Abstract
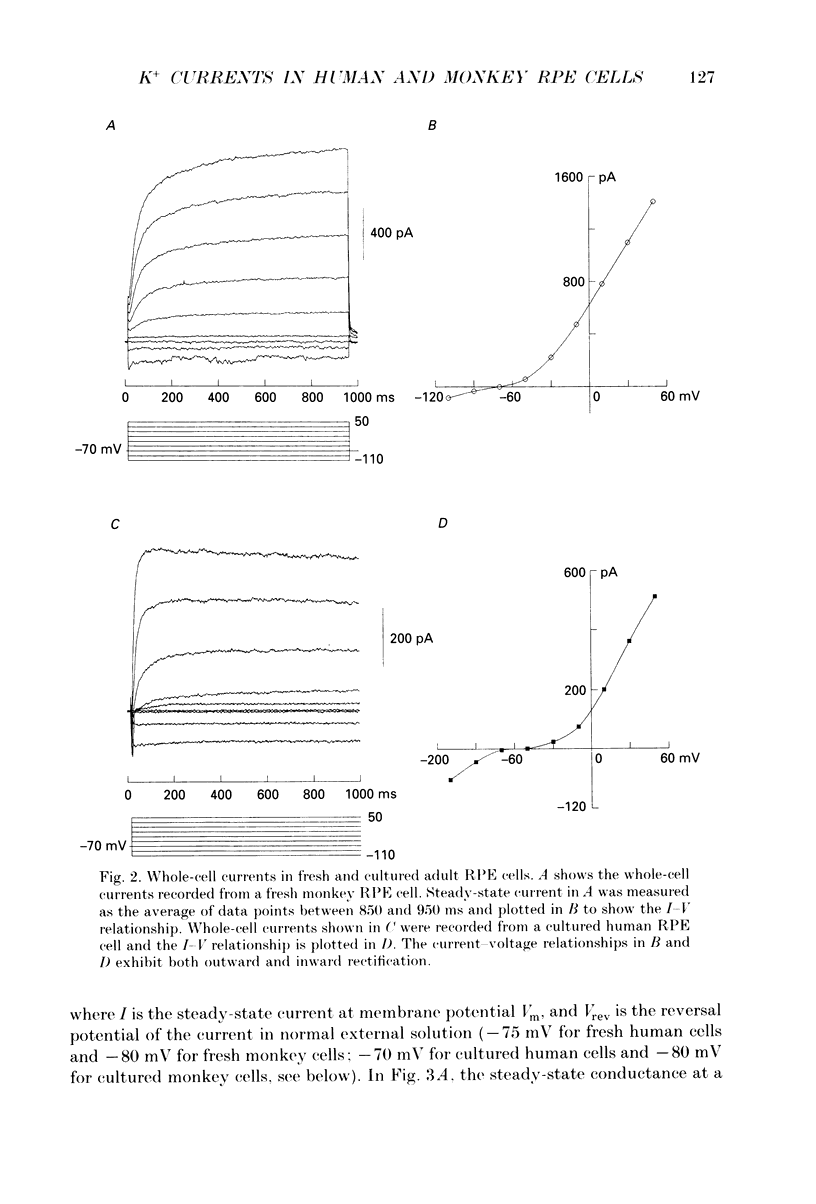
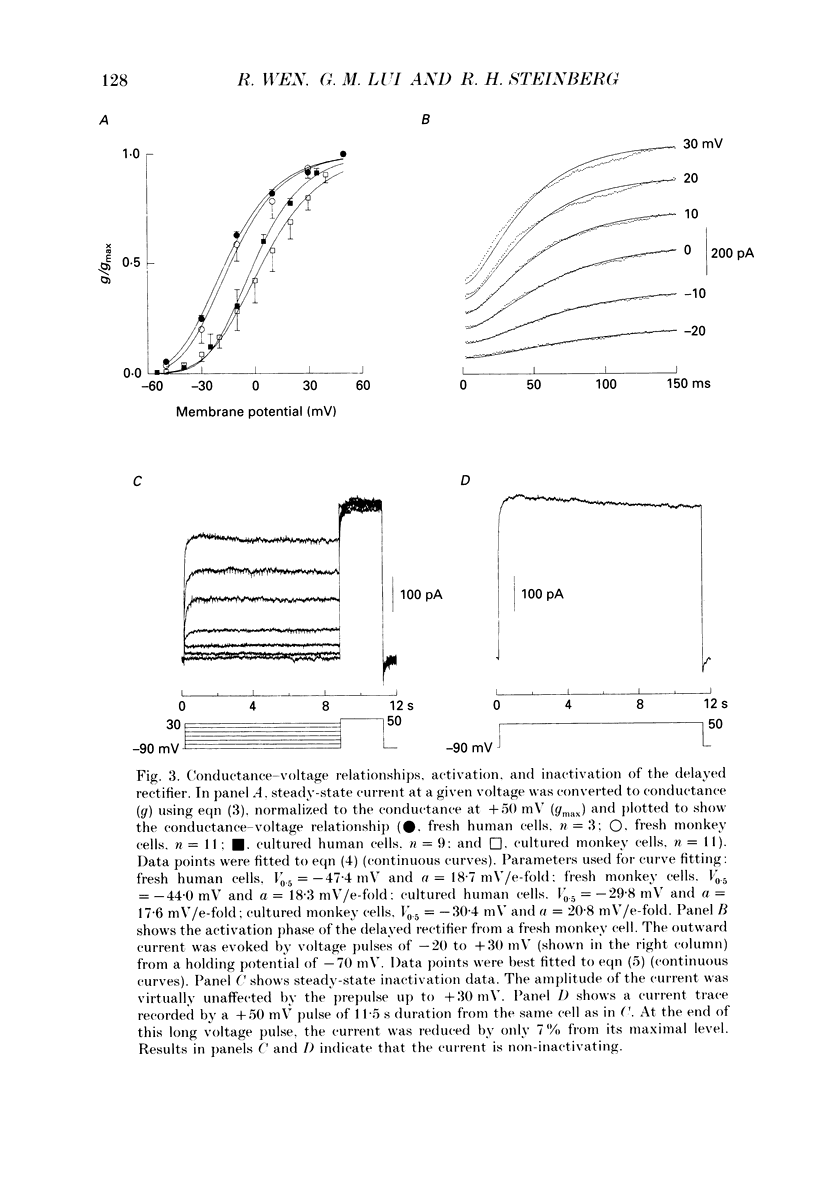
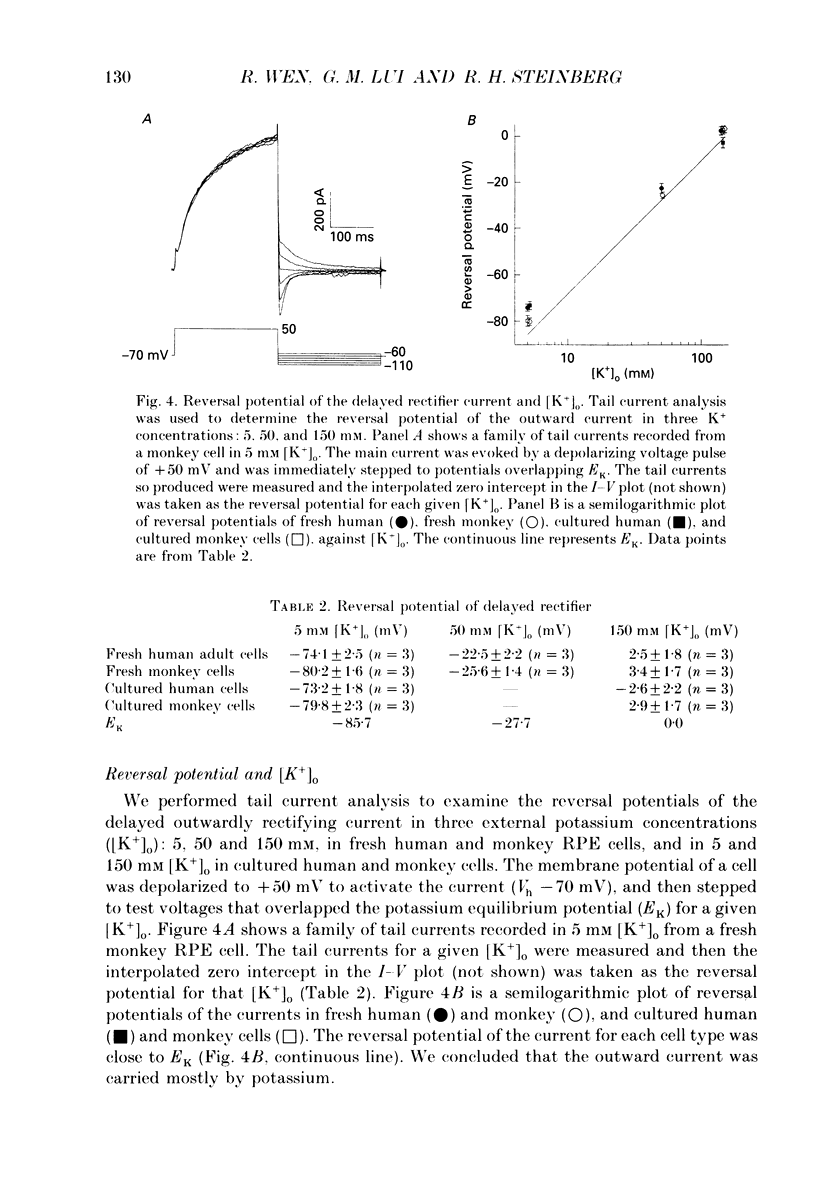
1. Whole-cell potassium currents of freshly isolated human (adult and fetal) and monkey (adult) retinal pigment epithelial (RPE) cells, as well as cultured human and monkey RPE cells were studied using the patch-clamp technique. 2. In freshly isolated adult cells of both species, two currents were observed in the voltage range from -150 to +50 mV: an outwardly rectifying current and an inwardly rectifying current. These currents were also found in cultured cells of both species. 3. The outwardly rectifying current in freshly isolated adult human and monkey cells and some cultured cells was evoked by depolarizing voltage pulses more positive that -30 mV. The current activated with a sigmoidal time course after a brief delay, and was virtually non-inactivating. The conductance associated with the current was half-maximal at -16.4 mV for fresh human cells and -13.5 mV for fresh monkey cells, but was shifted 16.0 and 17.7 mV in the positive direction in cultured human