Abstract
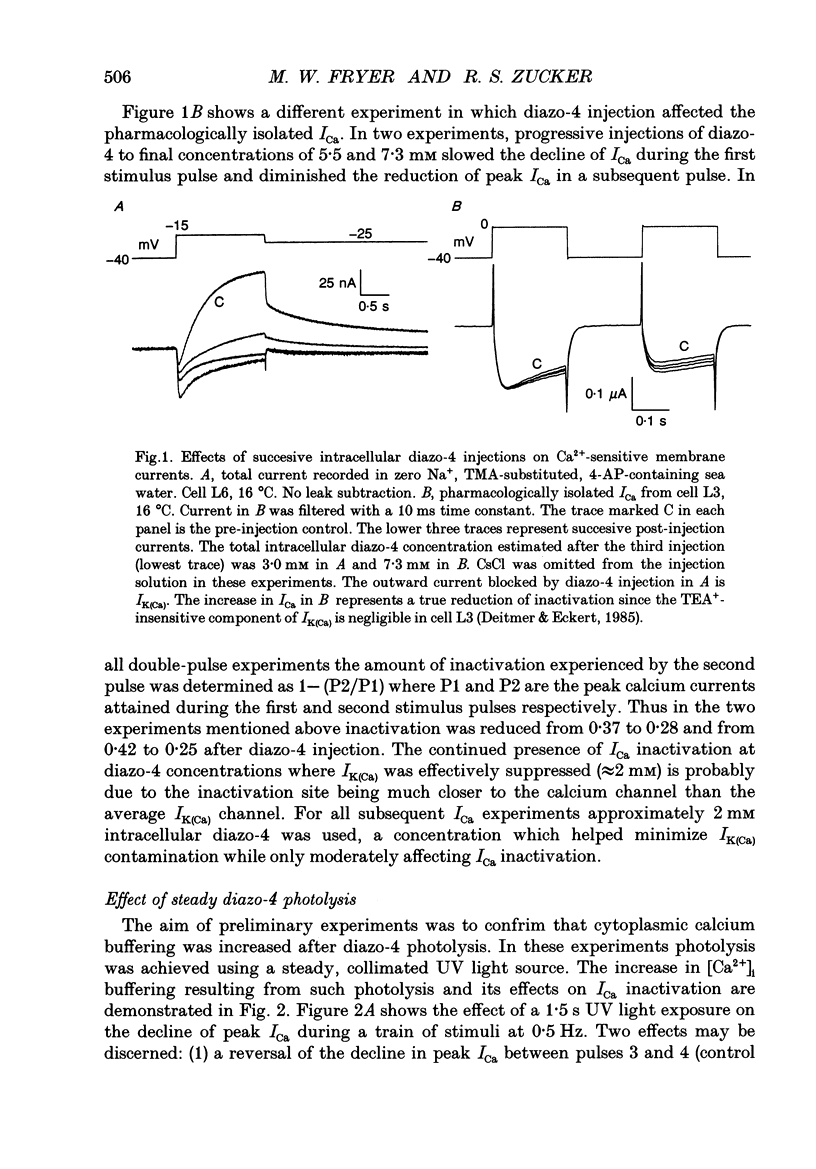
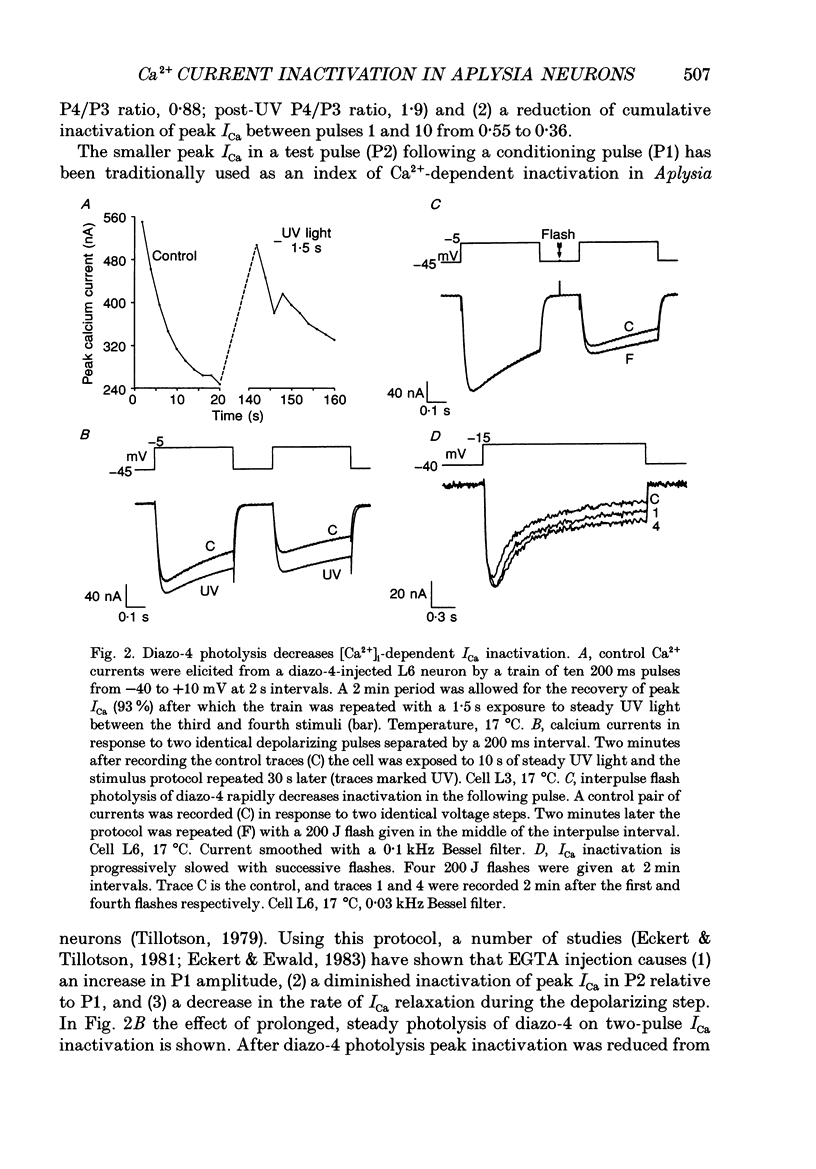
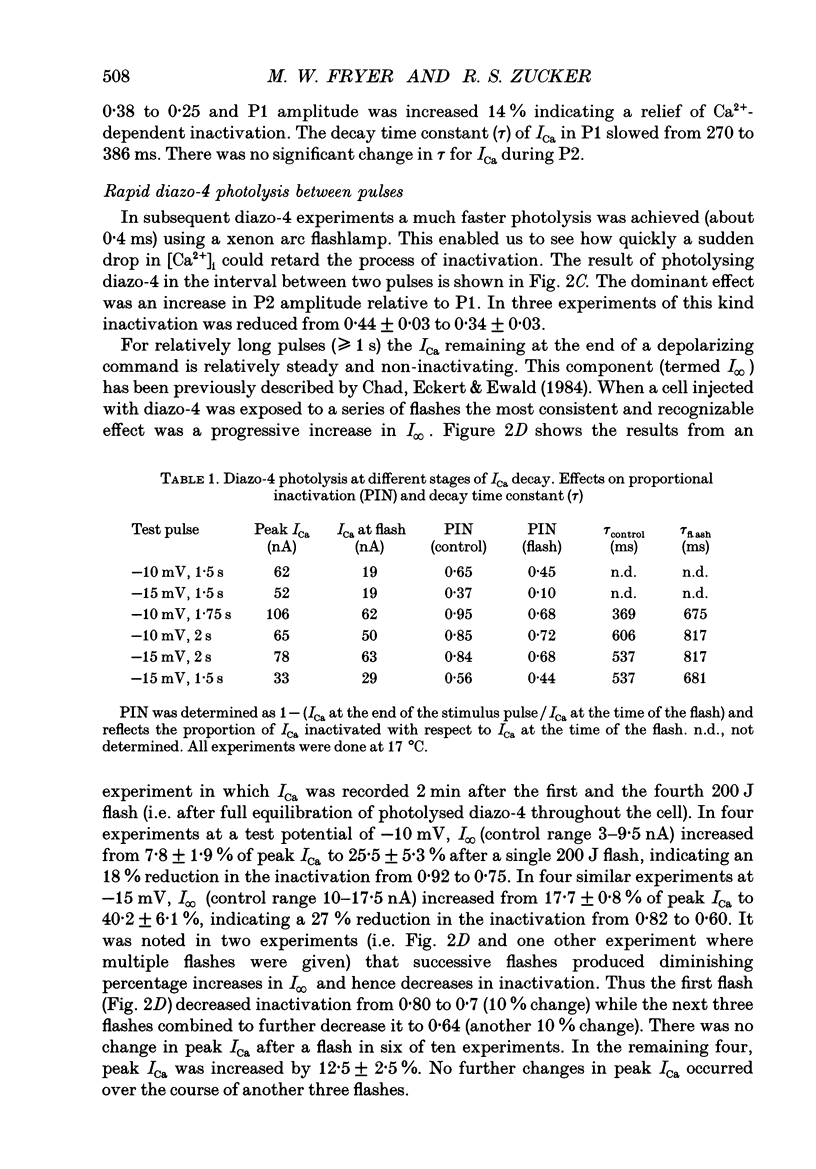
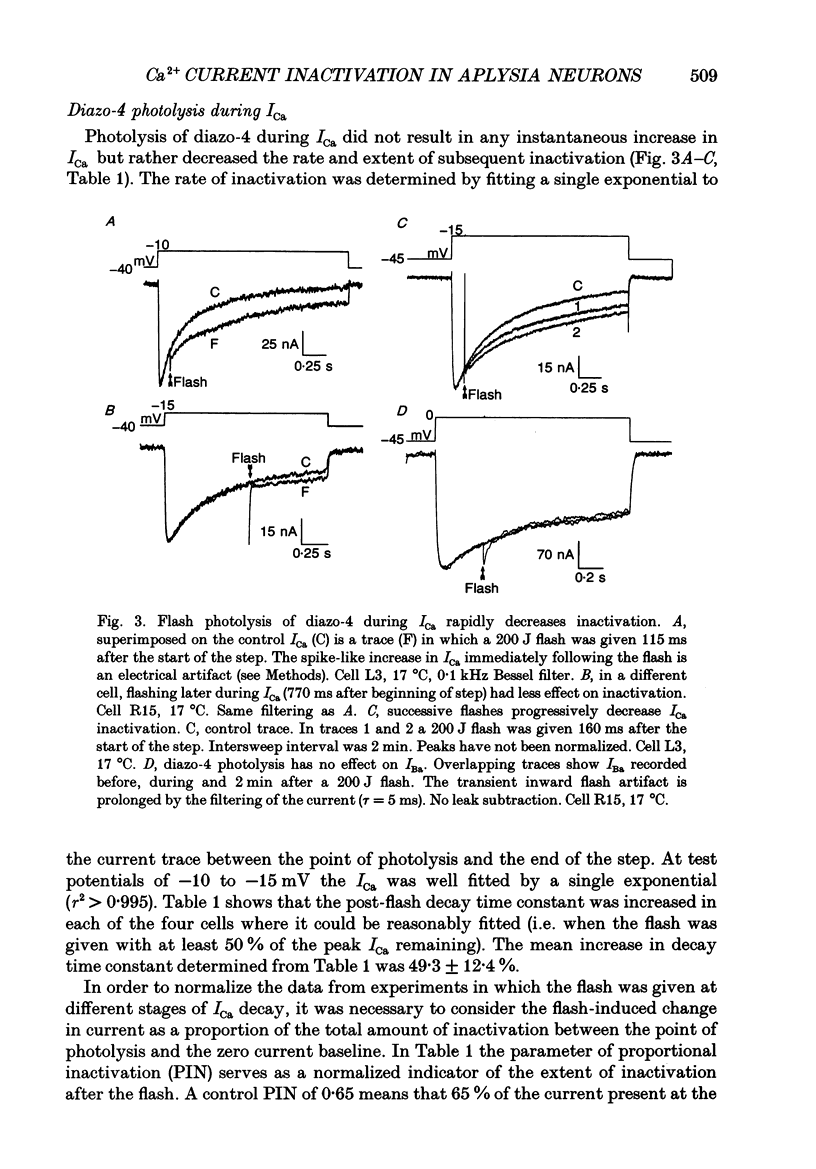
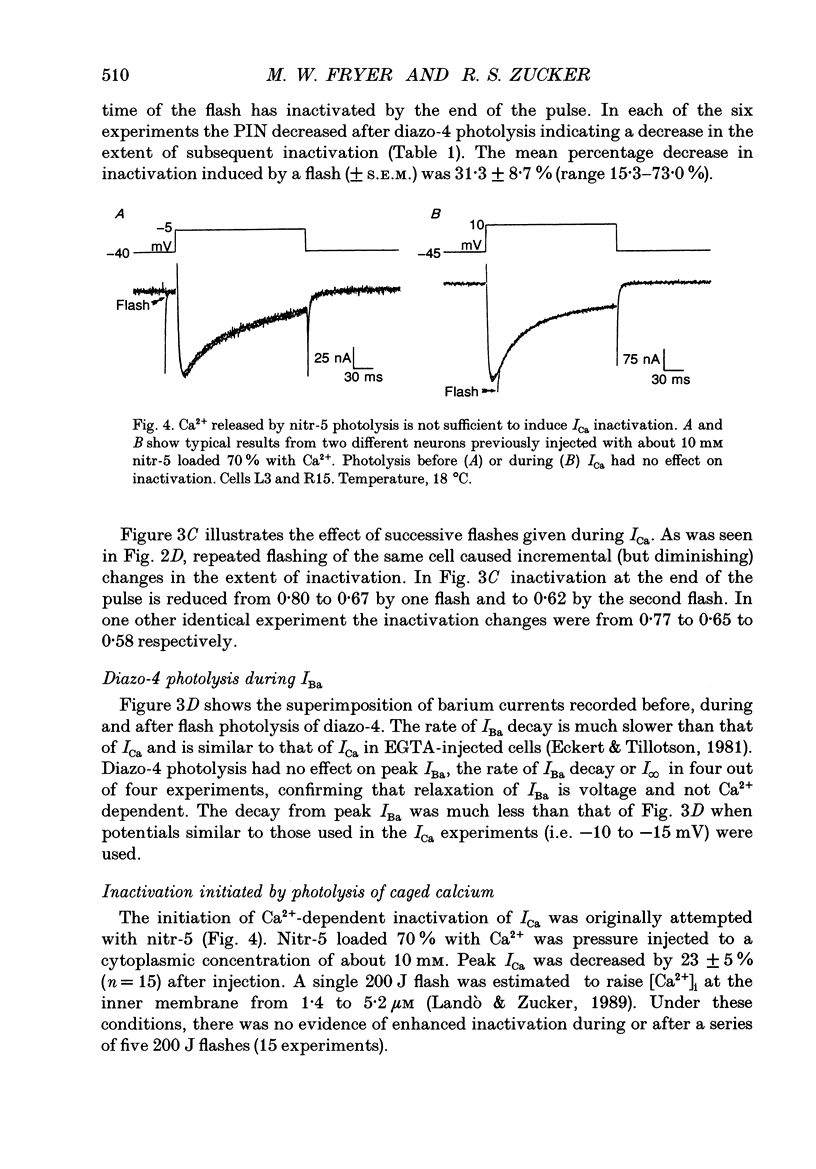
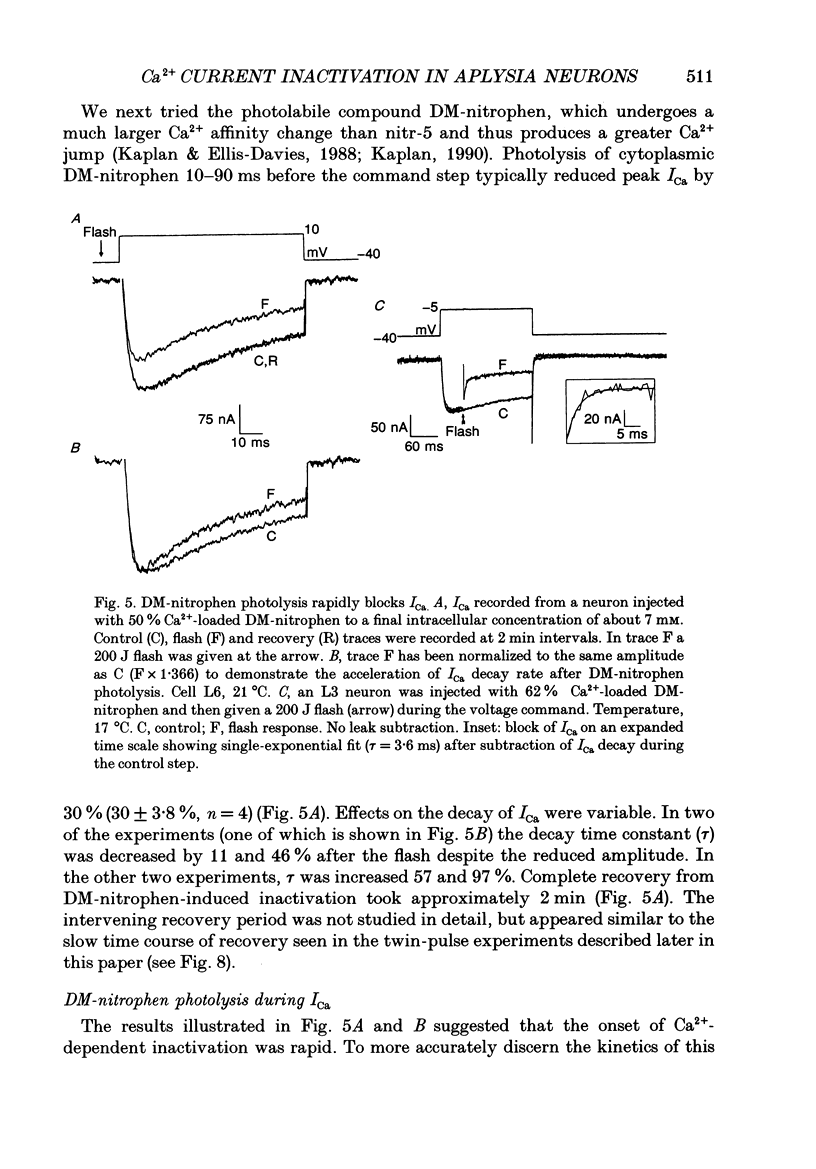
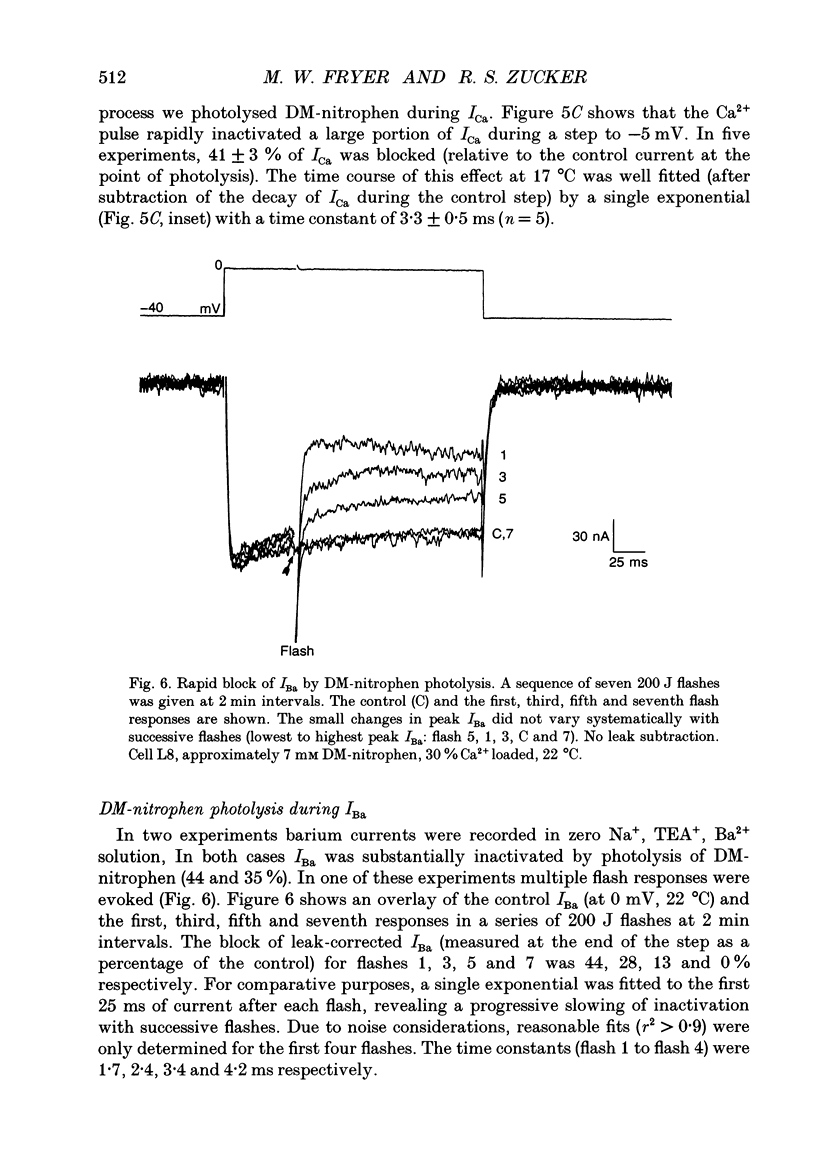
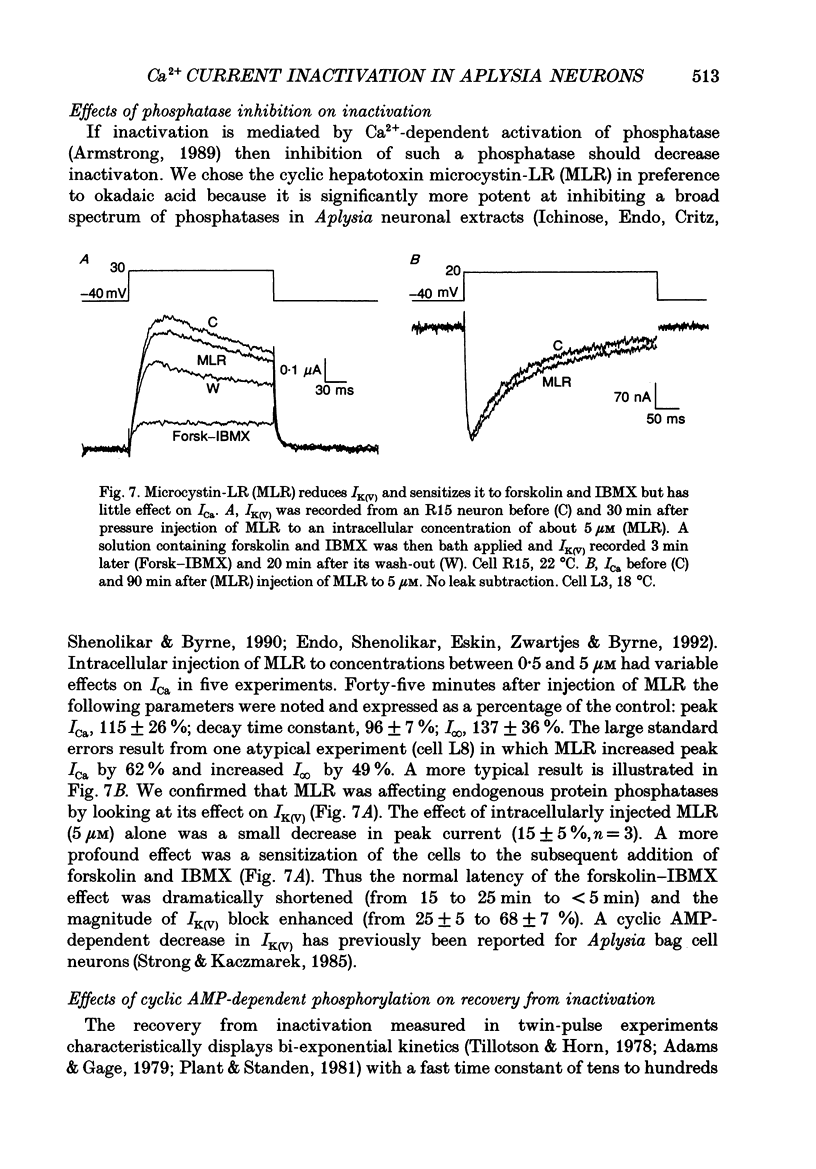
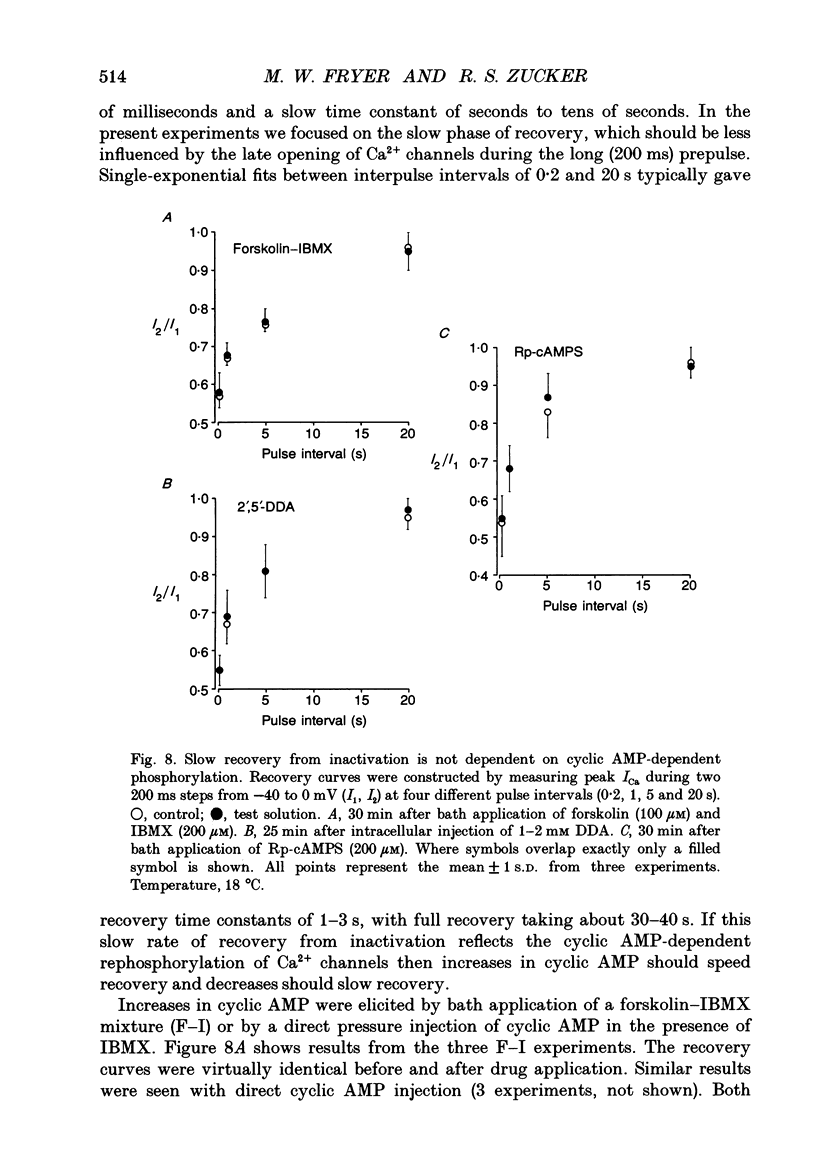
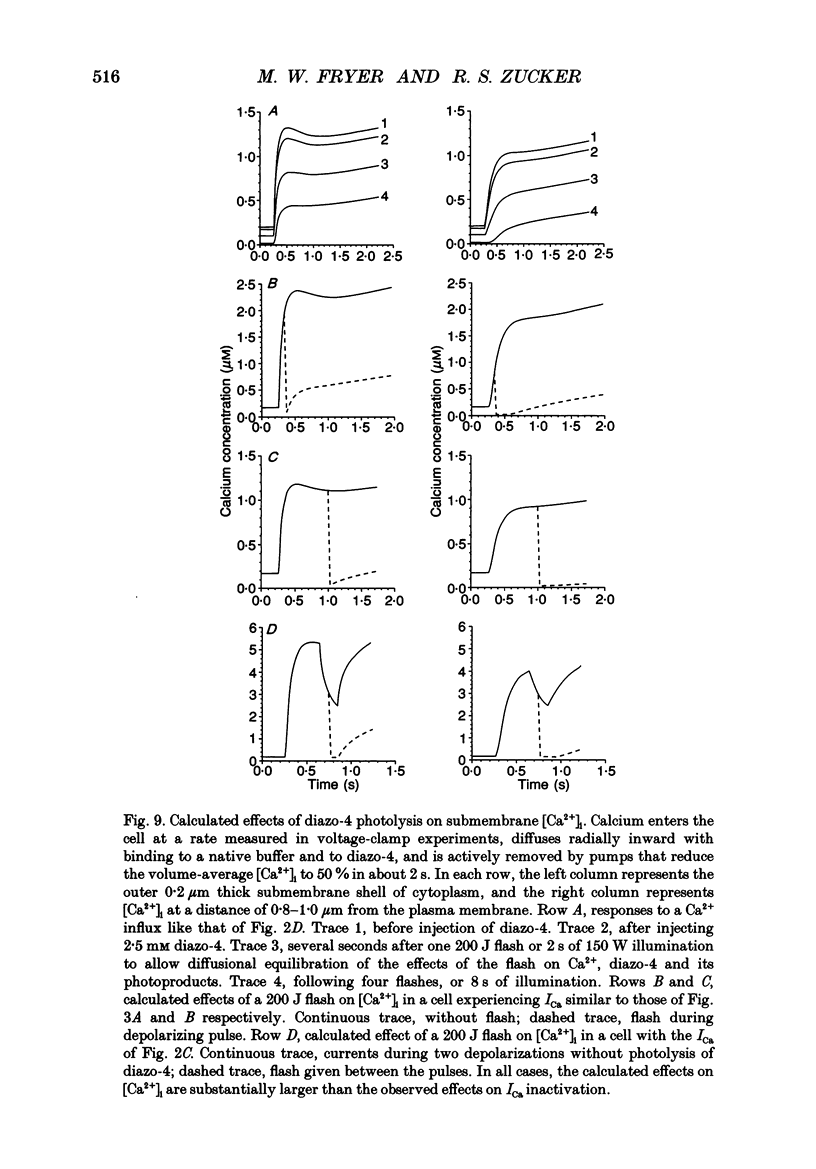
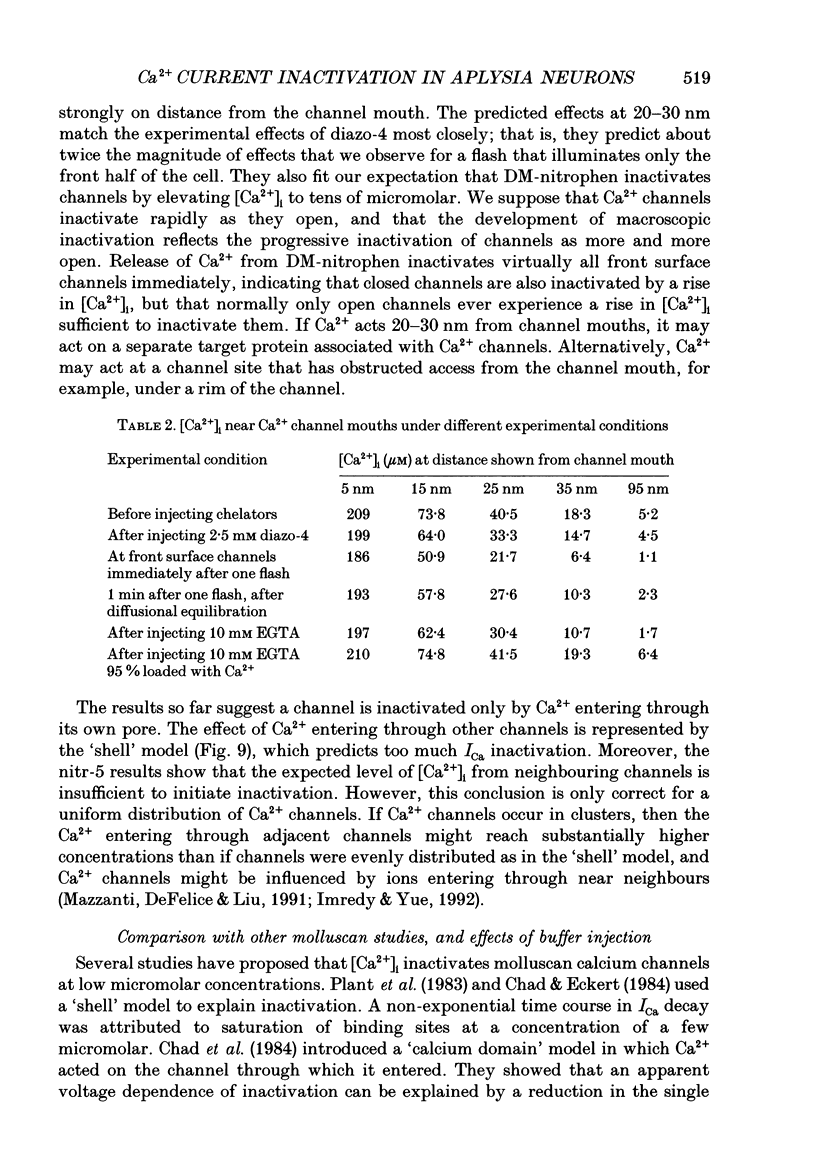
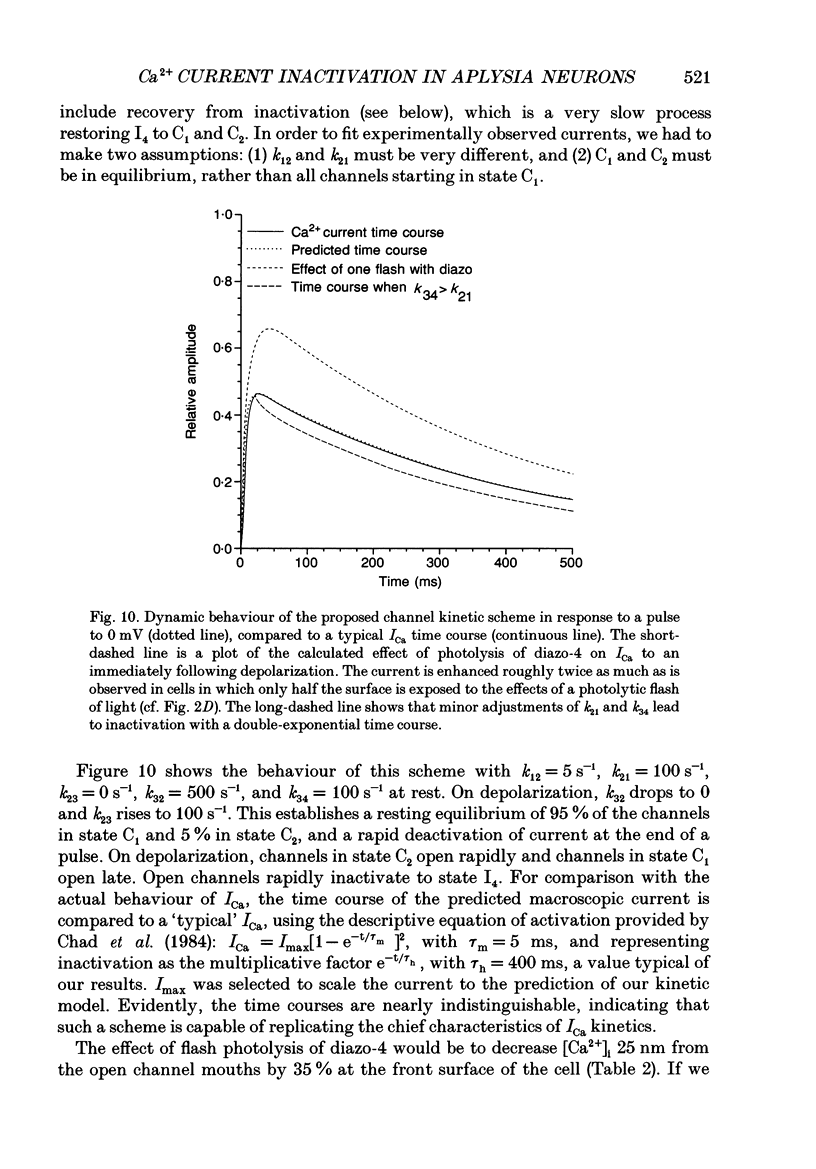
1. The kinetics and sensitivity of the Ca(2+)-dependent inactivation of calcium current (ICa) were examined in intact cell bodies from the abdominal ganglion of Aplysia californica under two-electrode voltage clamp. 2. Rapid changes in the level of intracellular free calcium ([Ca2+]i) were generated at the cell surface by photolytic release of Ca2+ (nitr-5 and dimethoxy nitrophen) or Ca2+ buffer (diazo-4). 3. Diazo-4 increased ICa by 10-15% and slowed the rate of ICa decay when photolysed before a test pulse or between a prepulse and a test pulse. The predominant effect of further light flashes was to increase the amount of non-inactivating current (I infinity) remaining at the end of long (> 1 s) depolarizing pulses. 4. A rapid increase in [Ca2+]i buffering during ICa inactivation did not cause a rapid recovery of current but merely reduced the rate and extent of subsequent inactivation. This effect was not seen when Ba2+ was the charge carrier. 5. Photolytic release of Ca2+ from nitr-5 produced estimated Ca2+ jumps of 3-4 microM at the front surface of the cell but failed to augment inactivation either before or during ICa. In contrast, photolysis of DM-nitrophen 10-90 ms before the test pulse decreased peak ICa by about 30%. A flash given during ICa rapidly blocked 41 +/- 3% of peak current with a time constant of 3-4 ms at 17 degrees C. Similar results were seen with the barium current (IBa). 