Abstract
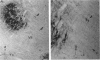
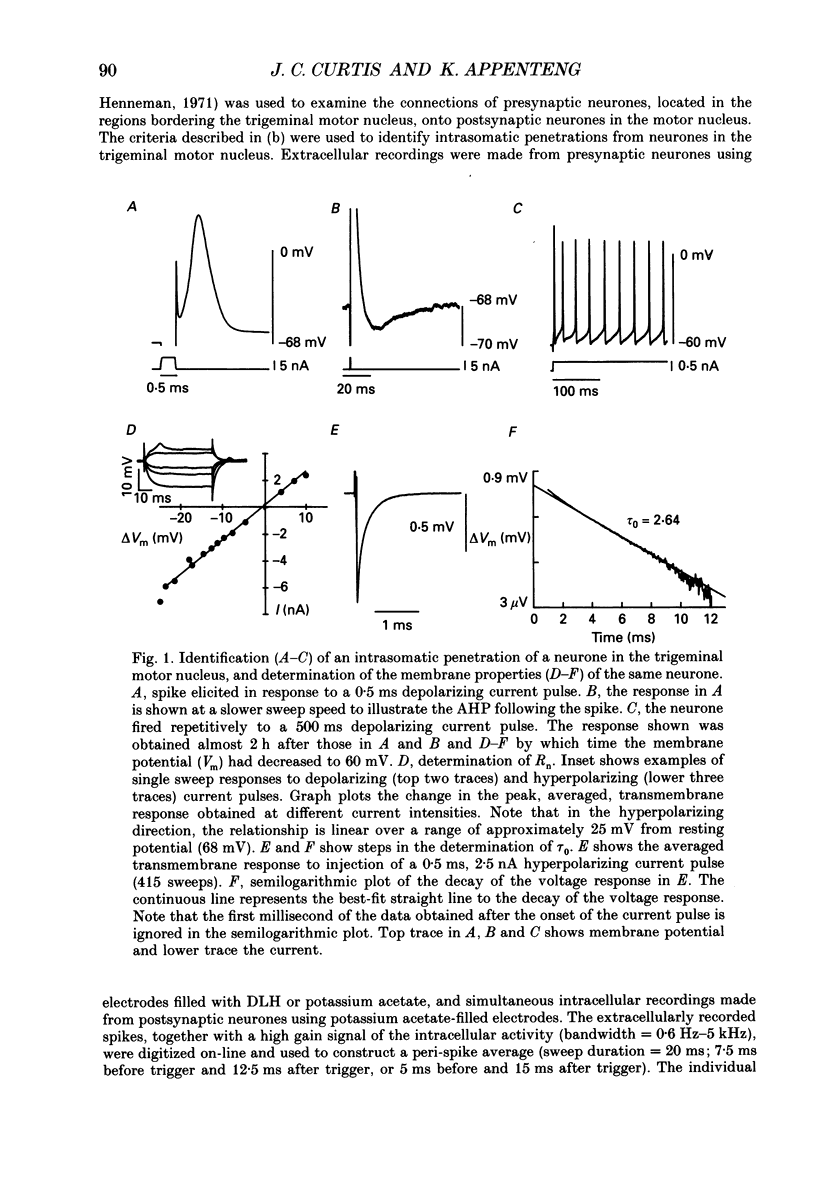
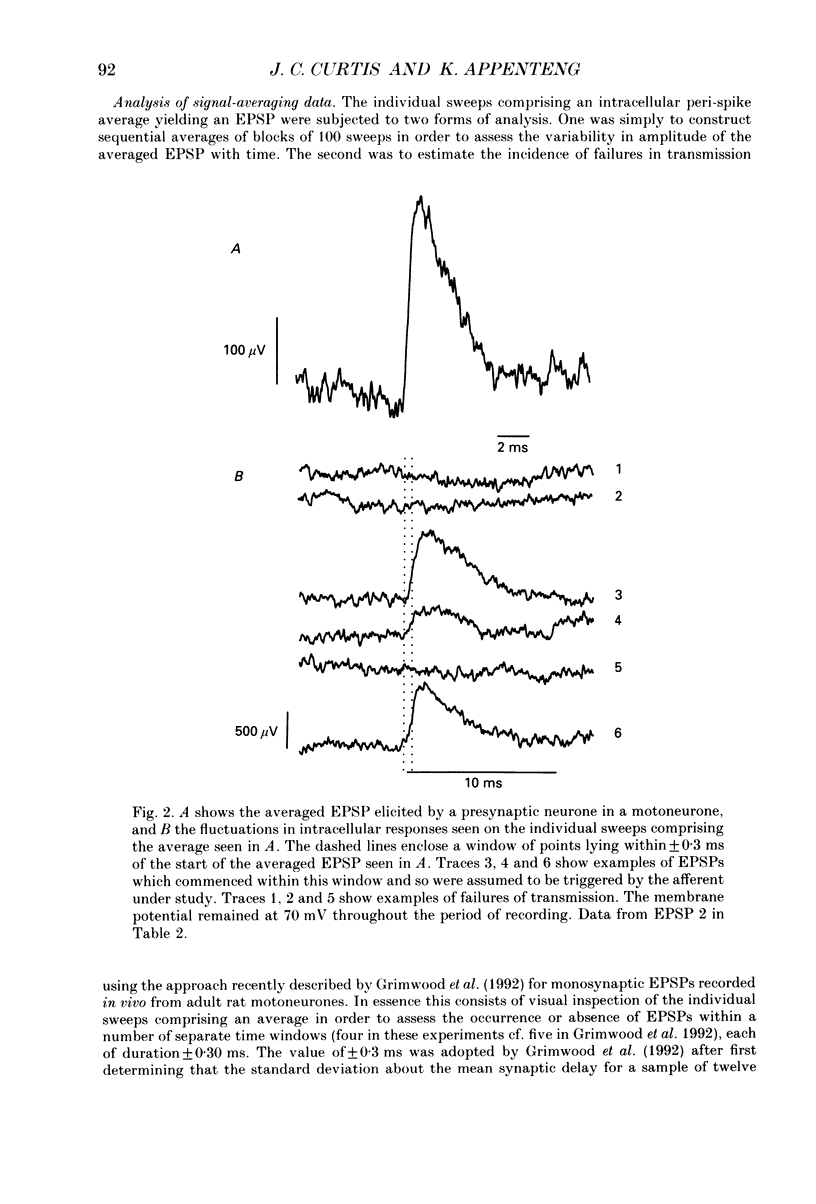
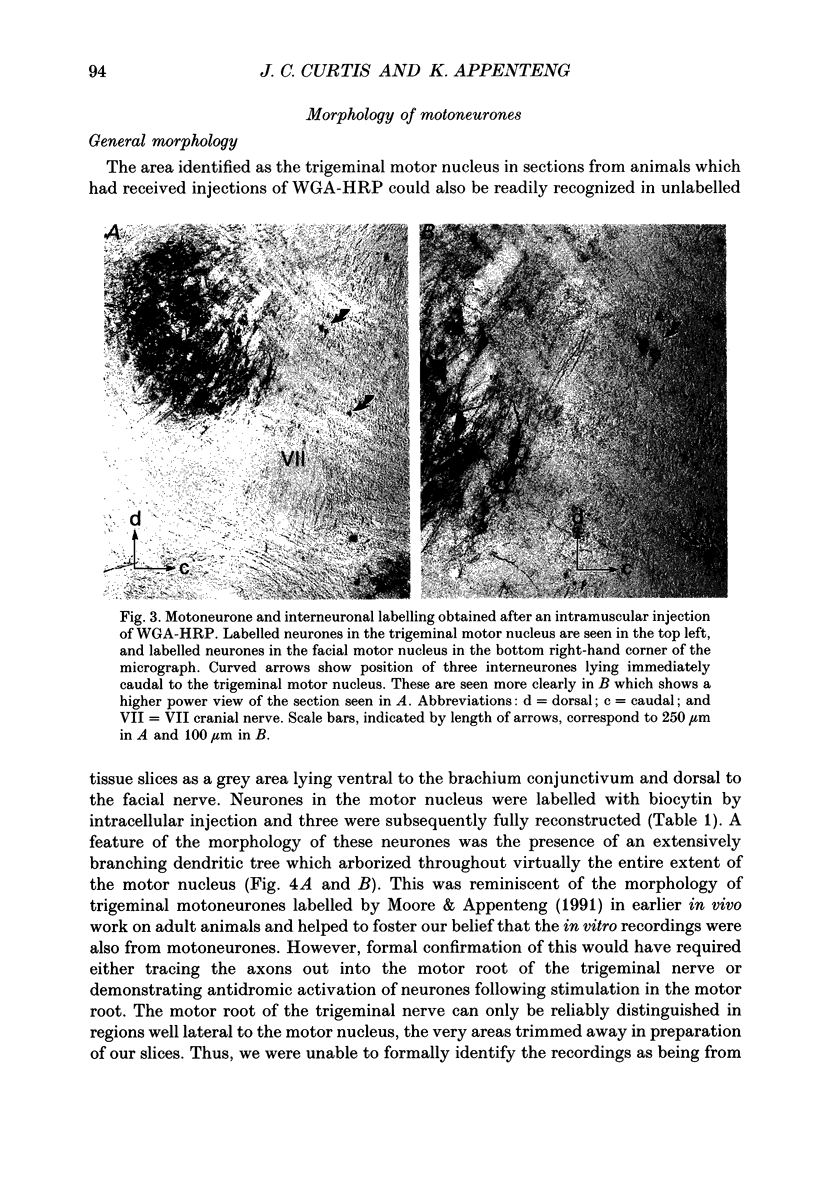
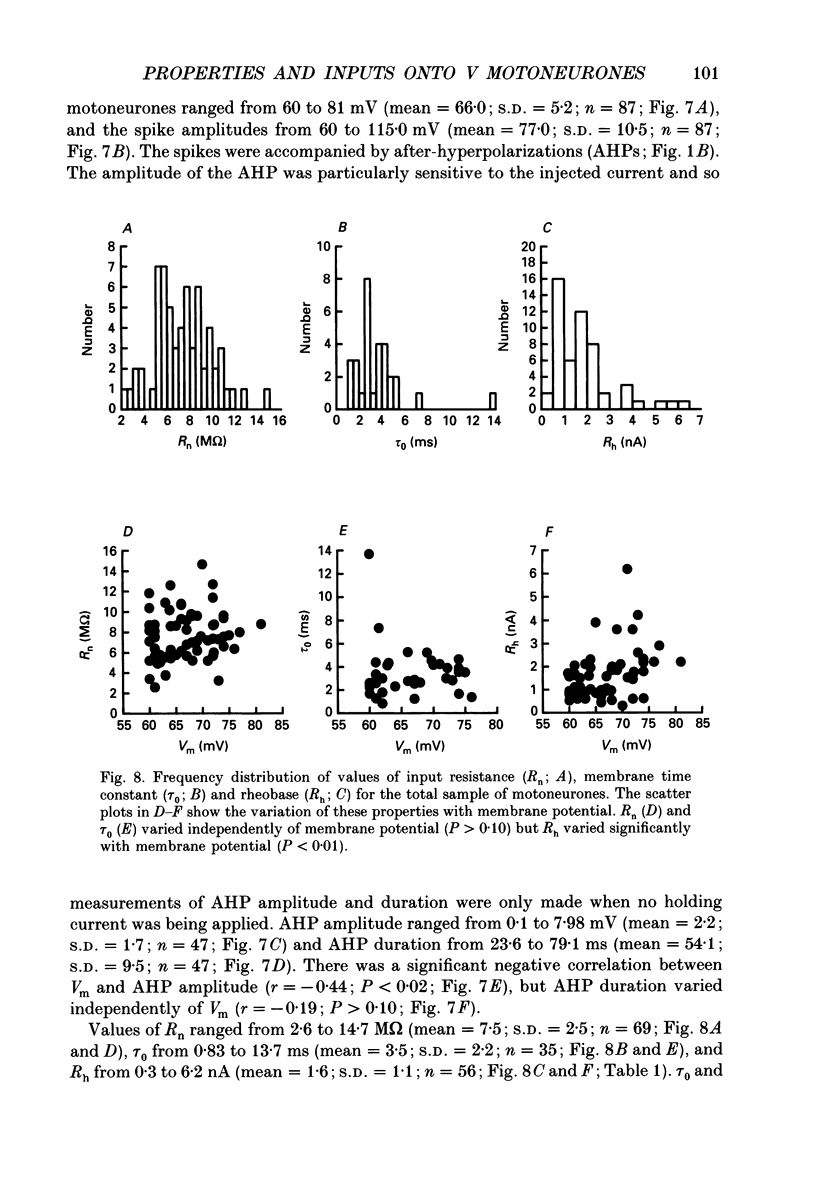
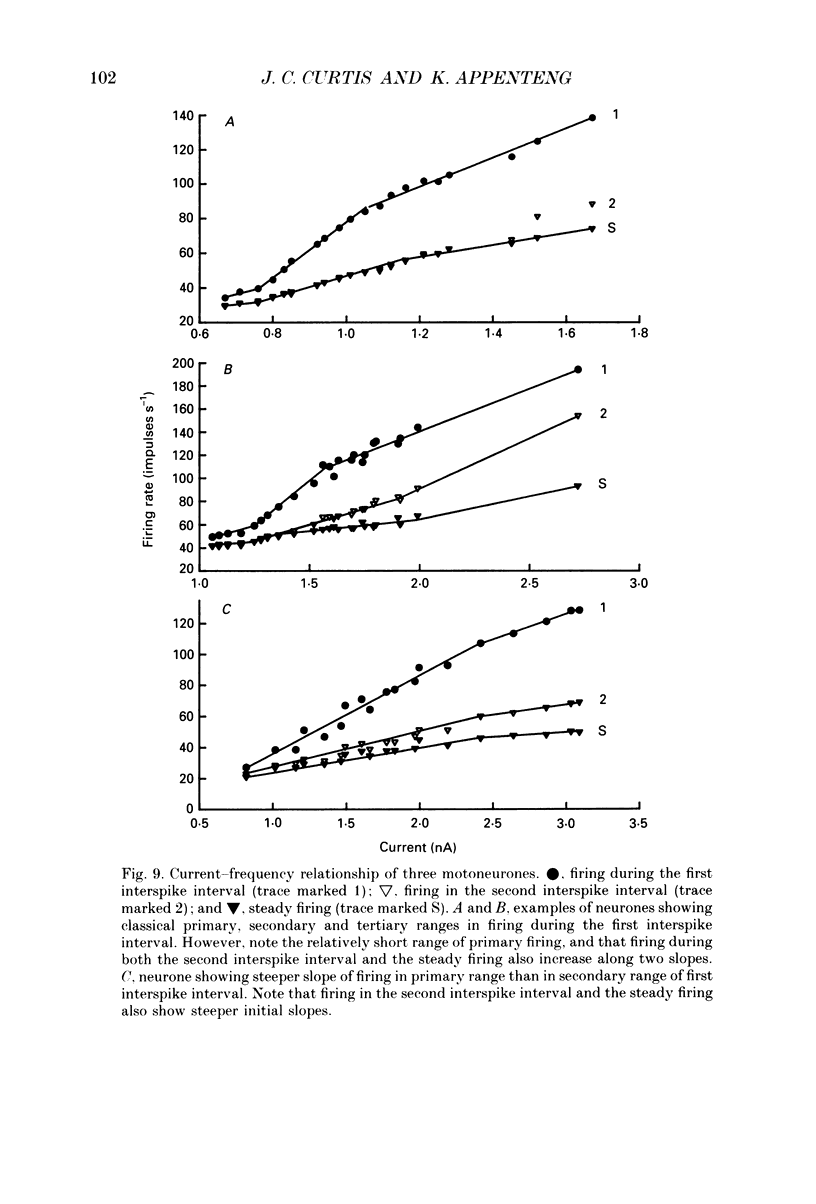
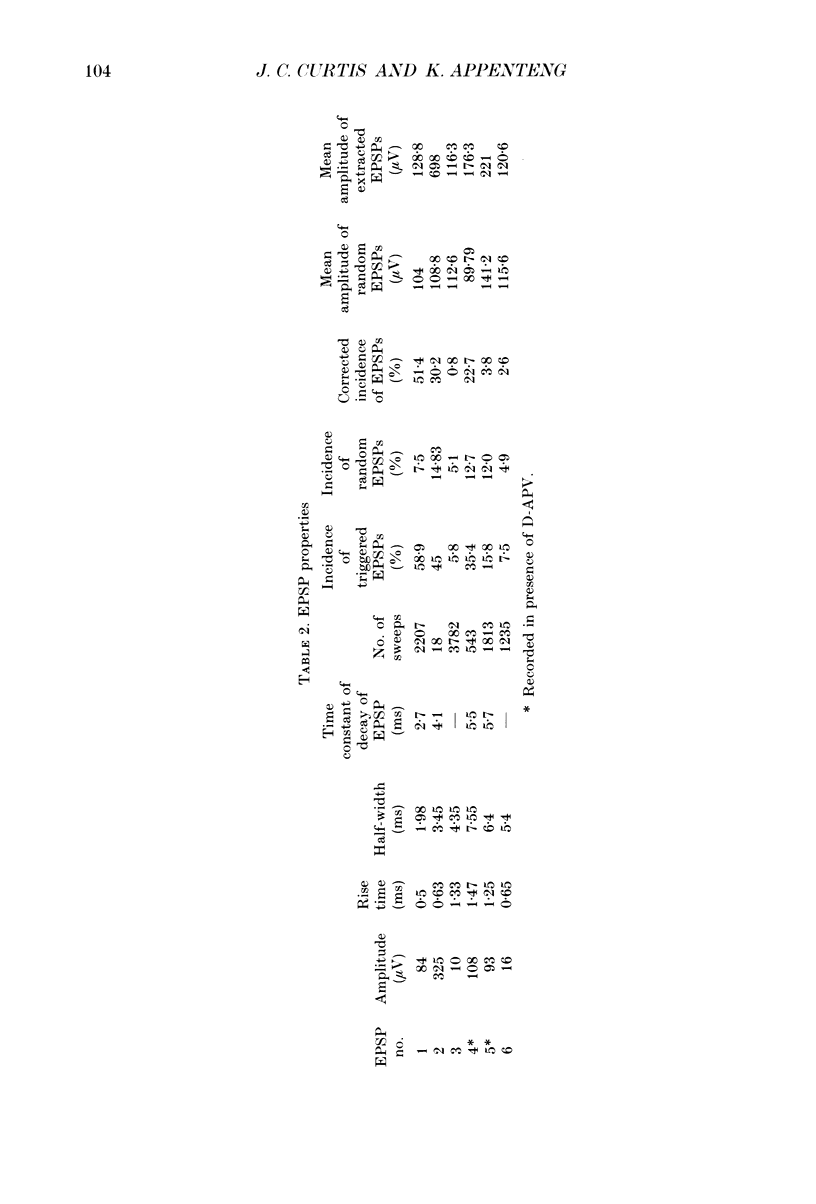
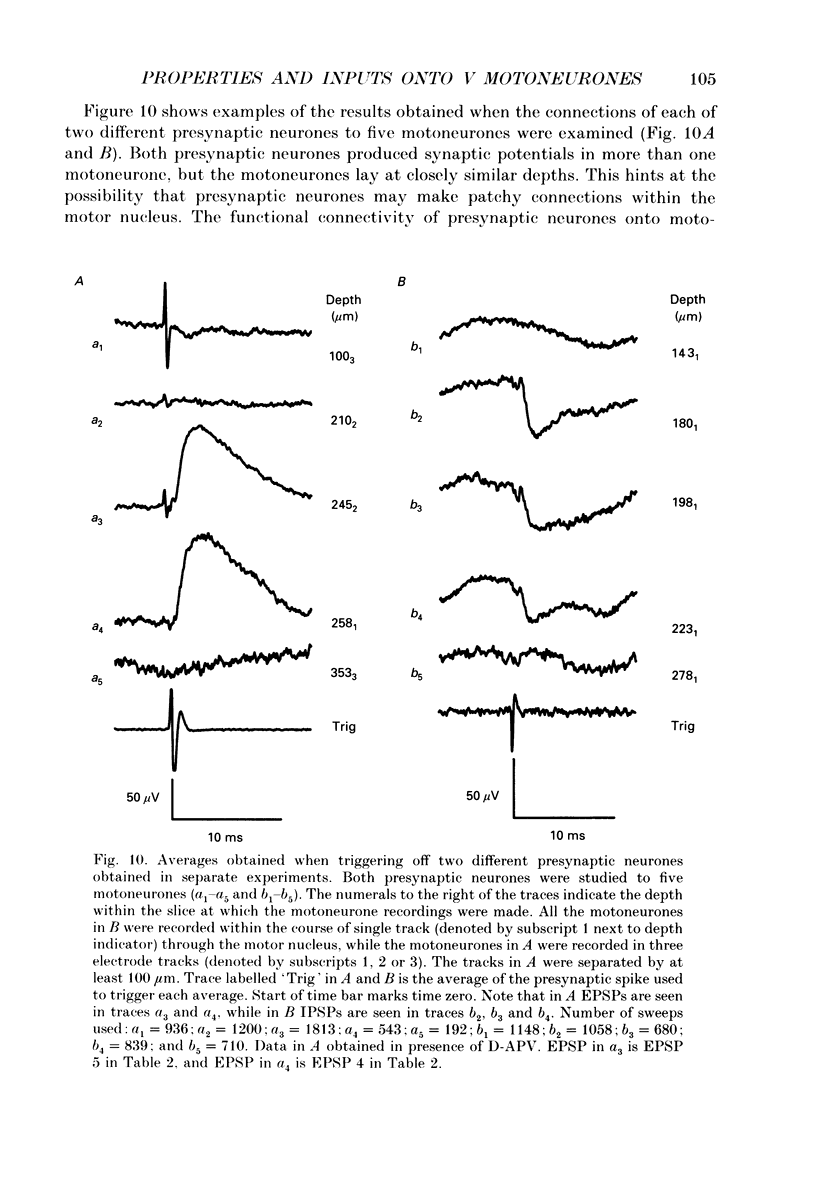
1. We have developed a tissue slice preparation which allows the study of the actions of single presynaptic neurones onto single trigeminal motoneurones in the immature rat. Our aim in this first stage of the work has been to assess the validity of this preparation as a model for responses obtained in vivo from trigeminal motoneurones in adult rats. We have quantified the integrative properties of the motoneurones and also the variability in transmission at synapses of single presynaptic neurones onto the motoneurones. This data has then been compared to similar published data obtained from adult (rat) trigeminal motoneurones in vivo. 2. Quantitative reconstructions were made of the morphology of three motoneurones which had been labelled with biocytin by intracellular injection. The neurones gave off six to nine dendrites, of mean length 522 microns (S.D. = 160; n = 22), which branched on average 10.5 times to produce 11.45 end-terminations per dendrite (S.D. = 8.57; n = 22). The mean surface area of the dendrites was 0.92 x 10(4) microns2 (S.D. = 0.67; n = 22), and, for individual cells, the ratio of the combined dendritic surface area