Abstract
Background
Guidelines specify steroids as therapy for acute exacerbation of chronic obstructive pulmonary disease (AECOPD). However, the duration of survival benefit associated with steroids and the optimal dosage of nebulized budesonide (NB) during hospitalization remain unclear.
Methods
We conducted a retrospective study of hospitalized AECOPD patients. The primary endpoint was all-cause mortality after discharge. Cox regression analysis was used to determine the impact of steroid therapy on survival.
Results
Wilcoxon analysis showed the positive impact of systemic corticosteroids (SCs) therapy on survival during the early stage of follow-up (P = 0.038). NB therapy was associated with a significantly reduced risk of death within six months after discharge (adjusted Hazard ratio (HR), 0.36; 95% confidence interval (CI) 0.15–0.88). Subgroup analysis suggested that fewer than two AEs in the previous year (adjusted HR 0.05; 95% CI 0.01–0.38), age > = 65 years (adjusted HR 0.31; 95% CI 0.11–0.90), body mass index (BMI) < 25 kg/m2 (adjusted HR 0.33; 95% CI 0.12–0.92), and smoking index > 40 packets/year (adjusted HR 0.17; 95% CI 0.04–0.79) were involved in this association. Finally, treatment with a total dose of NB < = 60 mg during hospitalization reduced six-month mortality compared to treatment without steroids (adjusted HR 0.39; 95% CI 0.17–0.92), but not the total dose of NB > 60 mg.
Conclusions
NB therapy for hospitalized AECOPD patients significantly reduced six-month mortality. Subgroup analysis showed that certain populations benefited more from NB therapy, and < = 60 mg NB might be suitable treatment for hospitalized AECOPD patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00408-024-00784-1.
Keywords: COPD, Systemic corticosteroids, Nebulized budesonide, Exacerbation, Mortality
Introduction
Chronic obstructive pulmonary disease (COPD) is a main cause of death worldwide and the third leading cause of death in China, resulting in a heavy economic and social burden [1, 2]. Acute exacerbation (AE) is a common and substantial adverse event during COPD, characterized by increased dyspnea and/or cough and sputum, and may be accompanied by tachypnea [3], which can lead to a significant increase in the frequency of subsequent and mortality [4].
The GOLD guidelines advocate corticosteroids as a highly efficient treatment for exacerbated COPD [3]. Many studies have shown that the use of systemic corticosteroids (SCs) reduced the rate of treatment failure and improved pulmonary function and oxygenation in AECOPD [5, 6]. Meanwhile, the morbidity of osteoporosis, hyperglycemia, pneumonia, and sepsis caused by glucocorticoids increased [7]. Given the adverse effects of glucocorticoids, nebulized budesonide (NB) is widely used in clinic practice to minimize glucocorticoid exposure. Multiple studies have given positive answers of whether NB is comparable to SCs [8–11], in terms of improving pulmonary function and blood gas, reducing the length of hospitalization, and in-hospital mortality. Therefore, NB may be a suitable alternative treatment for AE in certain patients. Compared to conventional doses (4 mg/day), high-dose (8 mg/day) NB was more effective in improving pulmonary function and symptoms in the early treatment of AECOPD [12]. However, there is currently no evidence to support the establishment of an optimal dose and duration of NB.
SCs, NB, and their combination for the treatment of AECOPD have been studied previously [13, 14]. However, most discussed outcomes or endpoints were limited to hospitalization. There is also a lack of evidence on whether glucocorticoid treatment during hospitalization can bring long-term benefits over six months or even more than six months after discharge [15]. We conducted this observational study to evaluate the long-term effects of glucocorticoids, and the optimal dose and duration of NB.