6. Microinjection of the potent phosphatase inhibitor microcystin-LR (5 microM) had variable effects on ICa inactivation and augmented the cyclic AMP-induced depression of the delayed rectifier (IK(V) by forskolin (100 microM) and 3-isobutyl-1-methylxanthine (IBMX; 200 microM). 7. Full recovery from inactivation measured in two-pulse experiments took at least 20 s. This slow recovery process was unaffected by increases in intracellular cyclic AMP elicited by direct injection or by bath application of forskolin and IBMX. It was also unaffected by decreases in cyclic AMP induced by injecting 2',5'-dideoxyadenosine (1 mM) or bath application of the Rp isomer of cyclic adenosine 3',5'-monophosphothioate (Rp-cAMPS; 200 microM). 8. A 'shell' model relating submembrane Ca2+ to inactivation was inconsistent with the experimental results since it greatly overestimated the effects of diazo-4 and predicted significant inactivation by nitr-5 photolysis. 9. A model linearly relating [Ca2+]i in a single Ca2+ channel 'domain' to inactivation more closely matched the experimental results with diazo-4 and DM-(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Gage P. W. Characteristics of sodium and calcium conductance changes produced by membrane depolarization in an Aplysia neurone. J Physiol. 1979 Apr;289:143–161. doi: 10.1113/jphysiol.1979.sp012729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemà S., Calissano P., Rusca G., Giuditta A. Identification of a calcium-binding, brain specific protein in the axoplasm of squid giant axons. J Neurochem. 