and monkey cells, respectively. The reversal potential of the current in both human and monkey cells matched the potassium equilibrium potential (EK) over a wide range of external potassium concentrations. This current was blocked by 20 mM tetraethylammonium. 4. A membrane current that exhibited inward rectification was observed with hyperpolarizing voltage pulses. The zero-current potential of this current was close to EK. This current was blocked by 2 mM Ba2+ and 2 mM Cs+. In cultured human and monkey cells, but not in fresh cells, this current exhibited an inactivation when voltage pulses were more negative than -120 mV. External Na+ was responsible for the inactivation, as the inactivation was removed in a Na(+)-free solution. 5. Membrane currents in freshly isolated fetal human RPE cells were remarkably different from those in adult cells. A transient outward current resembling the A-type potassium current was observed as the dominant membrane current in freshly isolated fetal human cells. This current activated when voltage pulses were more positive than -30 mV. It inactivated rapidly after reaching a maximal level. Application of 5 mM 4-aminopyridine (4-AP) completely blocked this current. Although this current was never observed in fresh adult cells, it was found in 33% of the cultured adult cells with similar kinetics, ion selectivity, and pharmacological properties. 6. In about 26% of the freshly isolated fetal human cells, a more slowly activating outward current, which resembled the delayed rectifier, was found to co-exist with the transient outward current.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
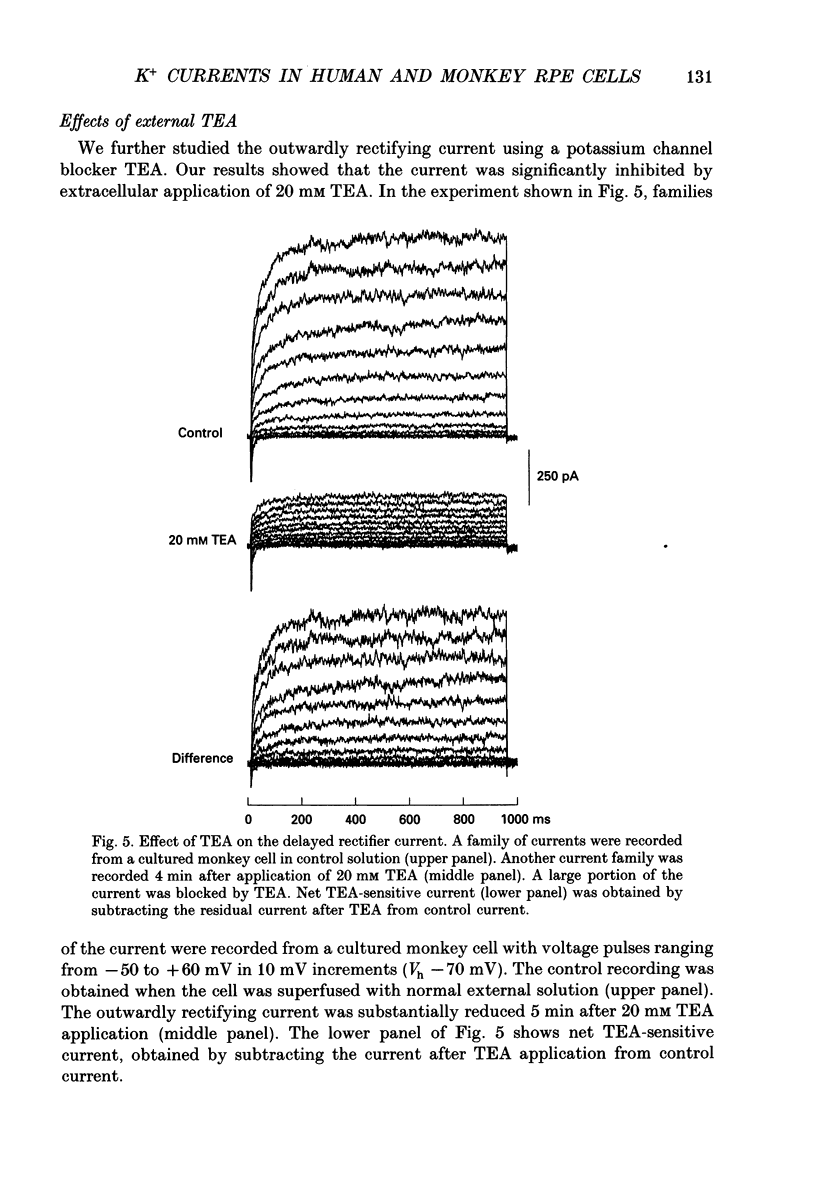
PDF







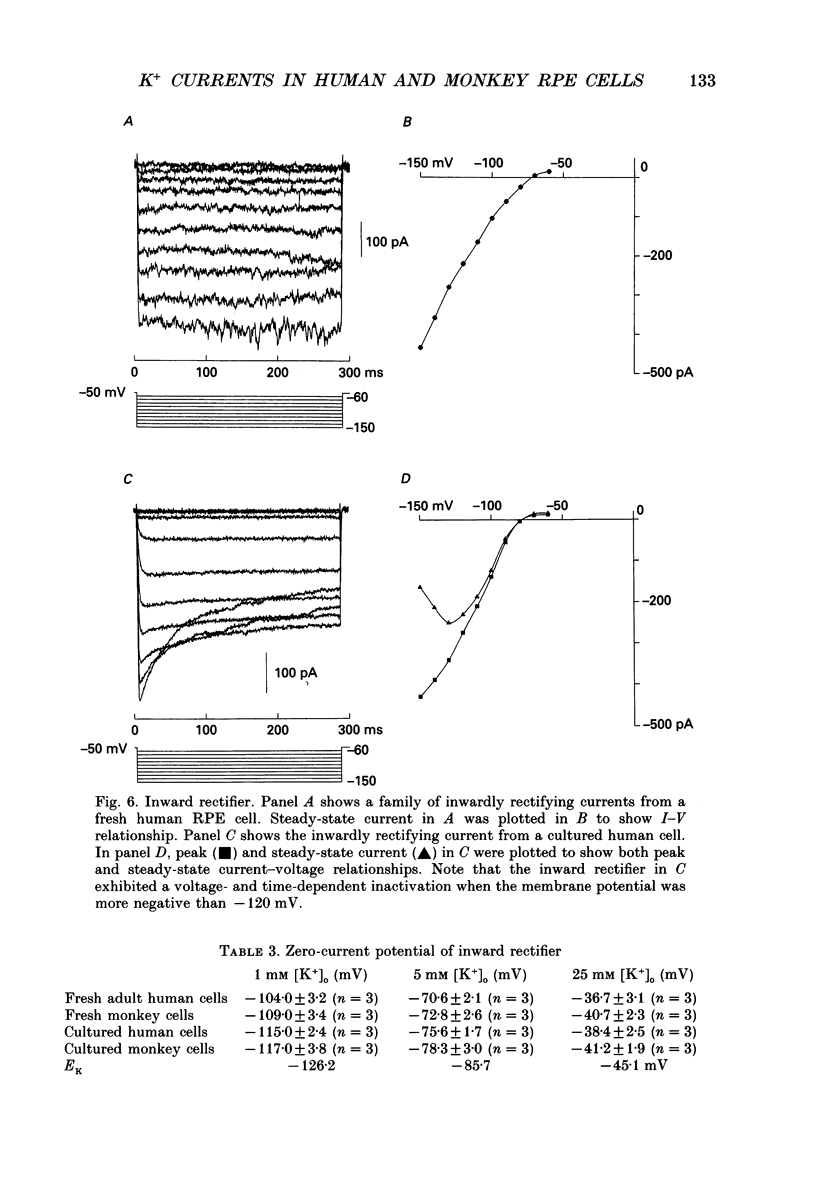
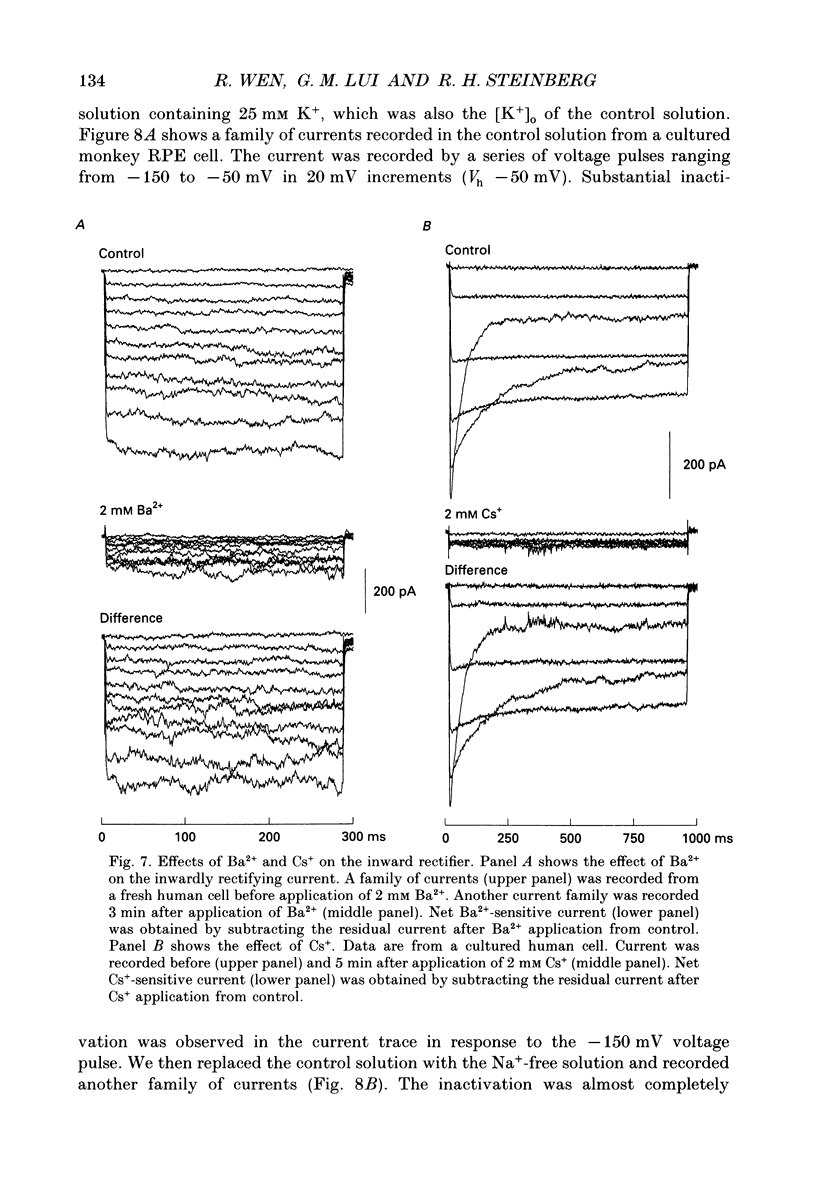
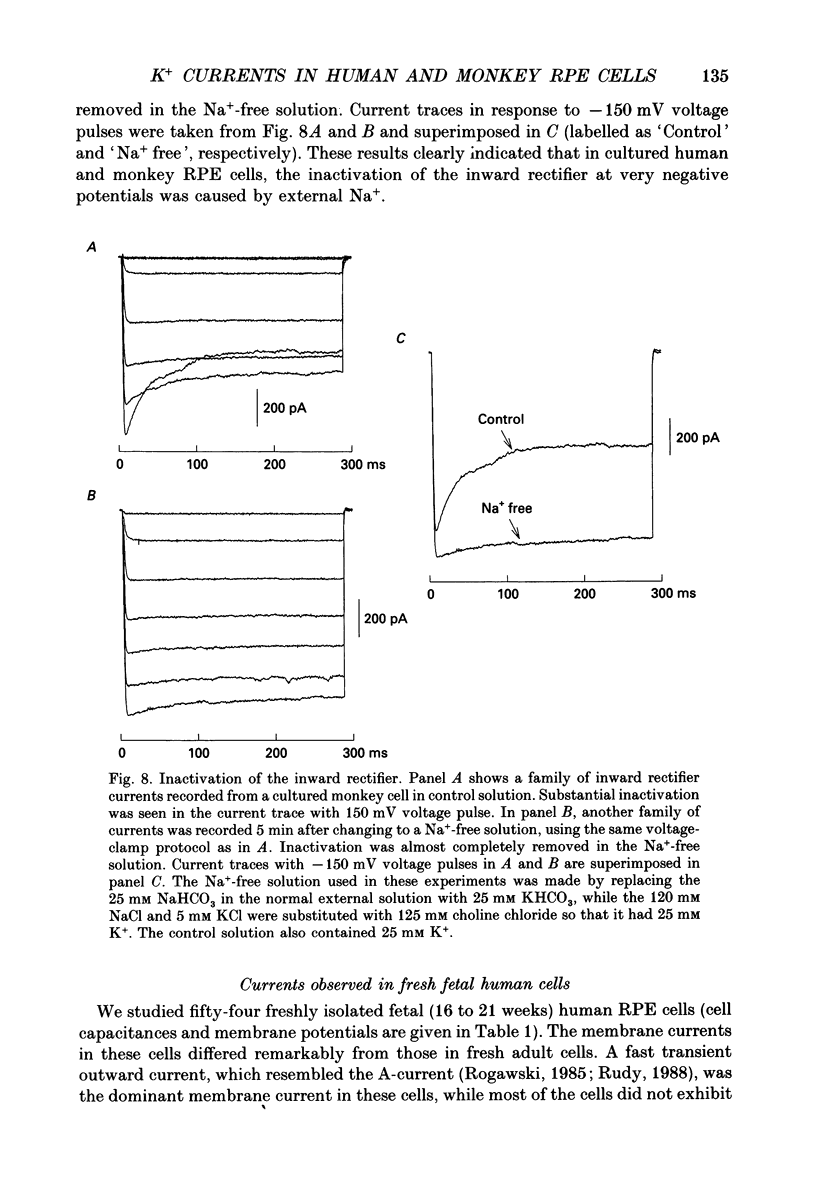







Images in this article
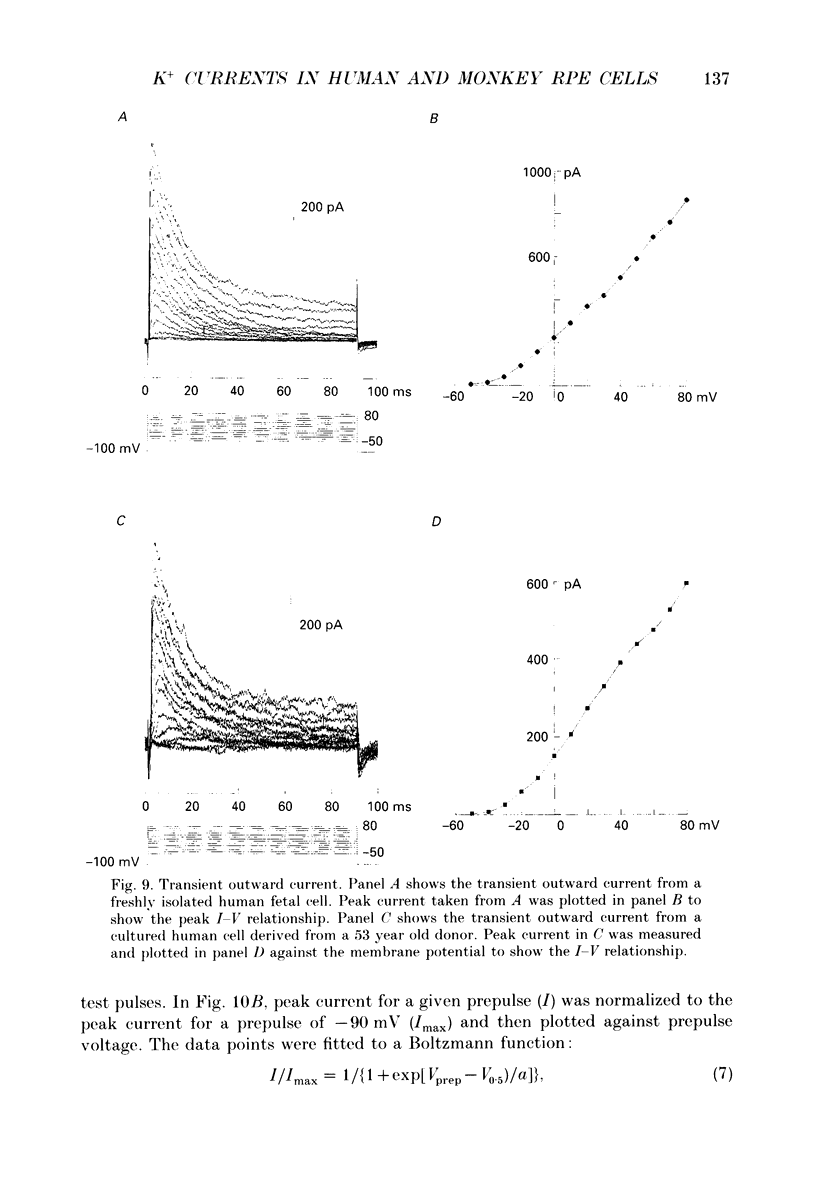
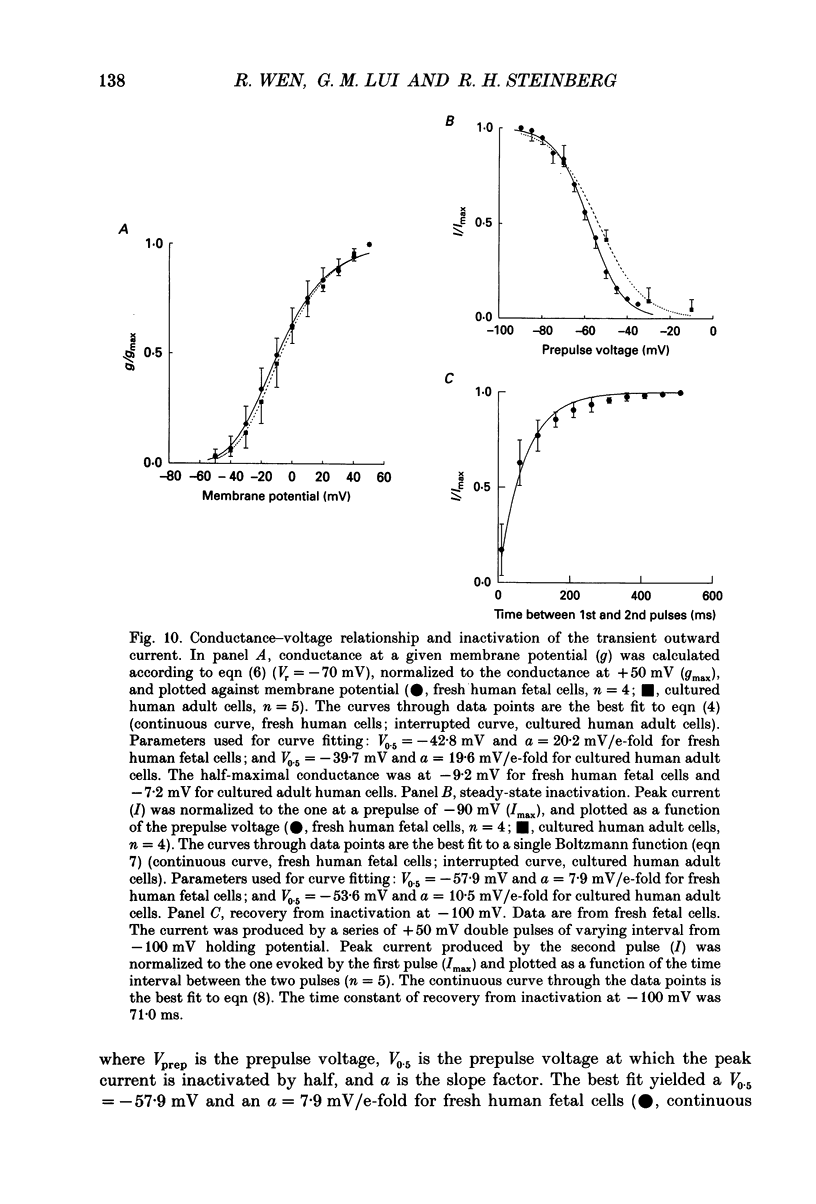
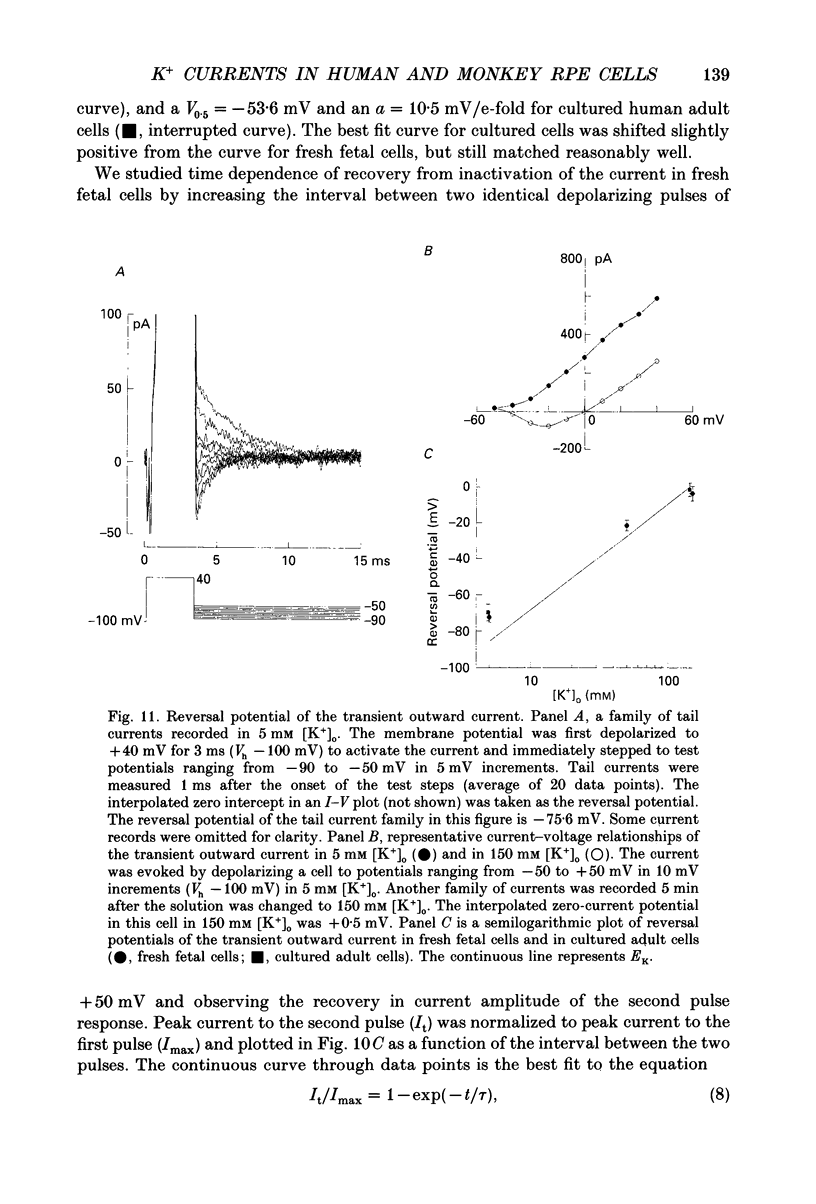
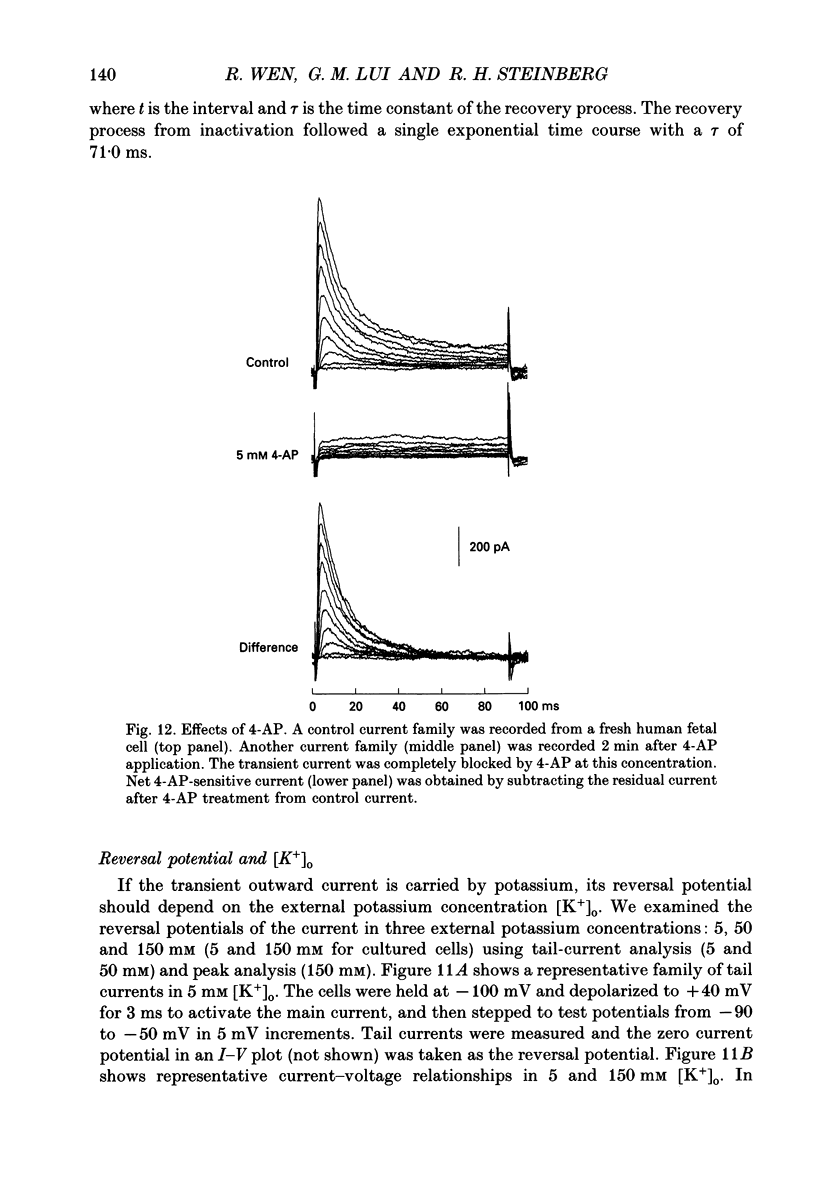
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barres B. A., Chun L. L., Corey D. P. Ion channels in vertebrate glia. Annu Rev Neurosci. 1990;13:441–474. doi: 10.1146/annurev.ne.13.030190.002301. [DOI] [PubMed] [Google Scholar]
