Abstract
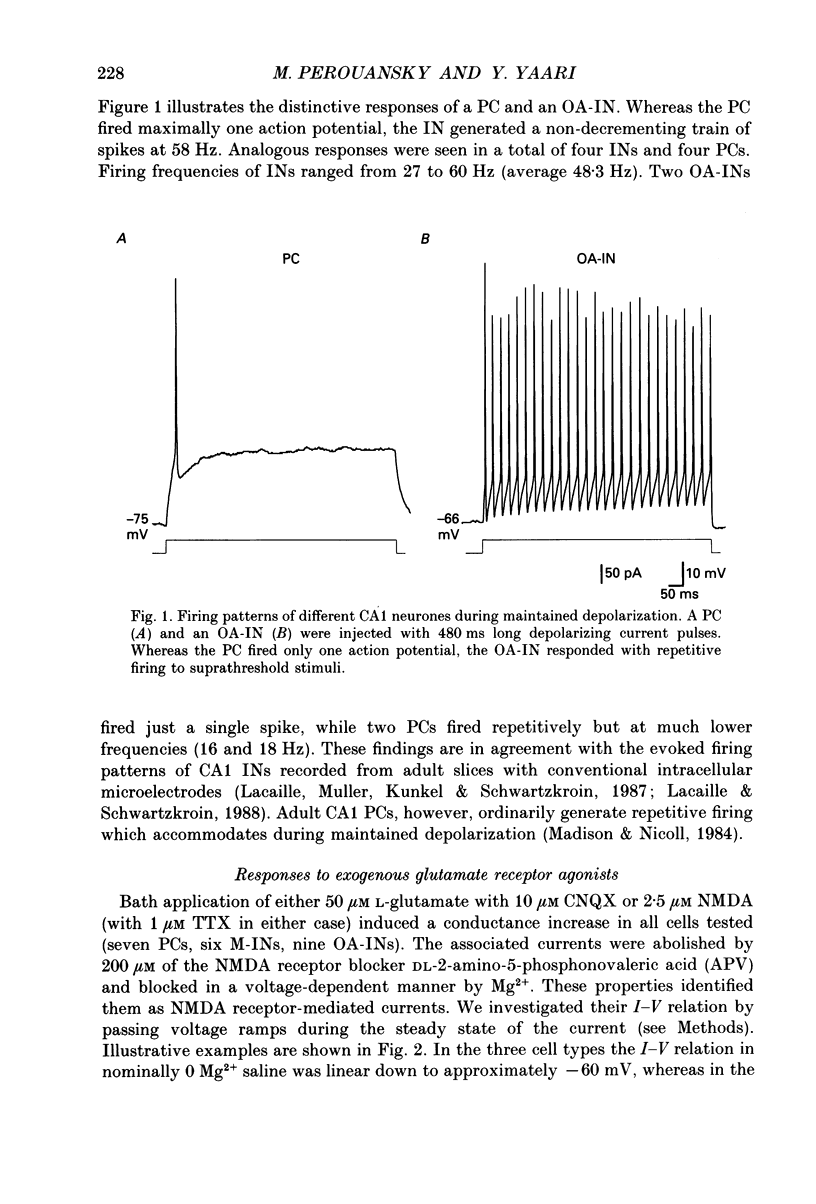
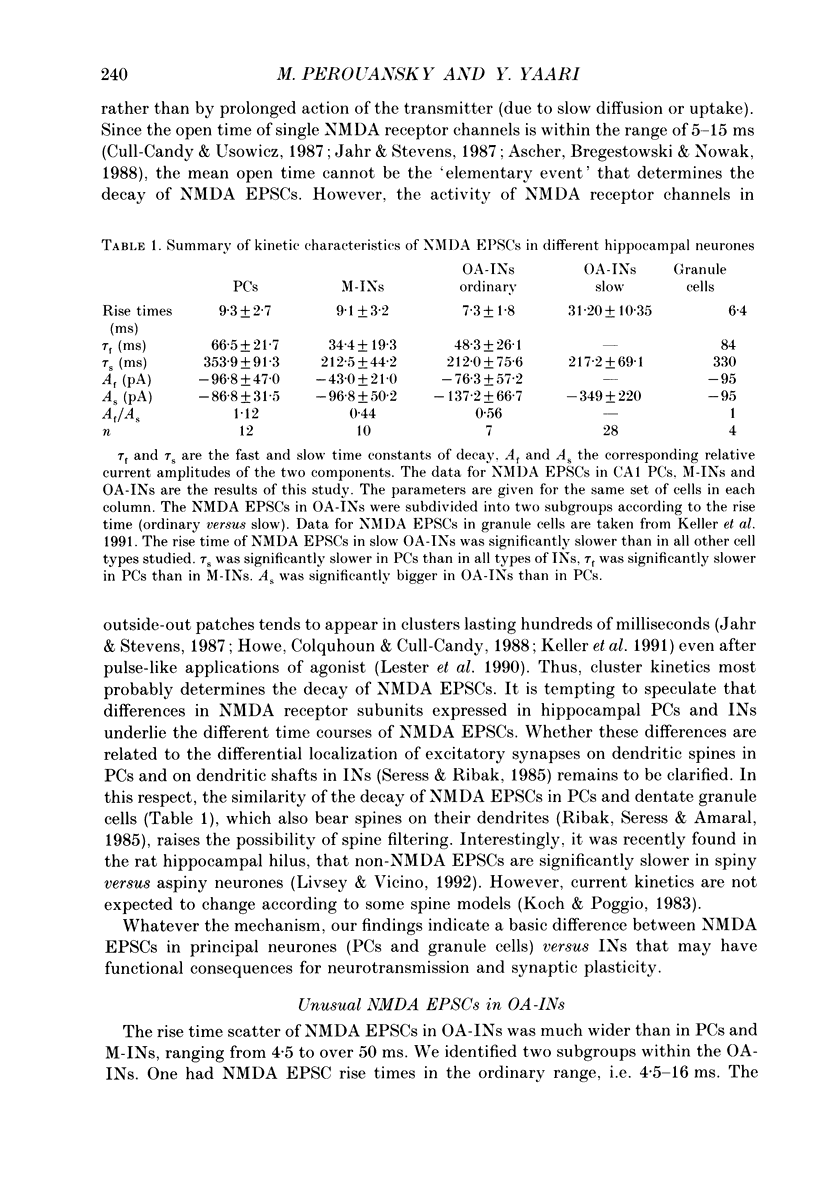
1. Whole-cell tight-seal recordings were obtained from visually identified pyramidal cells (PCs) and interneurones (INs) in the CA1 field of thin hippocampal slices from 13- to 23-day-old rats. The INs sampled were classified according to their location either in the molecular layer (M-INs) or in the oriens layer and alveus (OA-INs). PCs and INs differed in their mode of firing when depolarized by a prolonged current pulse. Whereas PCs fired a single action potential, most INs responded with non-accommodating high frequency spike firing. 2. In the presence of 1 microM tetrodotoxin (TTX), bath application of either 50 microM L-glutamate with 10 microM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) or 2.5 microM N-methyl-D-aspartate (NMDA), induced a similar conductance increase in PCs and INs that was completely blocked by 200 microM DL-2-amino-5-phosphonovaleric acid (APV). The NMDA receptor-mediated currents reversed around 4 mV and exhibited an area of negative slope conductance at potentials more negative than -20 to -30 mV in the presence of 1-2 mM Mg2+. 