1973 Mar;20(3):681–689. doi: 10.1111/j.1471-4159.1973.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Armstrong D. L. Calcium channel regulation by calcineurin, a Ca2+-activated phosphatase in mammalian brain. Trends Neurosci. 1989 Mar;12(3):117–122. doi: 10.1016/0166-2236(89)90168-9. [DOI] [PubMed] [Google Scholar]
- Armstrong D., Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J., Eckert R., Ewald D. Kinetics of calcium-dependent inactivation of calcium current in voltage-clamped neurones of Aplysia californica. J Physiol. 1984 Feb;347:279–300. doi: 10.1113/jphysiol.1984.sp015066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. Inactivation of calcium channels. Comp Biochem Physiol A Comp Physiol. 1989;93(1):95–105. doi: 10.1016/0300-9629(89)90196-5. [DOI] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Eckert R. Two components of Ca-dependent potassium current in identified neurons of Aplysia californica. Pflugers Arch. 1985 Apr;403(4):353–359. doi: 10.1007/BF00589246. [DOI] [PubMed] [Google Scholar]
- Delaney K. R., Zucker R. S. Calcium released by photolysis of DM-nitrophen stimulates transmitter release at squid giant synapse. J Physiol. 1990 Jul;426:473–498. doi: 10.1113/jphysiol.1990.sp018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Eckert R., Ewald D. Inactivation of calcium conductance characterized by tail current measurements in neurones of Aplysia californica. J Physiol. 1983 Dec;345:549–565. doi: 10.1113/jphysiol.1983.sp014996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J Physiol. 1981 May;314:265–280. doi: 10.1113/jphysiol.1981.sp013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds B., Klein M., Dale N., Kandel E. R. Contributions of two types of calcium channels to synaptic transmission and plasticity. Science. 1990 Nov 23;250(4984):1142–1147. doi: 10.1126/science.2174573. [DOI] [PubMed] [Google Scholar]