Methods
Study Design and Patients
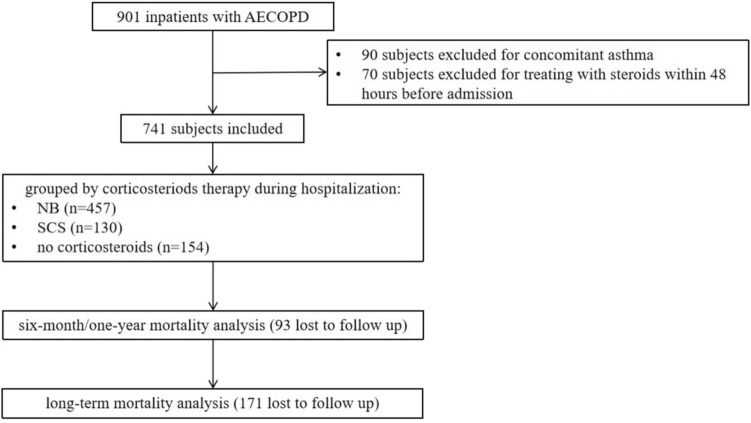
This study retrospectively examined a cohort of eligible hospitalized patients with AECOPD from September 2016 to July 2020 in the Department of Pulmonary and Critical Care Medicine of the Second Xiangya Hospital of Central South University. The Ethics Committee of the Second Xiangya Hospital of the Central South University (No. ChiCTR-POC-17010431) approved the research and the research was performed in accordance with Declaration of Helsinki. Written informed consent was obtained from all the participants. The inclusion criteria were as follows: (1) AECOPD diagnosed according to the 2016 GOLD guidelines [16] and (2) signed informed consent. Patients who had asthma, was treated with steroids within 48 h before admission, died during hospitalization, or refused to participate in the interviews were excluded from the study (Fig. 1). According to steroid treatment during hospitalization, the SCS group included patients treated with oral or intravenous steroids (alone or combined with NB). The NB group included patients treated with nebulized budesonide. The no-corticosteroid group included patients treated without steroids.
Fig. 1.
The flow diagram of eligible inpatients with AECOPD. AECOPD: acute exacerbation of chronic obstructive pulmonary disease; NB: nebulized budesonide; SCS: systemic corticosteroid
Data Collection
The demographic and clinical data of the patients including sex, age, body mass index (BMI), smoking status, comorbidities, routine blood examination, blood gas analysis, spirometry, and six-minute walking distance (6 MWD) were collected,. Dyspnea and respiratory symptoms were evaluated using the modified Medical Research Council (mMRC) Dyspnea Scale and the COPD Assessment Test (CAT). The dosage and duration of corticosteroid regimens were recorded according to the patient’s medical instructions and medical records.
Follow-up
All patients were followed-up via telephone after discharge. The first-year follow-up information included the frequency of exacerbation and readmission due to AECOPD in the previous year, time of the first AE after discharge, and survival status. Subsequent annual follow-up inquired about survival constraints. We recorded their survival time until death or until December 2022 for survival analysis.
Outcomes
The primary endpoints were the one-year and long-term all-cause mortality after discharge. Secondary end points were the improvement during hospitalization and the condition of re-exacerbation during follow-up. Improvement during hospitalization was assessed by comparison of the same variable at admission and at discharge. Changes of symptom assessments and laboratory investigations were analyzed to illustrate the differences in recovery among the three group. The condition of re-exacerbation during one year of follow-up was recorded, including the occurrence of acute exacerbation, the degree and frequency.
Statistical Analysis
SPSS 26.0 (IBM, New York, USA) was used for all statistical analyses. Continuous variables are presented as mean and SD and were analyzed using the t-test. Nonparametric data are presented as median and interquartile range (IQR) and were analyzed using the Mann–Whitney test. For categorical variables, numbers and percentages were used, and the chi-square test was used for univariate analysis. Survival analysis was conducted using Kaplan–Meier survival curves and Cox proportional hazards regression modeling. The confounding variables we controlled in Cox regression analysis included indicators that have been reported to have a significant impact on mortality (age, baseline postbronchodilator FEV1/FVC, and the frequency of AE in the previous year) [17] as well as the characteristics of inter-group differences in the baseline table. Hazard ratios (HRs) and nominal 95% confidence intervals (CIs) were presented. Statistical significance was set at P < 0.05. Graphs were jointly completed using SPSS 26.0 and GraphPad Prism 9.
Results
There were 741 patients who met the inclusion criteria finally enrolled the study. The median age was 69 years, and 683 patients were male (92.17%). The median follow-up time in our study was 41 months and the maximum follow-up time was 74 months. A total of 216 patients (37.89%) died during the follow-up period. Among them, 87 (11.74%) died within one year, and nearly half of them (49) died within six months after discharge. There were 457 patients in the NB group, 130 in the SCS group, and 154 in the non-corticosteroid group.