to the total neuronal surface area ranged from 98.3 to 99.2% (n = 3). At dendritic branch points the ratio of the summed diameters of the daughter dendrites to the 3/2 power against the parent dendrite to the 3/2 power was 1.09 (S.D. = 0.21; n = 217), allowing branch points to be collapsed into a single cylinder. The equivalent cylinder diameter of the combined dendritic tree remained approximately constant over the proximal 25-40% of the equivalent electrical length of the dendritic tree and then showed tapering. The tapering could be ascribed to termination of dendrites at different electrical distances from the soma. 3. Electrical properties were determined for a total of eighty-seven motoneurones, all with membrane potentials more negative than 60 mV (mean = 66.0 mV; S.D. = 5.2) and spikes which overshot zero (mean spike amplitude = 77 mV; S.D. = 10.5; n = 87). The spikes were followed by after-hyperpolarizations (AHPs) of mean amplitude 2.2 mV (S.D. = 1.7; n = 47), and mean duration 54.1 ms (S.D. = 9.5; n = 47). The mean input resistance of the neurones was 7.5 M omega (S.D. = 2.5; n = 69), the mean membrane time constant was 3.5 ms (S.D. = 2.2; n = 35), and the mean rheobase was 1.6 nA (S.D. = 1.1; n = 56).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







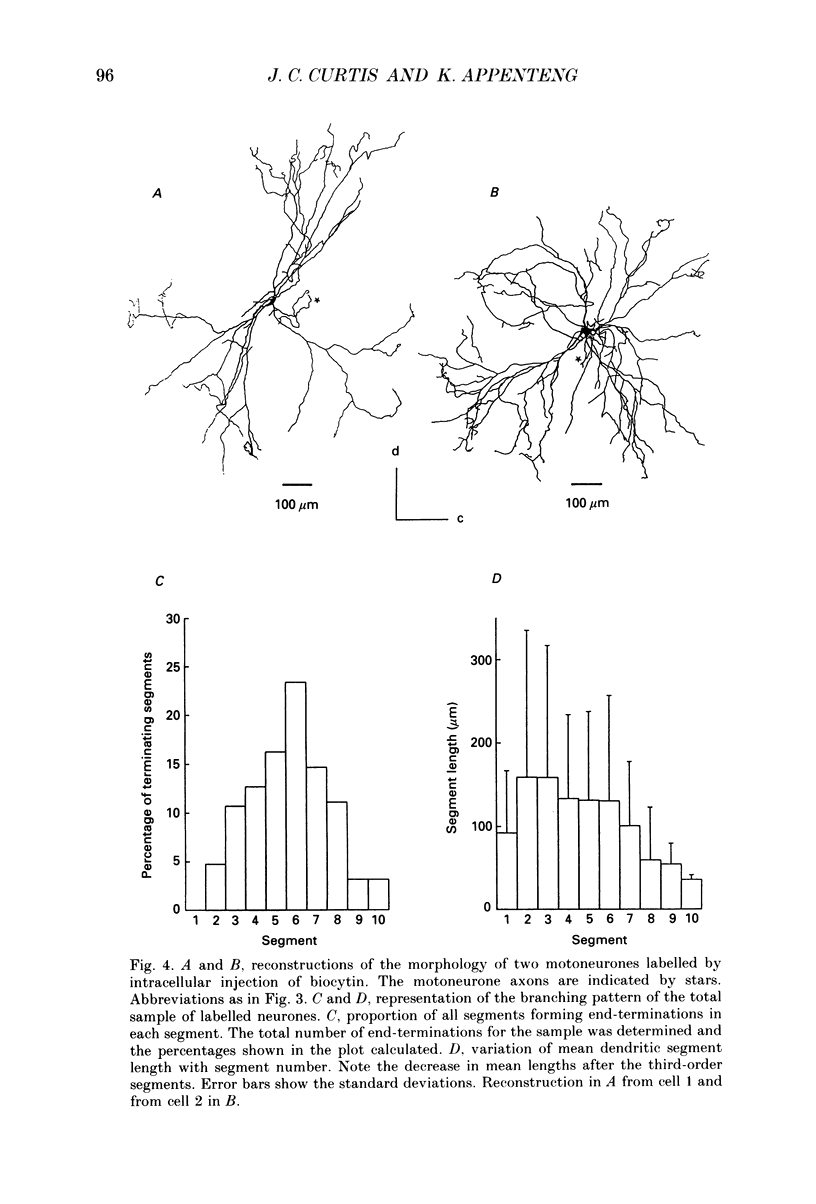
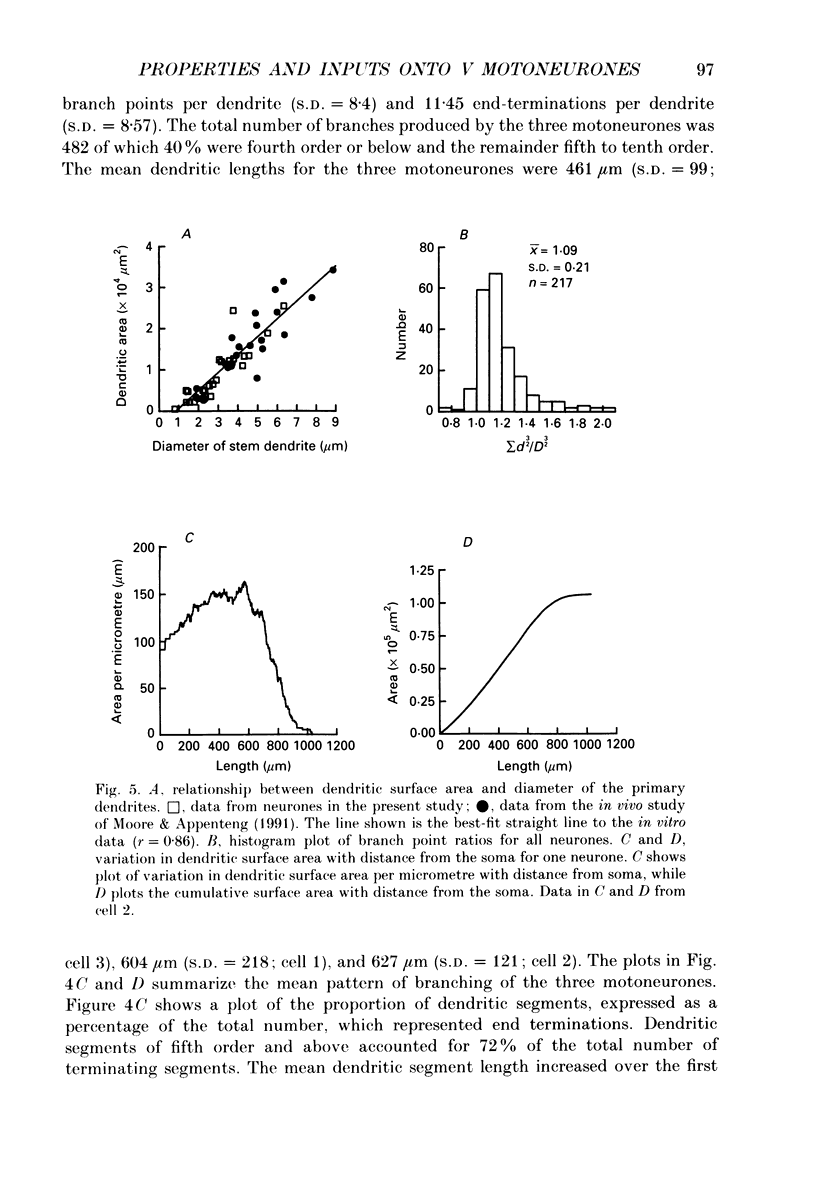
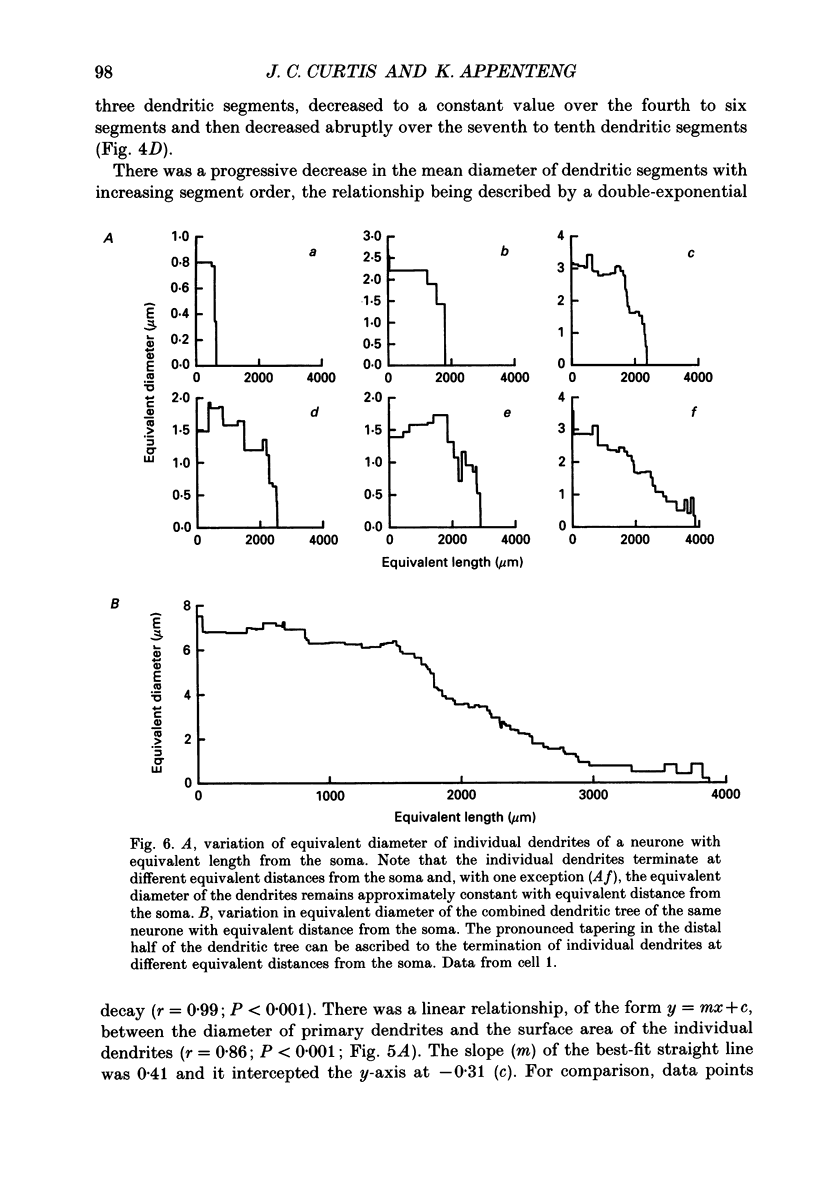

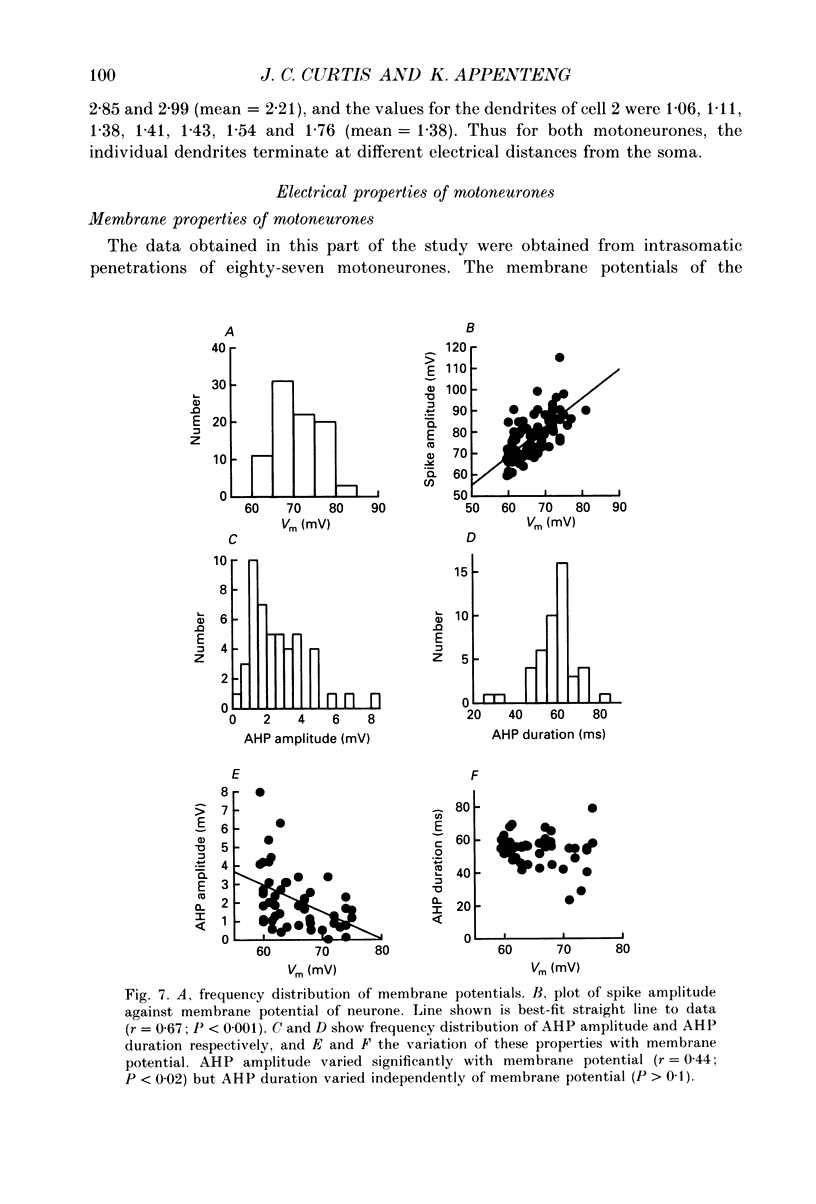