3. Dual-component excitatory postsynaptic currents (EPSCs) were evoked in PCs and INs by stimulating afferent fibres close to the neurone. The NMDA receptor-mediated component of the EPSCs (NMDA EPSC) was isolated by adding 10 microM CNQX to block non-NMDA receptors. The NMDA EPSCs in all cell types reversed around 1.5 mV and were abolished by 50 microM APV. 4. In saline containing 1 mM Mg2+, the peak current-voltage (I-V) relationship of NMDA EPSCs in PCs and INs showed an area of negative slope conductance at voltages more negative than -20 to -30 mV. In nominally Mg(2+)-free saline, the peak I-V relation was linear over a much wider voltage range in both cell types. 5. The 10-90% rise times of NMDA EPSCs at -60 mV ranged from 4.5 to 16 ms in PCs (mean 8.7 ms; n = 25) and in M-INs (mean 9.1 ms; n = 10). Their decay could be best fitted with the sum of two exponentials. The decay of NMDA EPSCs in PCs was significantly slower than that recorded in INs. The average fast (tau f) and slow (tau s) time constants of decay were, respectively, 66.5 and 353.9 ms in PCs, and 34.4 and 212.5 ms in M-INs.(ABSTRACT TRUNCATED AT 400 WORDS)
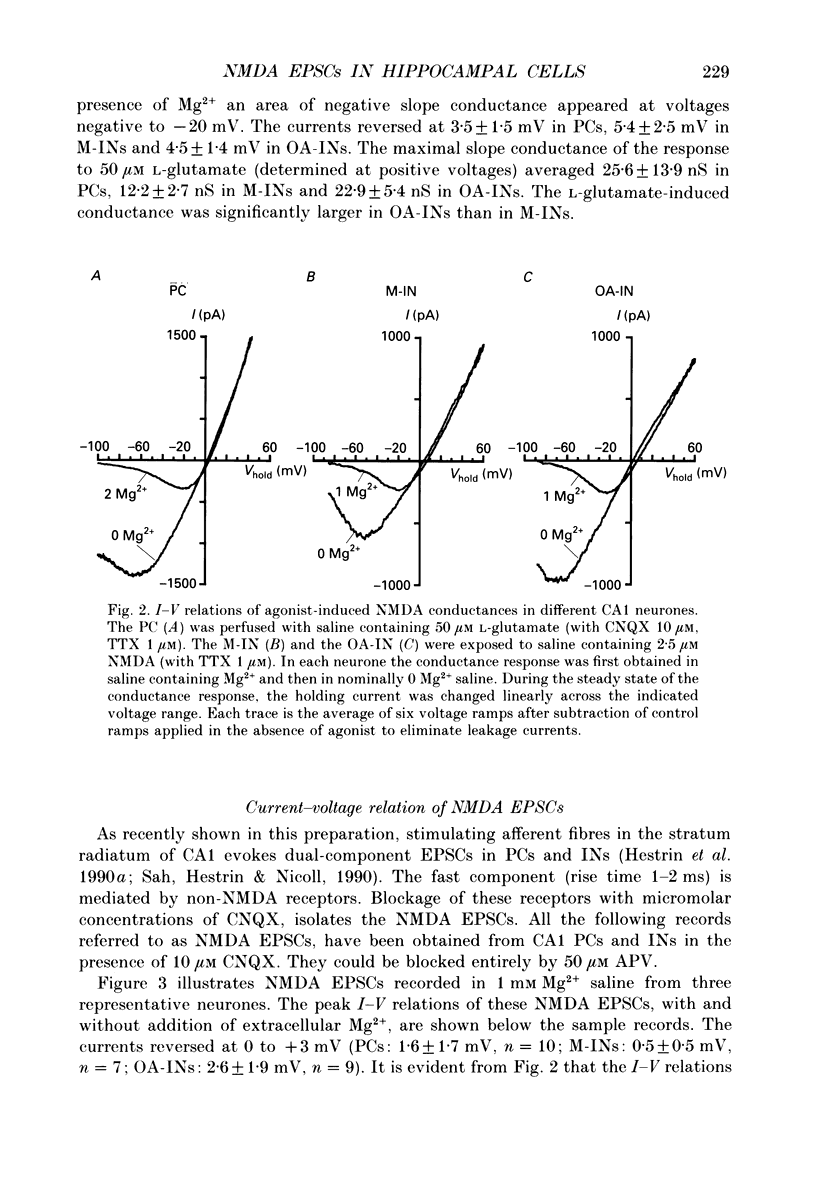
Full text
PDF



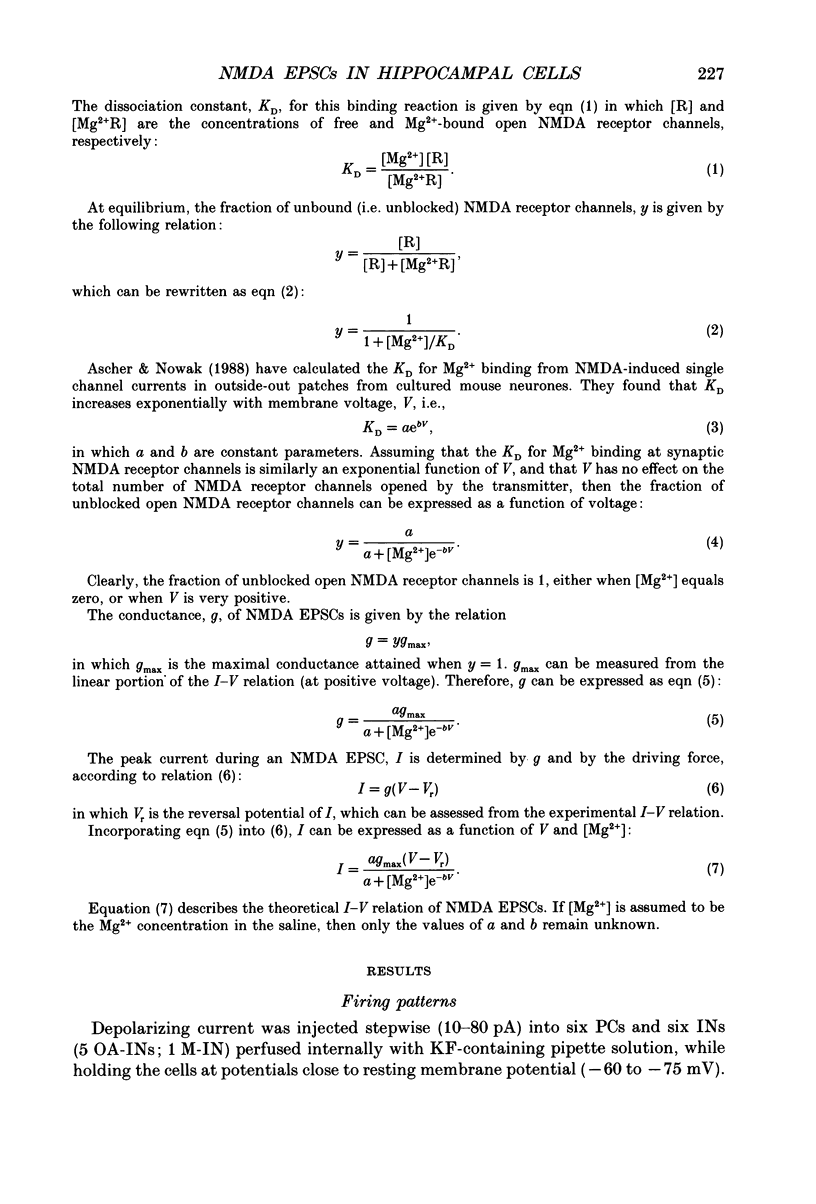
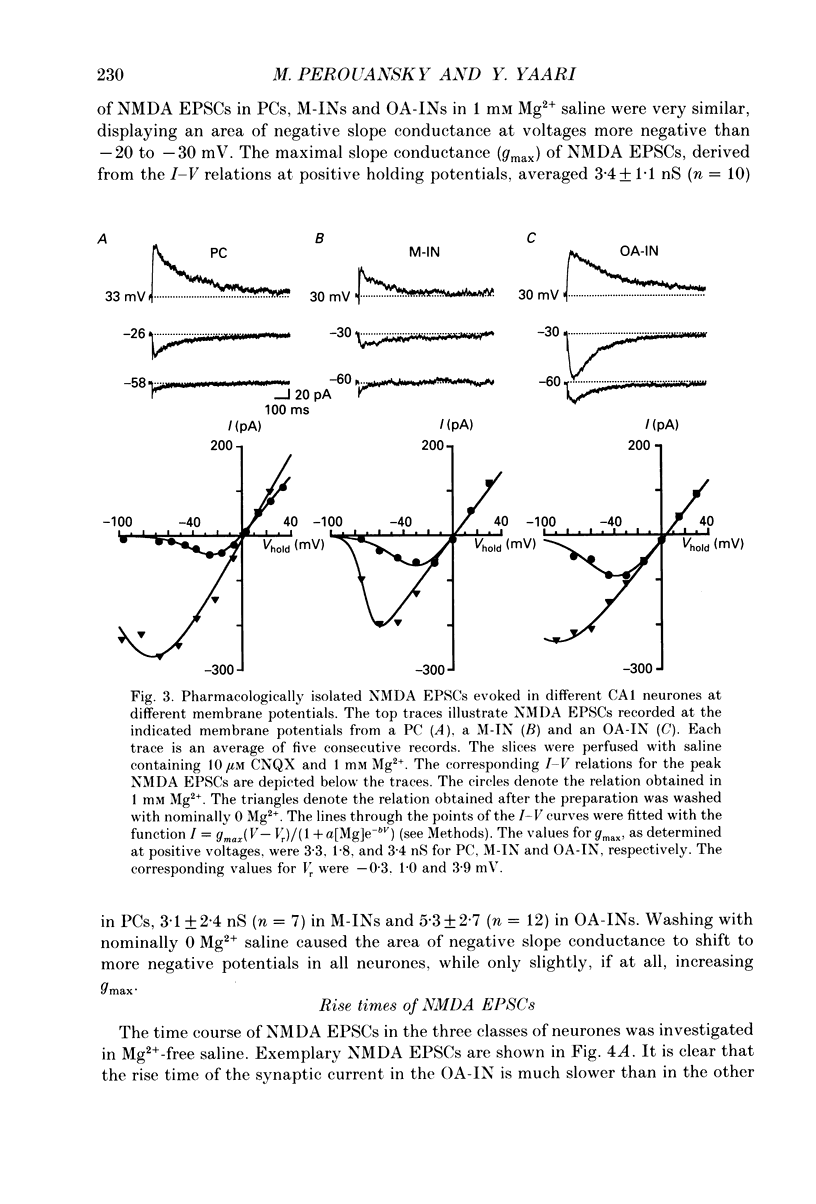
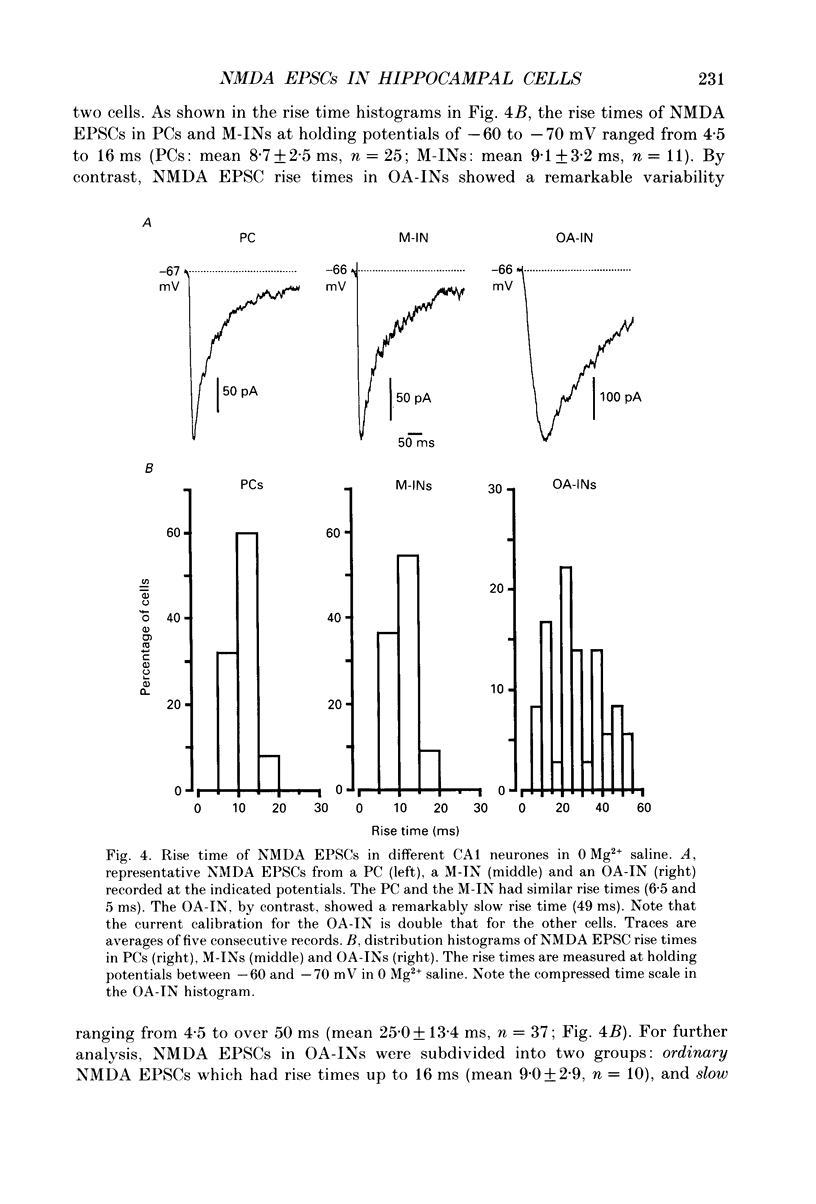
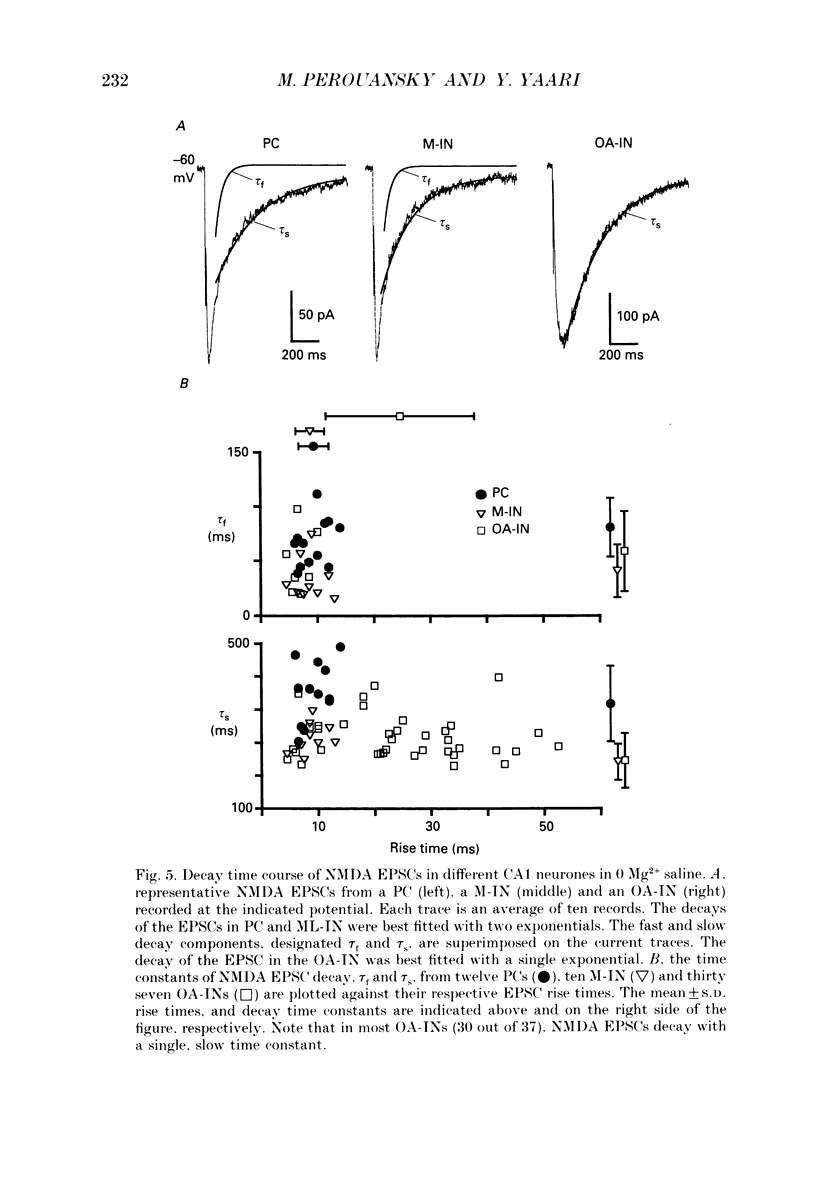

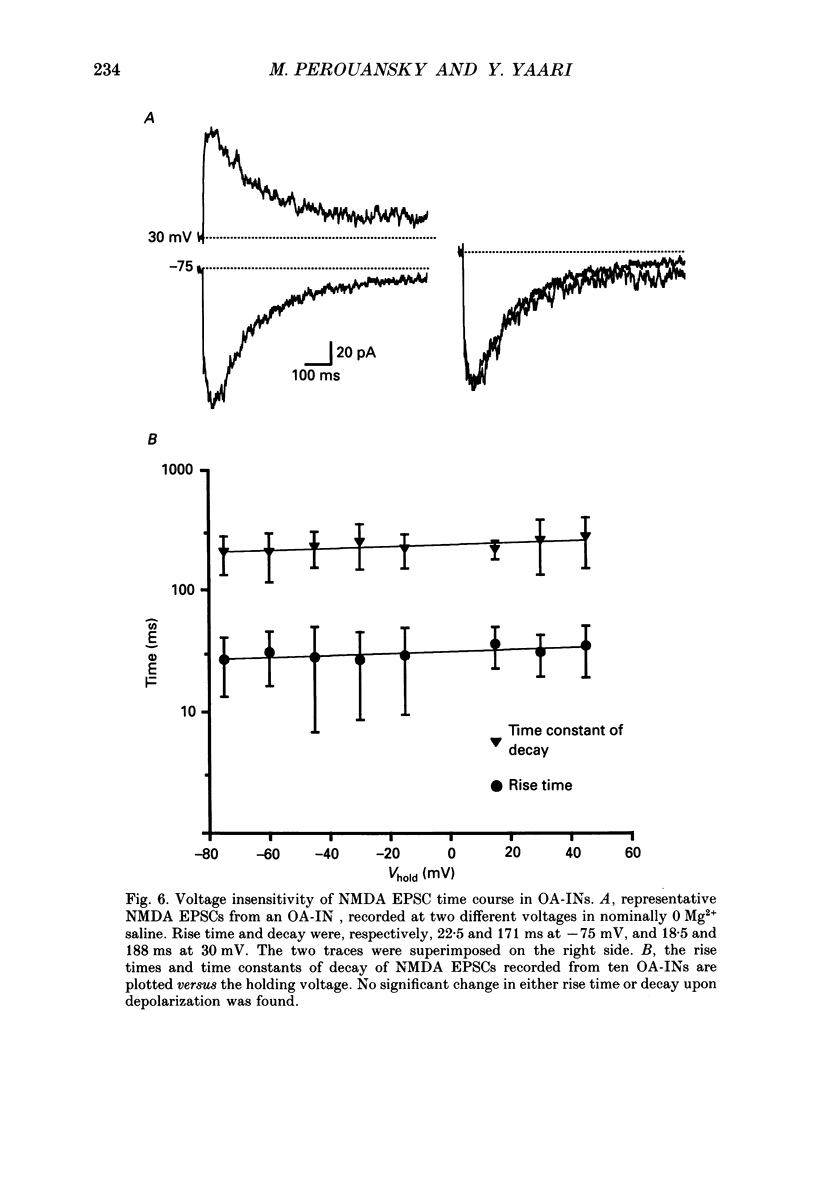