The SCS-Treated Patients had the Worst Baseline Condition at the Beginning of the AE Course
The baseline characteristics of the participants were showed in Table 1. The non-corticosteroid group showed the best overall condition compared with the other two groups. The proportion of patients with inhaler therapy during stable stage was also the lowest in the non-corticosteroid group compared to the NB group and the SCS group (50.6% vs. 60.4% vs. 72.3%, P = 0.014). Most patients were administered NB (61.7%) as steroid therapy. Physicians tended to use SCs to treat patients with the worst lung function, shortest 6 MWD, most severe dyspnea with the lowest PaO2, lowest SaO2, highest PaCO2 and highest frequency of AEs in the previous year. In terms of comorbidities and complications, patients (52.3%) complicated with respiratory failure were more likely to be treated with SCs and those (26.0%) complicated with cor pulmonale were more likely to be treated with NB.
Table 1.
Baseline characteristics
| Variables | no corticosteroids | NB | SCS | P-value |
|---|---|---|---|---|
| (n = 154) | (n = 457) | (n = 130) | ||
| Age (years) | 69 (64, 76) | 68 (62, 75) | 68 (63, 75) | 0.504 |
| Male (%) | 142 (92.2) | 418 (91.5) | 123 (94.6) | 0.499 |
| Body mass index (kg/m2) | 21.4 (18.9, 23.7) | 21.1 (18.7, 24.1) | 22.0 (19.0, 24.4) | 0.410 |
| Smoking index (packets/year) | 40.0 (15.0, 53.1) | 40.0 (20.0, 50.0) | 40.0 (20.0, 60.0) | 0.512 |
| Spirometry (post-bronchodilation) | ||||
| FVC (L) | 2.2 (1.8, 2.9) | 2.1 (1.7, 2.5) | 2.0 (1.6, 2.5)a | 0.021 |
| FEV1 (L) | 0.9 (0.6, 1.4) | 0.8 (0.6, 1.0)a | 0.7 (0.5, 0.9)a | 0.000 |
| FEV1% predicted (%) | 41.7 (26.4, 58.2) | 33.1 (24.2, 43.5)a | 30.2 (21.5, 37.7)a | 0.000 |
| FEV1/FVC (%) | 43.6 (31.1, 54.7) | 37.8 (30.1, 47.5) | 33.8 (29.6, 43.9)a | 0.002 |
| 6 MWD (m) | 220.0 (100.0, 365.0) | 234.0 (100.0, 374.0) | 205.0 (82.5, 302.0) | 0.106 |
| mMRC | 3 (2, 3) | 3 (2, 4)a | 4 (3, 4)a,b | 0.000 |
| CAT | 21.2 ± 7.9 | 22.86 ± 7.4a | 24.4 ± 6.7a | 0.004 |
| Comorbidities and complications | ||||
| Coronary heart disease (%) | 29 (18.8) | 74 (16.2) | 22 (16.9) | 0.751 |
| Hypertension (%) | 50 (32.5) | 166 (36.3) | 50 (38.5) | 0.550 |
| Diabetes (%) | 21 (13.6) | 52 (11.4) | 19 (14.6) | 0.538 |
| pneumonia (%) | 19 (12.3) | 67 (14.7) | 16 (12.3) | 0.668 |
| bronchiectasia (%) | 23 (14.9) | 71 (15.5) | 15 (11.5) | 0.523 |
| respiratory failure (%) | 38 (24.7) | 201 (44.0)a | 68 (52.3)a | 0.000 |
| prior pulmonary TB (%) | 48 (31.2) | 146 (31.9) | 38 (29.2) | 0.840 |
| cor pulmonale (%) | 25 (16.2) | 119 (26.0)a | 27 (20.8) | 0.035 |
| Frequency of AEs in the last 12 months (times) | 2 (1, 2) | 2 (1, 3) | 2 (1, 3)a | 0.021 |
| Inhaler therapy during stable stage | 0.014 | |||
| No inhaler, % | 76 (49.4) | 181 (39.6)a | 36 (27.7)a,b | |
| LAMA/LABA/LABA + LAMA, % | 10 (6.5) | 33 (7.2) | 9 (6.9) | |
| LABA + ICS, % | 27 (17.5) | 78 (17.1) | 31 (23.8) | |
| Triple therapy, % | 41 (26.6) | 165 (36.1)a | 54 (41.5)a | |
| Laboratory investigations on admission | ||||
| WBC count (× 106/L) | 7215.0 (6010.0, 9642.5) | 6800.0 (5440.0, 8830.0) | 7505.0 (6000.0, 10,007.5)b | 0.004 |
| Neutrophil count (× 106/L) | 5525.0 (3990.0, 7872.5) | 4835.0 (3780.0, 6610.0) | 5690.0 (4175.0, 8290.0)b | 0.001 |