- Endo S., Shenolikar S., Eskin A., Zwartjes R. E., Byrne J. H. Characterization of neuronal protein phosphatases in Aplysia californica. J Neurochem. 1992 Mar;58(3):975–982. doi: 10.1111/j.1471-4159.1992.tb09351.x. [DOI] [PubMed] [Google Scholar]
- Giannattasio B., Jones S. W., Scarpa A. Calcium currents in the A7r5 smooth muscle-derived cell line. Calcium-dependent and voltage-dependent inactivation. J Gen Physiol. 1991 Nov;98(5):987–1003. doi: 10.1085/jgp.98.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grega D. S., Macdonald R. L. Activators of adenylate cyclase and cyclic AMP prolong calcium-dependent action potentials of mouse sensory neurons in culture by reducing a voltage-dependent potassium conductance. J Neurosci. 1987 Mar;7(3):700–707. doi: 10.1523/JNEUROSCI.07-03-00700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick M. J., Lux H. D., Swandulla D., Zucker H. Voltage-dependent and calcium-dependent inactivation of calcium channel current in identified snail neurones. J Physiol. 1989 May;412:197–220. doi: 10.1113/jphysiol.1989.sp017611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. W., Lederer W. J. Ca2+ and voltage inactivate Ca2+ channels in guinea-pig ventricular myocytes through independent mechanisms. J Physiol. 1991 Dec;444:257–268. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J., Mieskes G., Rüegg J. C., Takai A., Trautwein W. Effects of a protein phosphatase inhibitor, okadaic acid, on membrane currents of isolated guinea-pig cardiac myocytes. Pflugers Arch. 1988 Aug;412(3):248–252. doi: 10.1007/BF00582504. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Trautwein W. Modification of L-type calcium current by intracellularly applied trypsin in guinea-pig ventricular myocytes. J Physiol. 1988 Oct;404:259–274. doi: 10.1113/jphysiol.1988.sp017289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M., Endo S., Critz S. D., Shenolikar S., Byrne J. H. Microcystin-LR, a potent protein phosphatase inhibitor, prolongs the serotonin- and cAMP-induced currents in sensory neurons of Aplysia californica. Brain Res. 1990 Nov 12;533(1):137–140. doi: 10.1016/0006-8993(90)91806-r. [DOI] [PubMed] [Google Scholar]
- Imredy J. P., Yue D. T. Submicroscopic Ca2+ diffusion mediates inhibitory coupling between individual Ca2+ channels. Neuron. 1992 Aug;9(2):197–207. doi: 10.1016/0896-6273(92)90159-b. [DOI] [PubMed] [Google Scholar]
- Kao J. P., Tsien R. Y. Ca2+ binding kinetics of fura-2 and azo-1 from temperature-jump relaxation measurements. Biophys J. 1988 Apr;53(4):635–639. doi: 10.1016/S0006-3495(88)83142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H., Ellis-Davies G. C. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H. Photochemical manipulation of divalent cation levels. Annu Rev Physiol. 1990;52:897–914. doi: 10.1146/annurev.ph.52.030190.004225. [DOI] [PubMed] [Google Scholar]
- Kay A. R. Inactivation kinetics of calcium current of acutely dissociated CA1 pyramidal cells of the mature guinea-pig hippocampus. J Physiol. 1991 Jun;437:27–48. doi: 10.1113/jphysiol.1991.sp018581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. H., Zucker R. S. Calcium-dependent inward current in Aplysia bursting pace-maker neurones. J Physiol. 