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appenteng K., Conyers L., Moore J. A. The monosynaptic excitatory connections of single trigeminal interneurones to the V motor nucleus of the rat. J Physiol. 1989 Oct;417:91–104. doi: 10.1113/jphysiol.1989.sp017792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenteng K., Girdlestone D. Transneuronal transport of wheat germ agglutinin-conjugated horseradish peroxidase into trigeminal interneurones of the rat. J Comp Neurol. 1987 Apr 15;258(3):387–396. doi: 10.1002/cne.902580307. [DOI] [PubMed] [Google Scholar]
- Baskys A., Malenka R. C. Agonists at metabotropic glutamate receptors presynaptically inhibit EPSCs in neonatal rat hippocampus. J Physiol. 1991 Dec;444:687–701. doi: 10.1113/jphysiol.1991.sp018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990 Aug 23;346(6286):724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Redman S. J. Cable properties of cat spinal motoneurones measured by combining voltage clamp, current clamp and intracellular staining. J Physiol. 1989 Feb;409:63–87. doi: 10.1113/jphysiol.1989.sp017485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S., Fleshman J. W., Glenn L. L., Burke R. E. Membrane area and dendritic structure in type-identified triceps surae alpha motoneurons. J Comp Neurol. 1987 Jan 1;255(1):68–81. doi: 10.1002/cne.902550106. [DOI] [PubMed] [Google Scholar]
- D'Albis A., Janmot C., Couteaux R. Species- and muscle type-dependence of perinatal isomyosin transitions. Int J Dev Biol. 1991 Mar;35(1):53–56. [PubMed] [Google Scholar]