| Eosinophil count (× 106/L) | 110.0 (50.0, 210.0) | 130.0 (50.0, 230.0) | 80.0 (30.0, 190.0)b | 0.005 |
| CRP (mg/l) | 9.4 (3.5, 34.0) | 9.0 (3.8, 23.8) | 8.4 (3.6, 31.9) | 0.990 |
| PCT (mg/l) | 0.10 (0.05, 0.14) | 0.05 (0.05, 0.13) | 0.05 (0.05, 0.12) | 0.159 |
| NT-pro BNP (pg/l) | 120.0 (50.0, 393.0) | 140.0 (50.0, 610.0) | 135.0 (50.0, 567.5) | 0.723 |
| PaO2 (mm/Hg) | 72.0 (62.5, 85.0) | 67.0 (55.0, 78.0)a | 65.3 (54.0, 76.0)a | 0.000 |
| PaCO2 (mm/Hg) | 46.0 (41.5, 53.5) | 50.0 (43.0, 62.0)a | 50.7 (43.0, 62.3)a | 0.001 |
| SaO2 (%) | 95.0 (92.0, 97.0) | 93.0 (88.0, 95.0)a | 92.0 (87.0, 95.0)a | 0.000 |
Date are presented as median (IQR) or n (%). aCompared to the no corticosteroids group, P < 0.05. bCompared to the NB group, P < 0.05
NB nebulized budesonide; SCS systemic corticosteroid; FVC forced vital capacity; FEV1 forced expiratory volume in 1 s; 6 MWD 6-min walking distance; mMRC modified Medical Research Council; CAT COPD Assessment Test; TB tuberculosis; AE acute exacerbation; COPD chronic obstructive pulmonary disease; CRP C-reactive protein; PCT Procalcitonin; NT-pro BNP N-Terminal Pro-Brain Natriuretic Peptide; PaO2 partial pressure of oxygen in the artery; PaCO2 partial pressure of carbon dioxide in arterial blood; SaO2 oxygen saturation in arterial blood; LAMA long-acting muscarinic receptor antagonist; LABA long-acting beta(2)-agonist; ICS inhaled corticosteroid
Nebulized Budesonide Therapy During Hospitalization was Independent Protective Factor of Six-Month Mortality
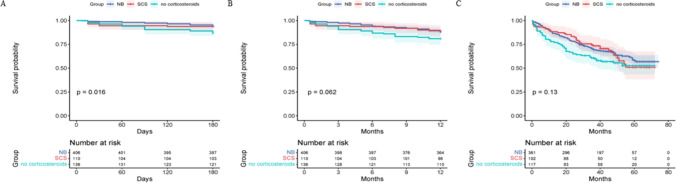
The changes in condition during hospitalization and follow-up were showed in Table 2. Patients in the NB group had significant improvement on mMRC (− 1 vs. 0, P = 0.014) and CAT (− 6 vs. − 4, P = 0.018) compared to patients in the non-corticosteroid group. SCS group had the highest proportion of patients treated with triple therapy during stable stage after discharge compare to the other two groups (68.5% vs. 62.1% vs. 50.6%, P = 0.006). Compared to the non-corticosteroid group, NB group showed a lower six-month mortality after discharge (5.9% vs. 13.2%, P = 0.017) (Table 2). Kaplan–Meier survival analysis further compared the impact of NB and SCs during hospitalization on six-month, one-year and long-term mortality over follow-up. Compared to patients treated without steroids, those treated with NB during hospitalization had a significantly lower risk of death over long-term follow-up (P = 0.047) (Fig. 2). SCS group showed a better survival trend than the non-corticosteroid group (Fig. 2). Although the results of the log-rank analysis did not reach statistical significance, the Wilcoxon analysis showed that SCs therapy had a positive impact on survival during the early stage of follow-up (P = 0.038). To eliminate the interference of disturbing factors including age, FEV1/FVC, frequency of AEs in the previous year, respiratory failure, cor pulmonale, mMRC, CAT and inhaler therapy after discharge, we used Cox proportional hazards regression modeling and found that NB therapy was an independent protective factor for six-month mortality afterward compared with treatment without steroid therapy (adjusted HR 0.36; 95% CI 0.15–0.88).
