Abstract
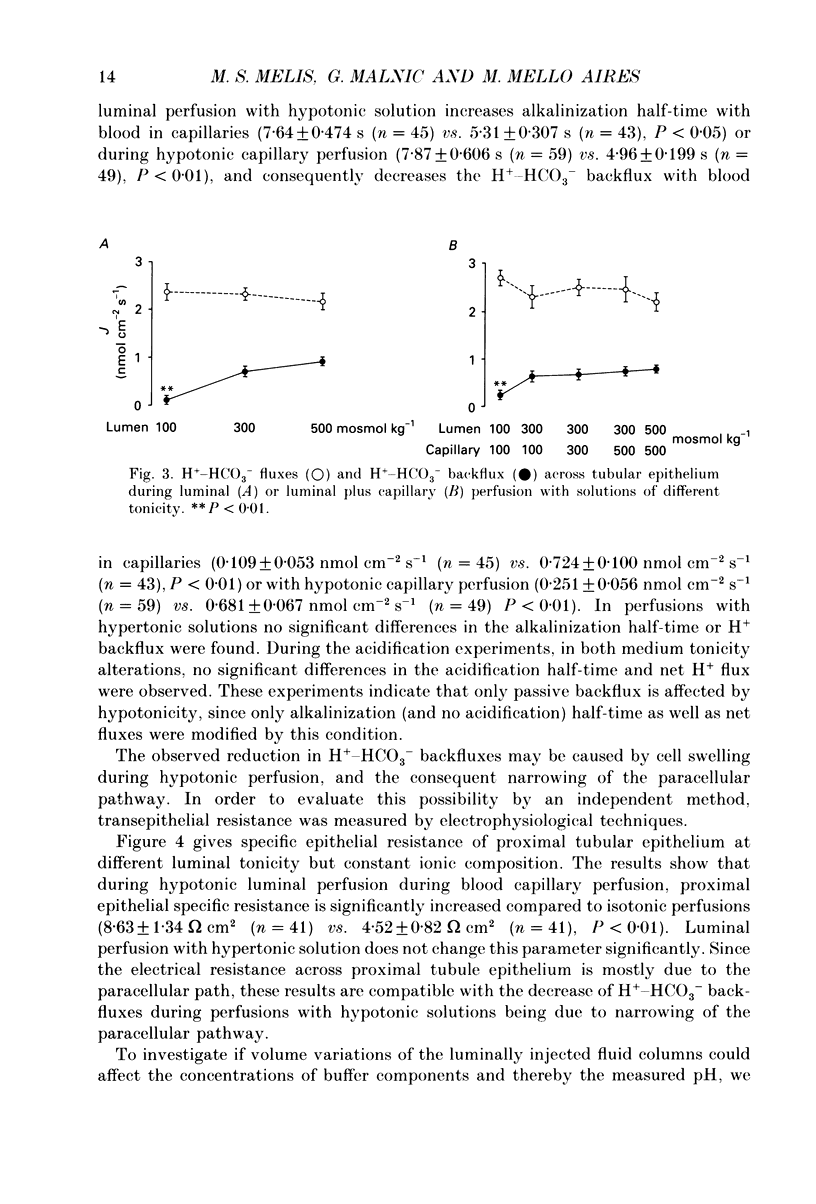
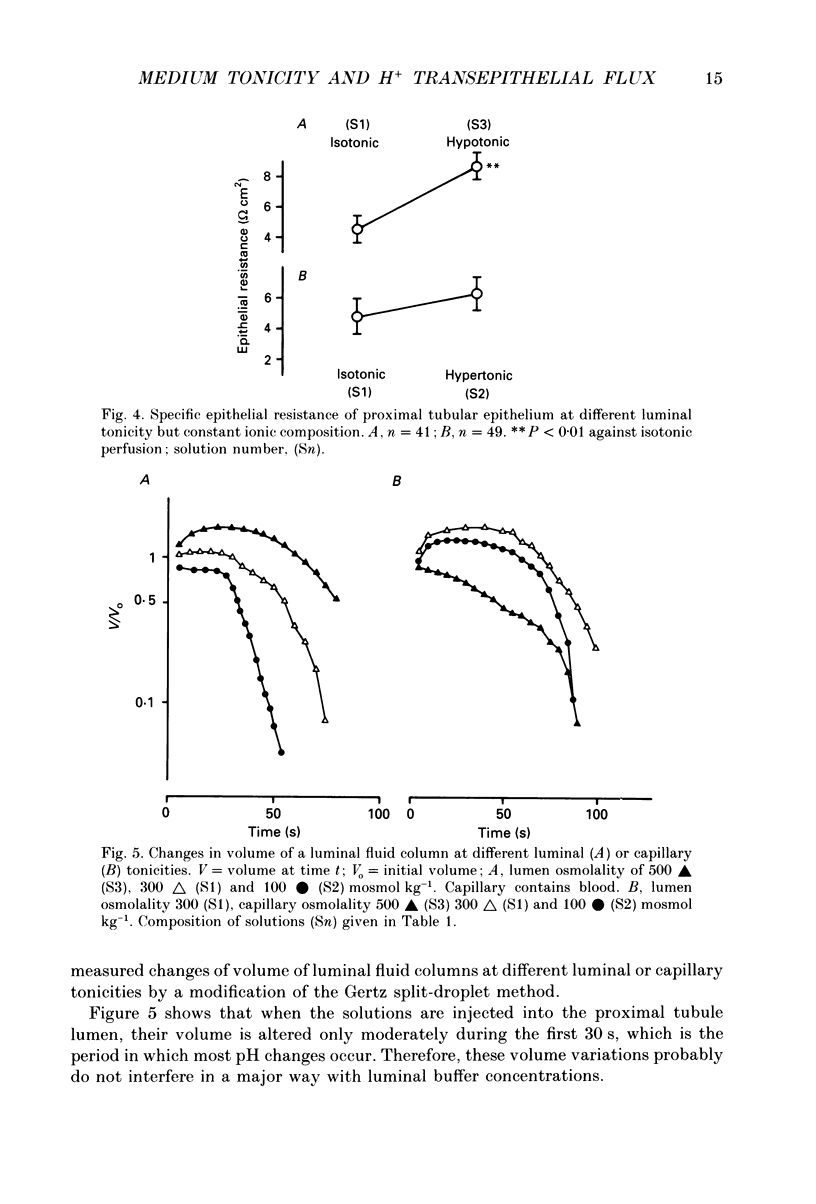
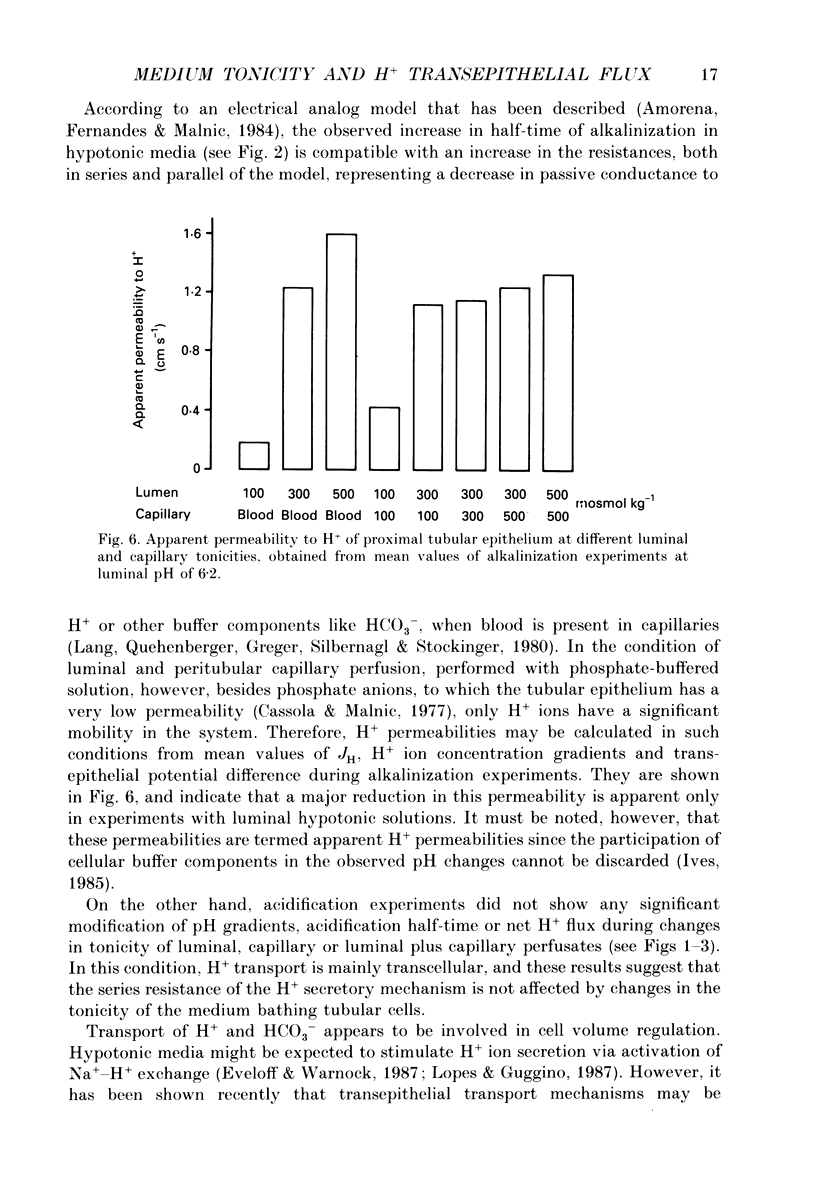
1. The effect of luminal and capillary perfusion with hypotonic or hypertonic solutions containing 25 mM NaHCO3 or NaH2PO4 plus NaCl, K+, Ca2+, Mg2+ and acetate at an osmolality of 100 or 500 mosmol kg-1 on rat proximal H+ secretion was estimated by monitoring luminal pH with Sb microelectrodes. The results were compared to perfusions with the same ionic concentration in which tonicity was adjusted to 300 mosmol kg-1 with raffinose. 2. The kinetics of acidification of luminally injected bicarbonate buffer permits evaluations of H(+)-HCO3-fluxes as well as stationary pH gradients; the kinetics of alkalinization of luminally injected acid phosphate buffer indicates H(+)-HCO3-backfluxes from blood to lumen. 3. In alkalinization experiments, luminal perfusion with hypotonic solution during presence of blood in capillaries or hypotonic capillary perfusion leads to a decrease of stationary pH, an increase of alkalinization half-time and consequently a decrease of passive H(+)-HCO3-backflux. 4. In alkalinization experiments, during luminal and/or capillary perfusions with hypertonic solutions, no significant differences in the stationary pH, alkalinization half-time and H(+)-HCO3-backflux were found. 5. During acidification experiments, with both hypo- and hypertonic perfusions, no significant differences in stationary pH, acidification half-time and H(+)-HCO3-flux were observed. 