- Fulton B. P., Walton K. Electrophysiological properties of neonatal rat motoneurones studied in vitro. J Physiol. 1986 Jan;370:651–678. doi: 10.1113/jphysiol.1986.sp015956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood P. D., Appenteng K., Curtis J. C. Monosynaptic EPSPs elicited by single interneurones and spindle afferents in trigeminal motoneurones of anaesthetized rats. J Physiol. 1992 Sep;455:641–662. doi: 10.1113/jphysiol.1992.sp019320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Pinter M. J. Relations among passive electrical properties of lumbar alpha-motoneurones of the cat. J Physiol. 1984 Nov;356:401–431. doi: 10.1113/jphysiol.1984.sp015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad G. G., Donnelly D. F., Getting P. A. Biophysical properties of hypoglossal neurons in vitro: intracellular studies in adult and neonatal rats. J Appl Physiol (1985) 1990 Oct;69(4):1509–1517. doi: 10.1152/jappl.1990.69.4.1509. [DOI] [PubMed] [Google Scholar]
- Harrison P. J., Jack J. J., Kullmann D. M. Monosynaptic EPSPs in cat lumbosacral motoneurones from group Ia afferents and fibres descending in the spinal cord. J Physiol. 1989 May;412:43–63. doi: 10.1113/jphysiol.1989.sp017603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E., Lüscher H. R., Mathis J. Simultaneously active and inactive synapses of single Ia fibres on cat spinal motoneurones. J Physiol. 