Table 2.
Condition changes during hospitalization and follow-up
| Variables | no corticosteroids | NB | SCS | P-value |
|---|---|---|---|---|
| (n = 154) | (n = 457) | (n = 130) | ||
| Changes of symptom assessments and laboratory investigations | ||||
| Δ6MWT (m) | 70.0 (33.5, 140.0) | 62.5 (19.8, 152.8) | 49.0 (15.0, 110.0) | 0.190 |
| ΔmMRC | 0 (− 1, 0) | − 1 (− 1, 0)a | 0 (− 1, 0) | 0.026 |
| ΔCAT | − 4.0 (− 8.0, − 1.0) | − 6.0 (− 11.0, − 2.0)a | − 5.5 (− 8.0, − 2.0)b | 0.019 |
| ΔWBC count (× 106/L) | − 1095.0 (− 2692.5, 257.7) | − 870.0 (− 2870.0, 270.0) | − 260.0 (− 2150.0, 1610.0)a,b | 0.016 |
| ΔNeutrophil count (× 106/L) | − 1260.0 (− 3062.5, − 10.0) | − 930.0 (− 2570.0, 275.0) | − 345.0 (− 2397.5, 1575.0)a | 0.024 |
| ΔEosinophil count (× 106/L) | 60.0 (0.0, 120.0) | 40.0 (− 10.0, 130.0) | 0 (− 32.5, 80.0)a | 0.012 |
| ΔPaO2 (mm/Hg) | 11.0 (1.8, 21.5) | 16.9 (0.5, 28.0) | 14.0 (2.3, 21.5) | 0.425 |
| ΔPaCO2 (mm/Hg) | − 1.5 (− 8.0, 1.8) | − 2.0 (− 8.8, 2.0) | − 1.0 (− 8.0, 3.3) | 0.636 |
| ΔSaO2 (%) | 3.0 (0.0, 6.0) | 4.0 (0.8, 9.0) | 3.0 (0.5, 8.0) | 0.396 |
| Inhaler therapy after discharge | 0.000 | |||
| No inhaler, % | 17 (11.0) | 13 (2.8)a | 4 (3.1)a | |
| LAMA/LABA/LABA + LAMA, % | 47 (30.5) | 107 (23.4) | 23 (17.7)a | |
| LABA + ICS, % | 12 (7.8) | 53 (11.6) | 14 (10.8) | |
| Triple therapy, % | 78 (50.6) | 284 (62.1)a | 89 (68.5)a | |
| One year of follow-up | ||||
| Time of first AE after discharge (months) | 4.0 (2.0, 8.5) | 5.0 (3.0, 8.5) | 4.0 (2.0, 6.0) | 0.269 |
| Frequency of AEs in the previous year (times) | 1 (0, 1) | 1 (0, 2) | 1 (0, 2) | 0.336 |
| Incidence of death within six months after discharge (%) | 18 (13.2) | 24 (5.9)a | 7 (6.4) | 0.017 |
| Incidence of death within one year after discharge (%) | 26 (19.1) | 49 (12.1) | 12 (10.9) | 0.080 |
| Long term of follow-up | ||||
| Incidence of death (%) | 53 (45.3) | 129 (35.7) | 36 (35.3) | 0.155 |
Data are presented as the median (IQR) or n (%)
NB nebulized budesonide; SCS systemic corticosteroid; 6 MWD 6-min walking distance; mMRC modified Medical Research Council; CAT COPD Assessment Test; PaO2 partial pressure of oxygen in the artery; PaCO2 partial pressure of carbon dioxide in arterial blood; SaO2 oxygen saturation in arterial blood; AE acute exacerbation; LAMA long-acting muscarinic receptor antagonist; LABA long-acting beta(2)-agonist; ICS inhaled corticosteroid
aCompared to the no corticosteroid group, P < 0.05. bCompared with the NB group, P < 0.05
ΔChanges in symptom assessments and examinations (discharge value – admission value)
Fig. 2.