6. Luminal perfusion with hypotonic solution increases specific epithelial resistance in the presence of blood in capillaries. Luminal perfusion with hypertonic solution does not change this parameter. 7. Volume changes, measured by the split-drop method, are slow during the first 30 s and do not explain the increased alkalinization half-time during luminal perfusion with hypotonic solution, since this is the period of fastest pH change. 8. Luminal perfusion with hypotonic solution decreases apparent H+ permeability in the presence of blood or hypotonic solution in capillaries. Hypertonic solutions in all experimental conditions had no significant effect on this parameter. 9. The data indicate that decrease of tonicity of fluids in contact with proximal tubule epithelium affects passive H(+)-HCO3-backflux, which proceeds in part through the shunt path, while acidification (H+ secretion), which is transcellular, is not affected by extracellular tonicity.
Full text
PDF


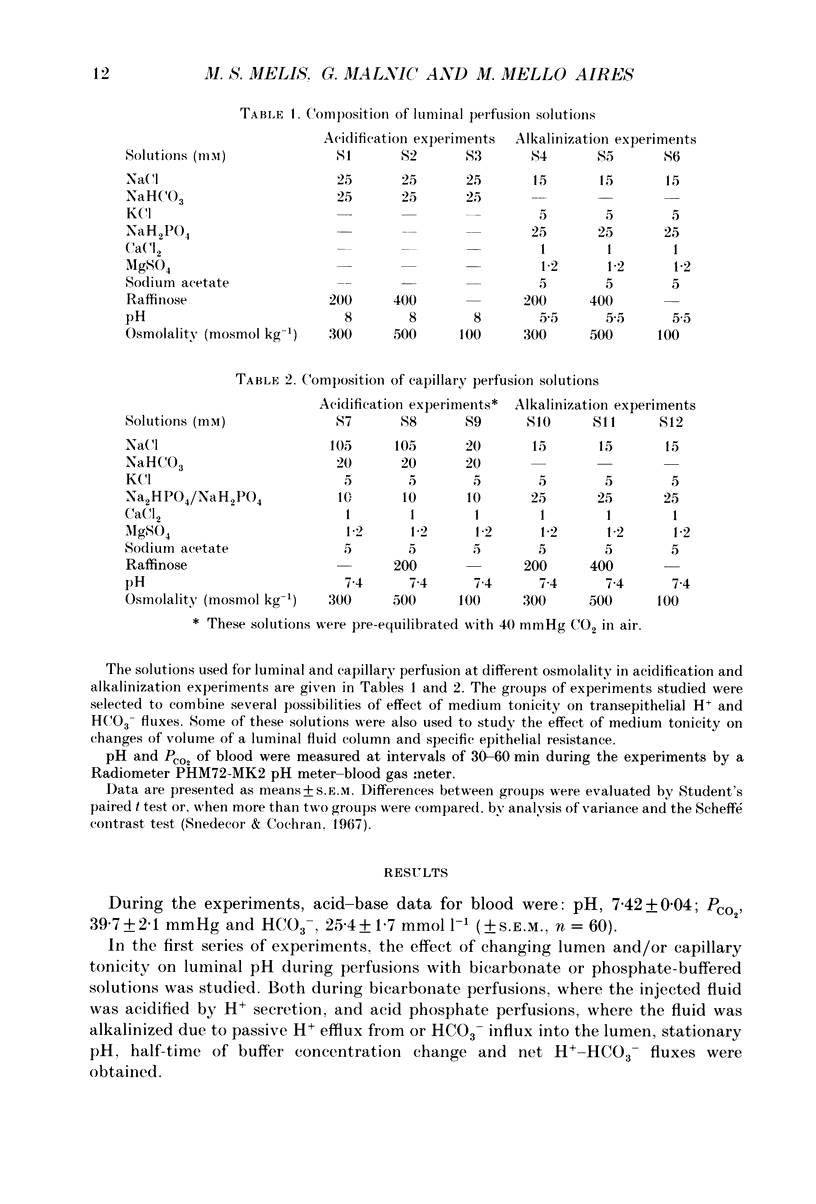
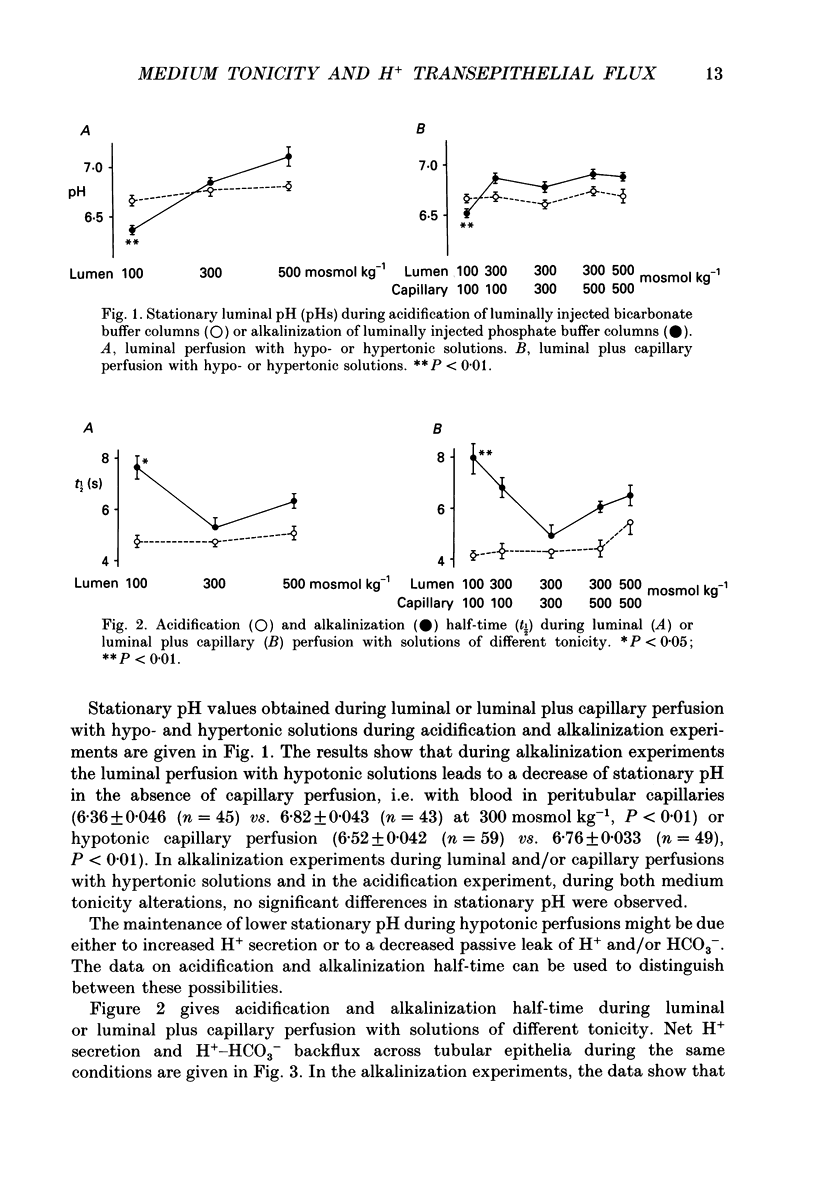




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amorena C., Fernandes D. T., Malnic G. Factors affecting proximal tubular acidification of non-bicarbonate buffer in the rat. J Physiol. 1984 Jul;352:31–48. doi: 10.1113/jphysiol.1984.sp015276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassola A. C., Malnic G. Phosphate transfer and tubular pH during renal stopped flow microperfusion experiments in the rat. Pflugers Arch. 1977 Jan 17;367(3):249–255. doi: 10.1007/BF00581362. [DOI] [PubMed] [Google Scholar]