1984 Jul;352:147–161. doi: 10.1113/jphysiol.1984.sp015283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K., Armstrong W. E. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988 Aug;25(1):1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez I., Rudomin P., Enriquez M. Differential effects of (-)-baclofen on Ia and descending monosynaptic EPSPs. Exp Brain Res. 1991;85(1):103–113. doi: 10.1007/BF00229991. [DOI] [PubMed] [Google Scholar]
- Kernell D. Functional properties of spinal motoneurons and gradation of muscle force. Adv Neurol. 1983;39:213–226. [PubMed] [Google Scholar]
- Klee M. R., Pierau F. K., Faber D. S. Temperature effects on resting potential and spike parameters of cat motoneurons. Exp Brain Res. 1974 Mar 29;19(5):478–492. doi: 10.1007/BF00236112. [DOI] [PubMed] [Google Scholar]
- Koerber H. R., Mendell L. M. Modulation of synaptic transmission at Ia-afferent fiber connections on motoneurons during high-frequency stimulation: role of postsynaptic target. J Neurophysiol. 1991 Mar;65(3):590–597. doi: 10.1152/jn.1991.65.3.590. [DOI] [PubMed] [Google Scholar]
- Larkman A., Stratford K., Jack J. Quantal analysis of excitatory synaptic action and depression in hippocampal slices. Nature. 1991 Mar 28;350(6316):344–347. doi: 10.1038/350344a0. [DOI] [PubMed] [Google Scholar]
- Larkman P. M., Penington N. J., Kelly J. S. Electrophysiology of adult rat facial motoneurones: the effects of serotonin (5-HT) in a novel in vitro brainstem slice. J Neurosci Methods. 1989 May;28(1-2):133–146. doi: 10.1016/0165-0270(89)90018-6. [DOI] [PubMed] [Google Scholar]