1985 May;362:107–130. doi: 10.1113/jphysiol.1985.sp015666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinks M. H., Klee C. B., Pant H. C., Gainer H. Identification and quantification of calcium-binding proteins in squid axoplasm. J Neurosci. 1988 Jun;8(6):2172–2182. doi: 10.1523/JNEUROSCI.08-06-02172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landò L., Zucker R. S. "Caged calcium" in Aplysia pacemaker neurons. Characterization of calcium-activated potassium and nonspecific cation currents. J Gen Physiol. 1989 Jun;93(6):1017–1060. doi: 10.1085/jgp.93.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Brown A. M. Single channel studies on inactivation of calcium currents. Science. 1984 Jul 27;225(4660):432–434. doi: 10.1126/science.6330896. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Ca channel gating during cardiac action potentials. Biophys J. 1990 Oct;58(4):1059–1065. doi: 10.1016/S0006-3495(90)82448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J., Liu Y. M. Gating of L-type Ca2+ channels in embryonic chick ventricle cells: dependence on voltage, current and channel density. J Physiol. 1991 Nov;443:307–334. doi: 10.1113/jphysiol.1991.sp018835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Davies N. W., Kaplan J. H., Lux H. D. Inactivation and block of calcium channels by photo-released Ca2+ in dorsal root ganglion neurons. Science. 1988 Aug 12;241(4867):842–844. doi: 10.1126/science.2457253. [DOI] [PubMed] [Google Scholar]
- Plant T. D., Standen N. B. Calcium current inactivation in identified neurones of Helix aspersa. J Physiol. 1981 Dec;321:273–285. doi: 10.1113/jphysiol.1981.sp013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant T. D., Standen N. B., Ward T. A. The effects of injection of calcium ions and calcium chelators on calcium channel inactivation in Helix neurones. J Physiol. 