- De Mello G. B., Lopes A. G., Malnic G. Conductances, diffusion and streaming potentials in the rat proximal tubule. J Physiol. 1976 Sep;260(3):553–569. doi: 10.1113/jphysiol.1976.sp011531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBona D. R., Civan M. M. Pathways for movement of ions and water across toad urinary bladder. I. Anatomic site of transepithelial shunt pathways. J Membr Biol. 1973;12(2):101–128. doi: 10.1007/BF01869994. [DOI] [PubMed] [Google Scholar]
- Eveloff J. L., Warnock D. G. Activation of ion transport systems during cell volume regulation. Am J Physiol. 1987 Jan;252(1 Pt 2):F1–10. doi: 10.1152/ajprenal.1987.252.1.F1. [DOI] [PubMed] [Google Scholar]
- Gagnon J., Ouimet D., Nguyen H., Laprade R., Le Grimellec C., Carrière S., Cardinal J. Cell volume regulation in the proximal convoluted tubule. Am J Physiol. 1982 Oct;243(4):F408–F415. doi: 10.1152/ajprenal.1982.243.4.F408. [DOI] [PubMed] [Google Scholar]
- Giebisch G., Malnic G., De Mello G. B., De Mello Aires M. Kinetics of luminal acidification in cortical tubules of the rat kidney. J Physiol. 1977 Jun;267(3):571–599. doi: 10.1113/jphysiol.1977.sp011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles R., Duchene C., Lambert I. Effect of osmotic shocks on rabbit kidney cortex slices. Am J Physiol. 1983 Jun;244(6):F696–F705. doi: 10.1152/ajprenal.1983.244.6.F696. [DOI] [PubMed] [Google Scholar]
- Györy A. Z., Kweifio-Okai G., Ng J. Hypo- and hyperosmolal saline and raffinose on kidney cortical cell volume at 37 degrees C. Am J Physiol. 1981 Mar;240(3):F180–F184. doi: 10.1152/ajprenal.1981.240.3.F180. [DOI] [PubMed] [Google Scholar]