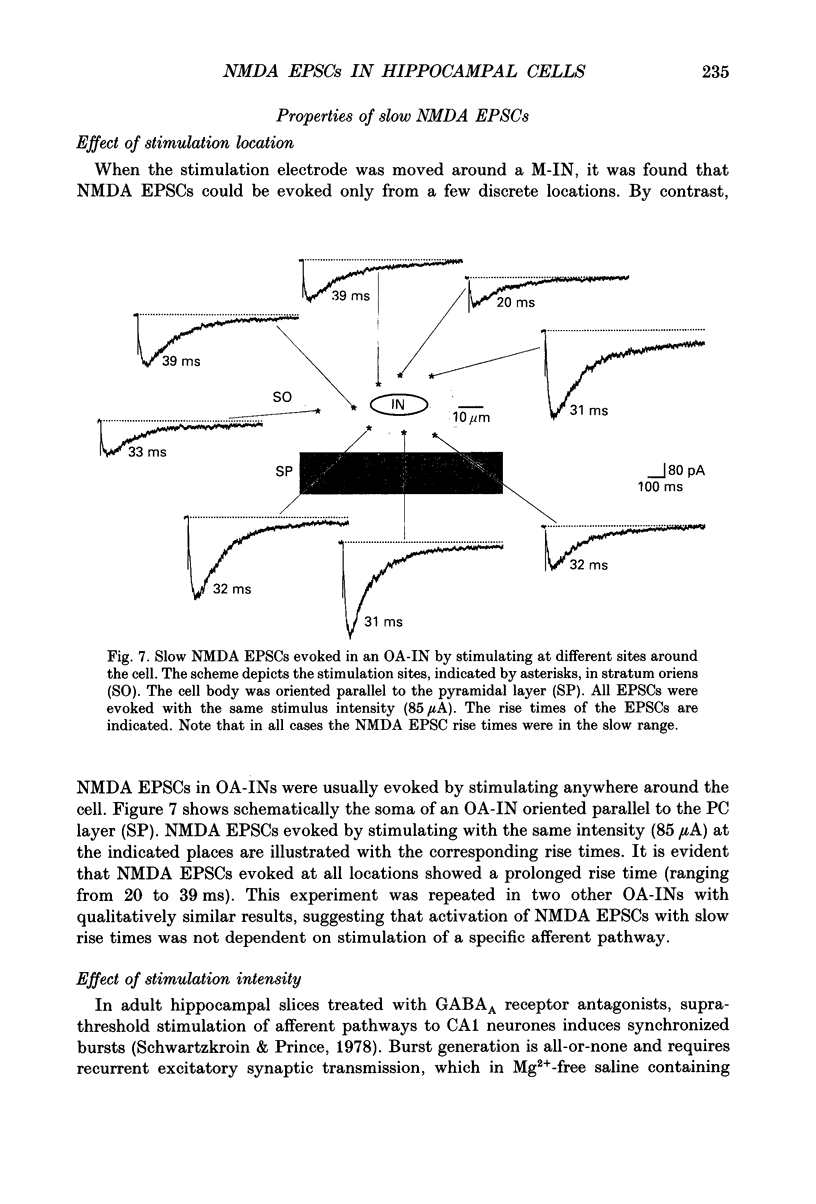
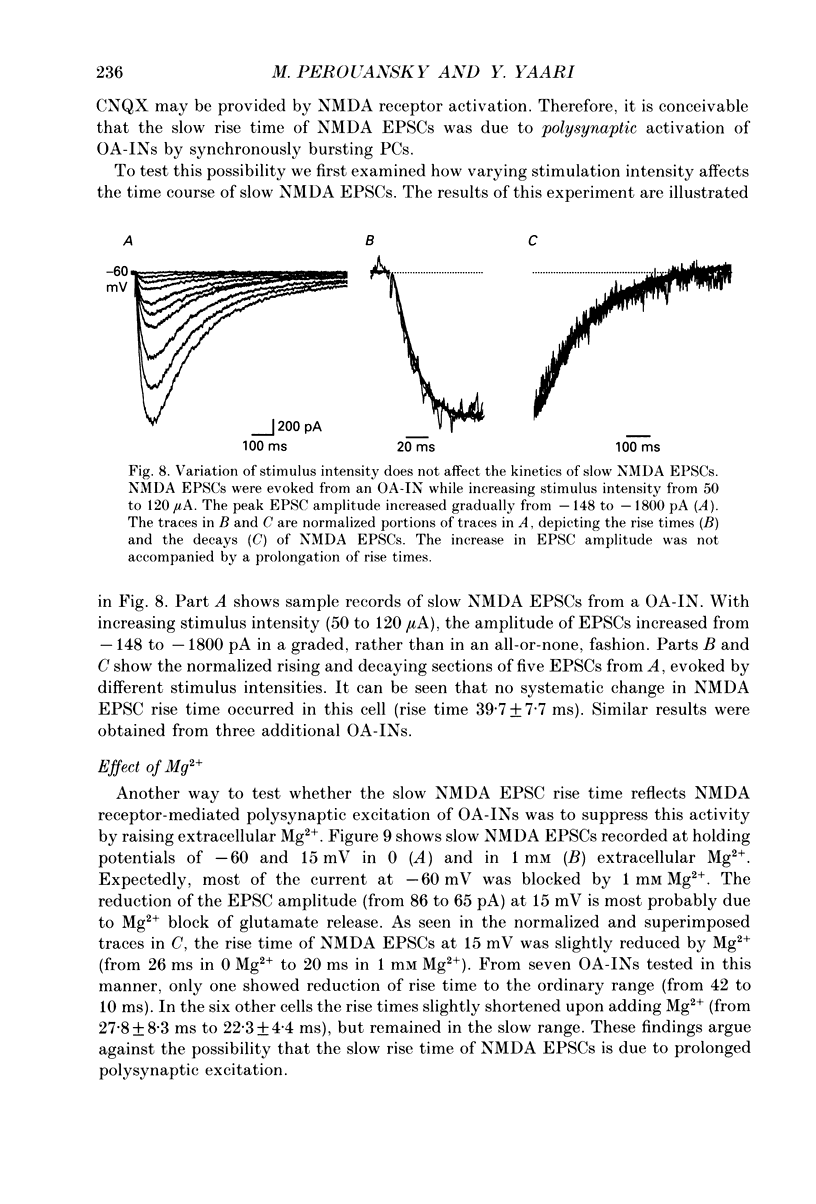
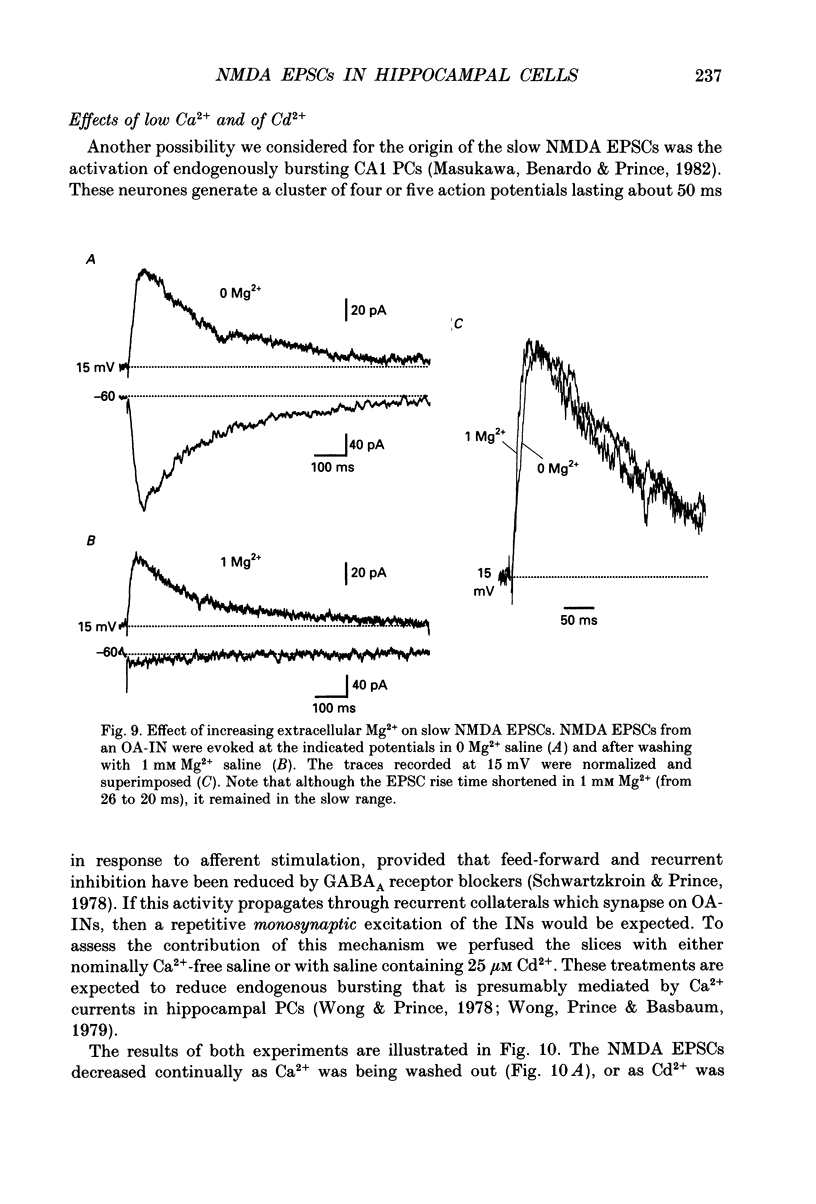
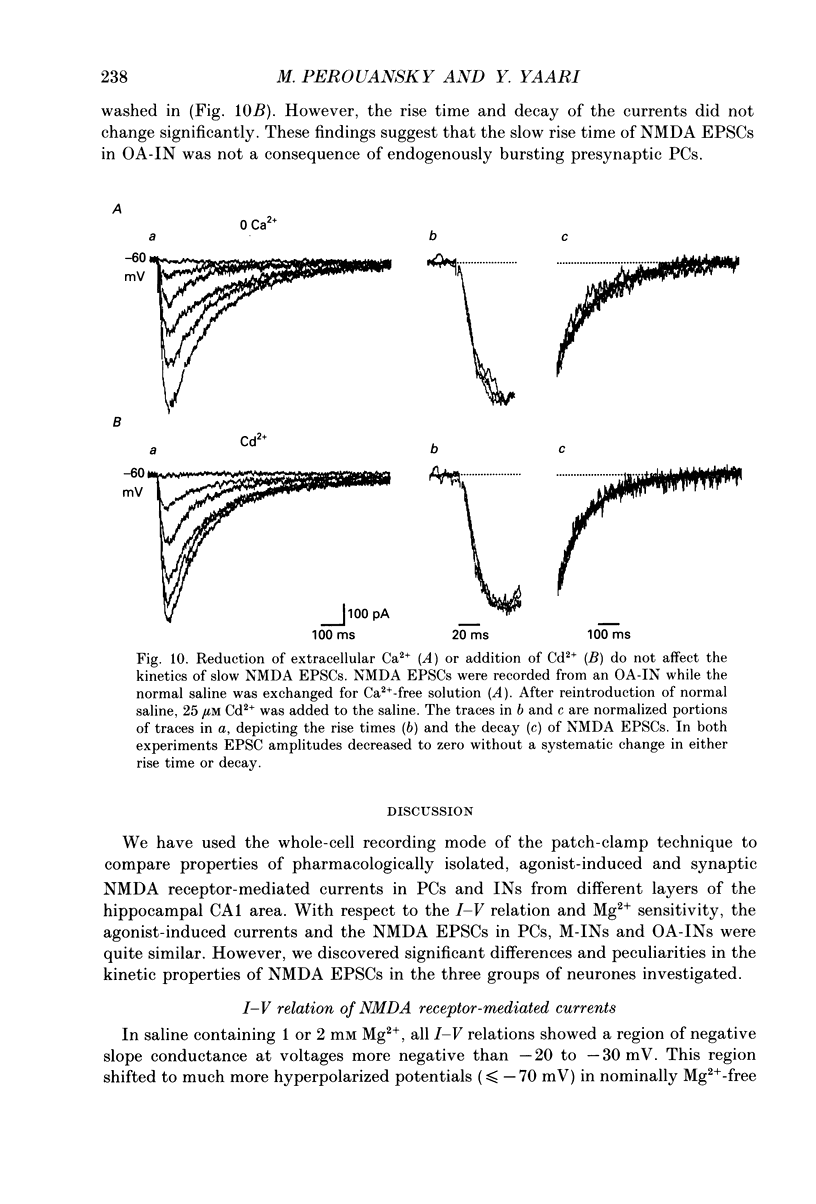





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Leefmans F. J., Gamiño S. M., Giraldez F., González-Serratos H. Intracellular free magnesium in frog skeletal muscle fibres measured with ion-selective micro-electrodes. J Physiol. 1986 Sep;378:461–483. doi: 10.1113/jphysiol.1986.sp016230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen M., Lambert J. D., Jensen M. S. Effects of new non-N-methyl-D-aspartate antagonists on synaptic transmission in the in vitro rat hippocampus. J Physiol. 1989 Jul;414:317–336. doi: 10.1113/jphysiol.1989.sp017690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Bregestovski P., Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988 May;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch P. J., Grossman C. J., Hayes A. G. 6,7-Dinitro-quinoxaline-2,3-dion and 6-nitro,7-cyano-quinoxaline-2,3-dion antagonise responses to NMDA in the rat spinal cord via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988 Oct 26;156(1):177–180. doi: 10.1016/0014-2999(88)90163-x. [DOI] [PubMed] [Google Scholar]