1983 Jan;334:189–212. doi: 10.1113/jphysiol.1983.sp014489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. P., Johnson J. D., Potter J. D. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+. Biophys J. 1981 Jun;34(3):559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman A., Keizer J., Rinzel J. Domain model for Ca2(+)-inactivation of Ca2+ channels at low channel density. Biophys J. 1990 Oct;58(4):985–995. doi: 10.1016/S0006-3495(90)82443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Zucker R. S. Aequorin response facilitation and intracellular calcium accumulation in molluscan neurones. J Physiol. 1980 Mar;300:167–196. doi: 10.1113/jphysiol.1980.sp013157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A binding-site model for calcium channel inactivation that depends on calcium entry. Proc R Soc Lond B Biol Sci. 1982 Dec 22;217(1206):101–110. doi: 10.1098/rspb.1982.0097. [DOI] [PubMed] [Google Scholar]
- Strong J. A., Kaczmarek L. K. Multiple components of delayed potassium current in peptidergic neurons of Aplysia: modulation by an activator of adenylate cyclase. J Neurosci. 1986 Mar;6(3):814–822. doi: 10.1523/JNEUROSCI.06-03-00814.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D., Horn R. Inactivation without facilitation of calcium conductance in caesium-loaded neurones of Aplysia. Nature. 1978 May 25;273(5660):312–314. doi: 10.1038/273312a0. [DOI] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Zucker R. S. Control of cytoplasmic calcium with photolabile tetracarboxylate 2-nitrobenzhydrol chelators. Biophys J. 1986 Nov;50(5):843–853. doi: 10.1016/S0006-3495(86)83525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Y., Salter M. W., MacDonald J. F. Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science. 1991 Sep 6;253(5024):1132–1135. doi: 10.1126/science.1653455. [DOI] [PubMed] [Google Scholar]