- Ikonomov O., Simon M., Frömter E. Electrophysiological studies on lateral intercellular spaces of Necturus gallbladder epithelium. Pflugers Arch. 1985 Mar;403(3):301–307. doi: 10.1007/BF00583604. [DOI] [PubMed] [Google Scholar]
- Ives H. E. Proton/hydroxyl permeability of proximal tubule brush border vesicles. Am J Physiol. 1985 Jan;248(1 Pt 2):F78–F86. doi: 10.1152/ajprenal.1985.248.1.F78. [DOI] [PubMed] [Google Scholar]
- Kinne-Saffran E., Beauwens R., Kinne R. An ATP-driven proton pump in brush-border membranes from rat renal cortex. J Membr Biol. 1982;64(1-2):67–76. doi: 10.1007/BF01870769. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Properties of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1980 Jun;238(6):F461–F469. doi: 10.1152/ajprenal.1980.238.6.F461. [DOI] [PubMed] [Google Scholar]
- Kirk K. L., DiBona D. R., Schafer J. A. Regulatory volume decrease in perfused proximal nephron: evidence for a dumping of cell K+. Am J Physiol. 1987 May;252(5 Pt 2):F933–F942. doi: 10.1152/ajprenal.1987.252.5.F933. [DOI] [PubMed] [Google Scholar]
- Lang F., Quehenberger P., Greger R., Silbernagl S., Stockinger P. Evidence for a bicarbonate leak in the proximal tubule of the rat kidney. Pflugers Arch. 1980 Aug;386(3):239–244. doi: 10.1007/BF00587474. [DOI] [PubMed] [Google Scholar]
- Linshaw M. A., Grantham J. J. Effect of collagenase and ouabain on renal cell volume in hypotonic media. Am J Physiol. 1980 Jun;238(6):F491–F498. doi: 10.1152/ajprenal.1980.238.6.F491. [DOI] [PubMed] [Google Scholar]