- Lev-Tov A., Pinco M. In vitro studies of prolonged synaptic depression in the neonatal rat spinal cord. J Physiol. 1992 Feb;447:149–169. doi: 10.1113/jphysiol.1992.sp018996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R. Transmission between pairs of hippocampal slice neurons: quantal levels, oscillations, and LTP. Science. 1991 May 3;252(5006):722–724. doi: 10.1126/science.1850871. [DOI] [PubMed] [Google Scholar]
- Malinow R., Tsien R. W. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990 Jul 12;346(6280):177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Moore J. A., Appenteng K. Contrasting effects of urethane and pentobarbitone anaesthesia on the electrical properties of rat jaw-elevator motoneurones. Brain Res. 1990 Jul 16;523(1):139–142. doi: 10.1016/0006-8993(90)91647-y. [DOI] [PubMed] [Google Scholar]
- Moore J. A., Appenteng K. The morphology and electrical geometry of rat jaw-elevator motoneurones. J Physiol. 1991;440:325–343. doi: 10.1113/jphysiol.1991.sp018711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J., Appenteng K. The membrane properties and firing characteristics of rat jaw-elevator motoneurones. J Physiol. 1990 Apr;423:137–153. doi: 10.1113/jphysiol.1990.sp018015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosfeldt Laursen A., Rekling J. C. Electrophysiological properties of hypoglossal motoneurons of guinea-pigs studied in vitro. Neuroscience. 