- Barres B. A. New roles for glia. J Neurosci. 1991 Dec;11(12):3685–3694. doi: 10.1523/JNEUROSCI.11-12-03685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermans G., Vereecke J., Carmeliet E. The mechanism of the inactivation of the inward-rectifying K current during hyperpolarizing steps in guinea-pig ventricular myocytes. Pflugers Arch. 1987 Dec;410(6):604–613. doi: 10.1007/BF00581320. [DOI] [PubMed] [Google Scholar]
- Butler A., Wei A. G., Baker K., Salkoff L. A family of putative potassium channel genes in Drosophila. Science. 1989 Feb 17;243(4893):943–947. doi: 10.1126/science.2493160. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y. Functions and distribution of voltage-gated sodium and potassium channels in mammalian Schwann cells. Glia. 1991;4(6):541–558. doi: 10.1002/glia.440040602. [DOI] [PubMed] [Google Scholar]
- DeCoursey T. E., Chandy K. G., Gupta S., Cahalan M. D. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984 Feb 2;307(5950):465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- Fox J. A., Pfeffer B. A., Fain G. L. Single-channel recordings from cultured human retinal pigment epithelial cells. J Gen Physiol. 1988 Feb;91(2):193–222. doi: 10.1085/jgp.91.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hughes B. A., Steinberg R. H. Voltage-dependent currents in isolated cells of the frog retinal pigment epithelium. J Physiol. 1990 Sep;428:273–297. doi: 10.1113/jphysiol.1990.sp018212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. How might the diversity of potassium channels be generated? Trends Neurosci. 1990 Oct;13(10):415–419. doi: 10.1016/0166-2236(90)90123-r. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Voltage-sensitive ion channels. Cell. 1989 Jan 13;56(1):13–25. doi: 10.1016/0092-8674(89)90979-3. [DOI] [PubMed] [Google Scholar]
- Jauch P., Petersen O. H., Läuger P. Electrogenic properties of the sodium-alanine cotransporter in pancreatic acinar cells: I. Tight-seal whole-cell recordings. J Membr Biol. 1986;94(2):99–115. doi: 10.1007/BF01871191. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Cahalan M. D. The plasticity of ion channels: parallels between the nervous and immune systems. Trends Neurosci. 1988 May;11(5):214–218. doi: 10.1016/0166-2236(88)90129-4. [DOI] [PubMed] [Google Scholar]