- Lopes A. G., Guggino W. B. Volume regulation in the early proximal tubule of the Necturus kidney. J Membr Biol. 1987;97(2):117–125. doi: 10.1007/BF01869418. [DOI] [PubMed] [Google Scholar]
- Lopes A. G., de Mello-Aires M., Malnic G. Behavior of the antimony microelectrode in different buffer solutions. Braz J Med Biol Res. 1981 Apr;14(1):29–36. [PubMed] [Google Scholar]
- Malnic G. Robert F. Pitts memorial lecture. H+ secretion in renal cortical tubules: kinetic aspects. Kidney Int. 1987 Jul;32(1):136–150. doi: 10.1038/ki.1987.183. [DOI] [PubMed] [Google Scholar]
- Malnic G., Silva Netto C. R., Stamopoulos C. D., de Mello Aires M. Online measurement of fluid reabsorption in renal tubules. Med Biol Eng Comput. 1979 May;17(3):330–332. doi: 10.1007/BF02443818. [DOI] [PubMed] [Google Scholar]
- Malnic G., de Mello-Aires M. Kinetic study of bicarbonate reabsorption in proximal tubule of the rat. Am J Physiol. 1971 Jun;220(6):1759–1767. doi: 10.1152/ajplegacy.1971.220.6.1759. [DOI] [PubMed] [Google Scholar]
- Montrose-Rafizadeh C., Guggino W. B. Cell volume regulation in the nephron. Annu Rev Physiol. 1990;52:761–772. doi: 10.1146/annurev.ph.52.030190.003553. [DOI] [PubMed] [Google Scholar]
- Montrose M. H., Murer H. Polarity and kinetics of Na(+)-H+ exchange in cultured opossum kidney cells. Am J Physiol. 1990 Jul;259(1 Pt 1):C121–C133. doi: 10.1152/ajpcell.1990.259.1.C121. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Effects of luminal hyperosmolality on electrical pathways of Necturas gallbladder. Am J Physiol. 1977 Mar;232(3):C99–108. doi: 10.1152/ajpcell.1977.232.3.C99. [DOI] [PubMed] [Google Scholar]