- Blake J. F., Brown M. W., Collingridge G. L. CNQX blocks acidic amino acid induced depolarizations and synaptic components mediated by non-NMDA receptors in rat hippocampal slices. Neurosci Lett. 1988 Jun 29;89(2):182–186. doi: 10.1016/0304-3940(88)90378-3. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Prince D. A. Effects of local anesthetic QX-314 on the membrane properties of hippocampal pyramidal neurons. J Pharmacol Exp Ther. 1982 Mar;220(3):476–481. [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Frazier D. T., Narahashi T., Yamada M. The site of action and active form of local anesthetics. II. Experiments with quaternary compounds. J Pharmacol Exp Ther. 1970 Jan;171(1):45–51. [PubMed] [Google Scholar]
- Hablitz J. J. Picrotoxin-induced epileptiform activity in hippocampus: role of endogenous versus synaptic factors. J Neurophysiol. 1984 May;51(5):1011–1027. doi: 10.1152/jn.1984.51.5.1011. [DOI] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Nicoll R. A., Perkel D. J., Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990 Mar;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S., Sah P., Nicoll R. A. Mechanisms generating the time course of dual component excitatory synaptic currents recorded in hippocampal slices. Neuron. 1990 Sep;5(3):247–253. doi: 10.1016/0896-6273(90)90162-9. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Colquhoun D., Cull-Candy S. G. On the kinetics of large-conductance glutamate-receptor ion channels in rat cerebellar granule neurons. Proc R Soc Lond B Biol Sci. 1988 May 23;233(1273):407–422. doi: 10.1098/rspb.1988.0030. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Baughman R. W. Both NMDA and non-NMDA subtypes of glutamate receptors are concentrated at synapses on cerebral cortical neurons in culture. Neuron. 1991 Oct;7(4):593–603. doi: 10.1016/0896-6273(91)90372-7. [DOI] [PubMed] [Google Scholar]
- Kay A. R., Miles R., Wong R. K. Intracellular fluoride alters the kinetic properties of calcium currents facilitating the investigation of synaptic events in hippocampal neurons. J Neurosci. 1986 Oct;6(10):2915–2920. doi: 10.1523/JNEUROSCI.06-10-02915.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller B. U., Konnerth A., Yaari Y. Patch clamp analysis of excitatory synaptic currents in granule cells of rat hippocampus. J Physiol. 1991 Apr;435:275–293. doi: 10.1113/jphysiol.1991.sp018510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C., Poggio T. A theoretical analysis of electrical properties of spines. Proc R Soc Lond B Biol Sci. 1983 Jul 22;218(1213):455–477. doi: 10.1098/rspb.1983.0051. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Keller B. U., Ballanyi K., Yaari Y. Voltage sensitivity of NMDA-receptor mediated postsynaptic currents. Exp Brain Res. 1990;81(1):209–212. doi: 10.1007/BF00230117. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Effect of internal fluoride and phosphate on membrane currents during intracellular dialysis of nerve cells. Nature. 1975 Oct 23;257(5528):691–693. doi: 10.1038/257691a0. [DOI] [PubMed] [Google Scholar]
- Lacaille J. C., Mueller A. L., Kunkel D. D., Schwartzkroin P. A. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987 Jul;7(7):1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille J. C., Schwartzkroin P. A. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. I. Intracellular response characteristics, synaptic responses, and morphology. J Neurosci. 1988 Apr;8(4):1400–1410. doi: 10.1523/JNEUROSCI.08-04-01400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R. A., Clements J. D., Westbrook G. L., Jahr C. E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990 Aug 9;346(6284):565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Livsey C. T., Vicini S. Slower spontaneous excitatory postsynaptic currents in spiny versus aspiny hilar neurons. Neuron. 1992 Apr;8(4):745–755. doi: 10.1016/0896-6273(92)90095-u. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984 Sep;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa L. M., Benardo L. S., Prince D. A. Variations in electrophysiological properties of hippocampal neurons in different subfields. Brain Res. 1982 Jun 24;242(2):341–344. doi: 10.1016/0006-8993(82)90320-1. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McNamara J. O., Russell R. D., Rigsbee L., Bonhaus D. W. Anticonvulsant and antiepileptogenic actions of MK-801 in the kindling and electroshock models. Neuropharmacology. 1988 Jun;27(6):563–568. doi: 10.1016/0028-3908(88)90176-1. [DOI] [PubMed] [Google Scholar]
- Mody I., Stanton P. K., Heinemann U. Activation of N-methyl-D-aspartate receptors parallels changes in cellular and synaptic properties of dentate gyrus granule cells after kindling. J Neurophysiol. 1988 Mar;59(3):1033–1054. doi: 10.1152/jn.1988.59.3.1033. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ribak C. E., Seress L., Amaral D. G. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985 Oct;14(5):835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- Sah P., Hestrin S., Nicoll R. A. Properties of excitatory postsynaptic currents recorded in vitro from rat hippocampal interneurones. J Physiol. 