- Lin H., Kenyon E., Miller S. S. Na-dependent pHi regulatory mechanisms in native human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1992 Dec;33(13):3528–3538. [PubMed] [Google Scholar]
- MacDermott A. B., Weight F. F. Action potential repolarization may involve a transient, Ca2+-sensitive outward current in a vertebrate neurone. Nature. 1982 Nov 11;300(5888):185–188. doi: 10.1038/300185a0. [DOI] [PubMed] [Google Scholar]
- Miller S. S., Steinberg R. H. Active transport of ions across frog retinal pigment epithelium. Exp Eye Res. 1977 Sep;25(3):235–248. doi: 10.1016/0014-4835(77)90090-2. [DOI] [PubMed] [Google Scholar]
- Miller S. S., Steinberg R. H. Passive ionic properties of frog retinal pigment epithelium. J Membr Biol. 1977 Sep 15;36(4):337–372. doi: 10.1007/BF01868158. [DOI] [PubMed] [Google Scholar]
- Neill J. M., Barnstable C. J. Expression of the cell surface antigens RET-PE2 and N-CAM by rat retinal pigment epithelial cells during development and in tissue culture. Exp Eye Res. 1990 Nov;51(5):573–583. doi: 10.1016/0014-4835(90)90088-c. [DOI] [PubMed] [Google Scholar]