- Soleimani M., Grassi S. M., Aronson P. S. Stoichiometry of Na+-HCO-3 cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987 Apr;79(4):1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring K. R. A parallel path model for Necturus proximal tubule. J Membr Biol. 1973 Nov 8;13(4):323–352. doi: 10.1007/BF01868235. [DOI] [PubMed] [Google Scholar]
- Vieira F. L., Malnic G. Hydrogen ion secretion by rat renal cortical tubules as studied by an antimony microelectrode. Am J Physiol. 1968 Apr;214(4):710–718. doi: 10.1152/ajplegacy.1968.214.4.710. [DOI] [PubMed] [Google Scholar]
- Völkl H., Paulmichl M., Lang F. Cell volume regulation in renal cortical cells. Ren Physiol Biochem. 1988 May-Oct;11(3-5):158–173. doi: 10.1159/000173160. [DOI] [PubMed] [Google Scholar]
- Welling P. A., Linshaw M. A. Importance of anion in hypotonic volume regulation of rabbit proximal straight tubule. Am J Physiol. 1988 Nov;255(5 Pt 2):F853–F860. doi: 10.1152/ajprenal.1988.255.5.F853. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Burckhardt B. C., Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflugers Arch. 1985 Dec;405(4):360–366. doi: 10.1007/BF00595689. [DOI] [PubMed] [Google Scholar]
- de Bermudez L., Windhager E. E. Osmotically induced changes in electrical resistance of distal tubules of rat kidney. Am J Physiol. 1975 Dec;229(6):1536–1546. doi: 10.1152/ajplegacy.1975.229.6.1536. [DOI] [PubMed] [Google Scholar]


