rev Physiol. 1990;52:467–485. doi: 10.1146/annurev.ph.52.030190.002343. [DOI] [PubMed] [Google Scholar]