1990 Nov;430:605–616. doi: 10.1113/jphysiol.1990.sp018310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Prince D. A. Cellular and field potential properties of epileptogenic hippocampal slices. Brain Res. 1978 May 19;147(1):117–130. doi: 10.1016/0006-8993(78)90776-x. [DOI] [PubMed] [Google Scholar]
- Seress L., Ribak C. E. A combined Golgi-electron microscopic study of non-pyramidal neurons in the CA 1 area of the hippocampus. J Neurocytol. 1985 Oct;14(5):717–730. doi: 10.1007/BF01170824. [DOI] [PubMed] [Google Scholar]
- Smart T. G. Excitatory amino acids: the involvement of second messengers in the signal transduction process. Cell Mol Neurobiol. 1989 Jun;9(2):193–206. doi: 10.1007/BF00713028. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., Walker V. E., Flynn D. M. Glycine enhances NMDA-receptor mediated synaptic potentials in neocortical slices. Nature. 1989 Mar 30;338(6214):422–424. doi: 10.1038/338422a0. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A., Basbaum A. I. Intradendritic recordings from hippocampal neurons. Proc Natl Acad Sci U S A. 1979 Feb;76(2):986–990. doi: 10.1073/pnas.76.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A. Participation of calcium spikes during intrinsic burst firing in hippocampal neurons. Brain Res. 1978 Dec 29;159(2):385–390. doi: 10.1016/0006-8993(78)90544-9. [DOI] [PubMed] [Google Scholar]