1989;30(3):619–637. doi: 10.1016/0306-4522(89)90156-5. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Schwindt P. C., Crill W. E. Electrical properties of facial motoneurons in brainstem slices from guinea pig. Brain Res. 1989 Nov 13;502(1):127–142. doi: 10.1016/0006-8993(89)90468-x. [DOI] [PubMed] [Google Scholar]
- RALL W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959 Nov;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990 Jan;70(1):165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Røste G. K. Non-motoneurons in the facial and motor trigeminal nuclei projecting to the cerebellar flocculus in the cat. A fluorescent double-labelling and WGA-HRP study. Exp Brain Res. 1989;75(2):295–305. doi: 10.1007/BF00247935. [DOI] [PubMed] [Google Scholar]
- Saha S., Appenteng K., Batten T. F. Light and electron microscopical localisation of 5-HT-immunoreactive boutons in the rat trigeminal motor nucleus. Brain Res. 1991 Sep 13;559(1):145–148. doi: 10.1016/0006-8993(91)90297-9. [DOI] [PubMed] [Google Scholar]
- Saha S., Appenteng K., Batten T. F. Quantitative analysis and postsynaptic targets of GABA-immunoreactive boutons within the rat trigeminal motor nucleus. Brain Res. 1991 Oct 4;561(1):128–138. doi: 10.1016/0006-8993(91)90757-m. [DOI] [PubMed] [Google Scholar]