- Nilius B., Wohlrab W. Potassium channels and regulation of proliferation of human melanoma cells. J Physiol. 1992 Jan;445:537–548. doi: 10.1113/jphysiol.1992.sp018938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B., 2nd, Green D. G. Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electroretinogram. J Neurophysiol. 1976 Sep;39(5):1117–1133. doi: 10.1152/jn.1976.39.5.1117. [DOI] [PubMed] [Google Scholar]
- Oakley B., 2nd, Miller S. S., Steinberg R. H. Effect of intracellular potassium upon the electrogenic pump of frog retinal pigment epithelium. J Membr Biol. 1978 Dec 29;44(3-4):281–307. doi: 10.1007/BF01944225. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn R. H., Miller S. S. Ion transport mechanisms in native human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1992 Dec;33(13):3513–3527. [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Salkoff L. B., Wyman R. J. Ion currents in Drosophila flight muscles. J Physiol. 1983 Apr;337:687–709. doi: 10.1113/jphysiol.1983.sp014649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L. Drosophila mutants reveal two components of fast outward current. Nature. 1983 Mar 17;302(5905):249–251. doi: 10.1038/302249a0. [DOI] [PubMed] [Google Scholar]
- Song M. K., Lui G. M. Propagation of fetal human RPE cells: preservation of original culture morphology after serial passage. J Cell Physiol. 1990 Apr;143(1):196–203. doi: 10.1002/jcp.1041430127. [DOI] [PubMed] [Google Scholar]
- Sugimoto T., Tanabe Y., Shigemoto R., Iwai M., Takumi T., Ohkubo H., Nakanishi S. Immunohistochemical study of a rat membrane protein which induces a selective potassium permeation: its localization in the apical membrane portion of epithelial cells. J Membr Biol. 1990 Jan;113(1):39–47. doi: 10.1007/BF01869604. [DOI] [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- la Cour M., Lund-Andersen H., Zeuthen T. Potassium transport of the frog retinal pigment epithelium: autoregulation of potassium activity in the subretinal space. J Physiol. 1986 Jun;375:461–479. doi: 10.1113/jphysiol.1986.sp016128. [DOI] [PMC free article] [PubMed] [Google Scholar]