- Williams P. J., Pittman Q. J., MacVicar B. A. Ca(2+)- and voltage-dependent inactivation of Ca2+ currents in rat intermediate pituitary. Brain Res. 1991 Nov 8;564(1):12–18. doi: 10.1016/0006-8993(91)91345-2. [DOI] [PubMed] [Google Scholar]
- Yatani A., Wilson D. L., Brown A. M. Recovery of Ca currents from inactivation: the roles of Ca influx, membrane potential, and cellular metabolism. Cell Mol Neurobiol. 1983 Dec;3(4):381–395. doi: 10.1007/BF00734718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Backx P. H., Imredy J. P. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 1990 Dec 21;250(4988):1735–1738. doi: 10.1126/science.2176745. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Effects of photolabile calcium chelators on fluorescent calcium indicators. Cell Calcium. 1992 Jan;13(1):29–40. doi: 10.1016/0143-4160(92)90027-p. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Fogelson A. L. Relationship between transmitter release and presynaptic calcium influx when calcium enters through discrete channels. Proc Natl Acad Sci U S A. 1986 May;83(9):3032–3036. doi: 10.1073/pnas.83.9.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Tetraethylammonium contains an impurity which alkalizes cytoplasm and reduce calcium buffering in neurons. Brain Res. 1981 Mar 16;208(2):473–478. doi: 10.1016/0006-8993(81)90580-1. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. The calcium concentration clamp: spikes and reversible pulses using the photolabile chelator DM-nitrophen. Cell Calcium. 1993 Feb;14(2):87–100. doi: 10.1016/0143-4160(93)90079-l. [DOI] [PubMed] [Google Scholar]