- Sayer R. J., Redman S. J., Andersen P. Amplitude fluctuations in small EPSPs recorded from CA1 pyramidal cells in the guinea pig hippocampal slice. J Neurosci. 1989 Mar;9(3):840–850. doi: 10.1523/JNEUROSCI.09-03-00840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Liu G., Feldman J. L. Intracellular recording from phrenic motoneurons receiving respiratory drive in vitro. Neurosci Lett. 1988 May 16;88(1):27–32. doi: 10.1016/0304-3940(88)90310-2. [DOI] [PubMed] [Google Scholar]
- Sypert G. W., Fleshman J. W., Munson J. B. Comparison of monosynaptic actions of medial gastrocnemius group Ia and group II muscle spindle afferents on triceps surae motoneurons. J Neurophysiol. 1980 Oct;44(4):726–738. doi: 10.1152/jn.1980.44.4.726. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. J Physiol. 1990 Apr;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. M., Radpour S. Excitatory Connections Between CA1 Pyramidal Cells Revealed by Spike Triggered Averaging in Slices of Rat Hippocampus are Partially NMDA Receptor Mediated. Eur J Neurosci. 1991;3(6):587–601. doi: 10.1111/j.1460-9568.1991.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Ulfhake B., Cullheim S., Franson P. Postnatal development of cat hind limb motoneurons. I: Changes in length, branching structure, and spatial distribution of dendrites of cat triceps surae motoneurons. J Comp Neurol. 1988 Dec 1;278(1):69–87. doi: 10.1002/cne.902780105. [DOI] [PubMed] [Google Scholar]
- Ulfhake B., Cullheim S. Postnatal development of cat hind limb motoneurons. III: Changes in size of motoneurons supplying the triceps surae muscle. J Comp Neurol. 1988 Dec 1;278(1):103–120. doi: 10.1002/cne.902780107. [DOI] [PubMed] [Google Scholar]