Kaplan–Meier survival curves for the impact of NB and SCS on six-month (A), one-year (B) and long-term (C) mortality after discharge in AECOPD patients. A: NB vs. no corticosteroids, P = 0.005, SCS vs. no corticosteroids, P = 0.087, NB vs. SCS, P = 0.824; B: NB vs. no corticosteroids, P = 0.028, SCS vs. no corticosteroids, P = 0.082, NB vs. SCS, P = 0.786; C: NB vs. no corticosteroids, P = 0.047, SCS vs. no corticosteroids, P = 0.160, NB vs. SCS, P = 0.993. NB: nebulized budesonide; SCS: systemic corticosteroid
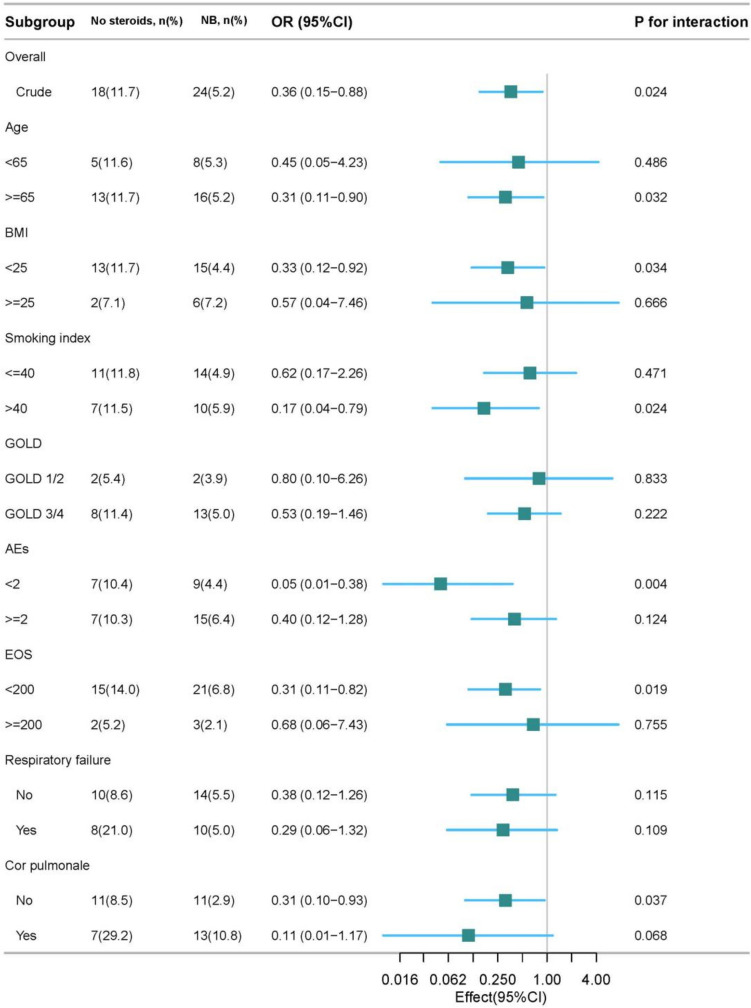
The Benefit of Nebulized Budesonide Therapy on Survival Prognosis was More Significant in Certain Subgroups
We conducted subgroup analysis and found that patients with NB therapy during hospitalization, especially in patients aged 65 and older (adjusted HR 0.31; 95% CI 0.11–0.90), patients with BMI of < 25 kg/m2 (adjusted HR 0.33; 95% CI 0.12–0.92), patients with smoking index of > 40 packets/year (adjusted HR 0.17; 95% CI 0.04–0.79), patients with less than 2 AEs in the previous year (adjusted HR 0.05; 95% CI 0.01–0.38), patients with blood eos count < 200 cells/µl (adjusted HR 0.31; 95% CI 0.11–0.82) or patients without concomitant cor pulmonale (adjusted HR 0.31; 95% CI 0.10–0.93) had a more significant reduction in six-month mortality (Fig. 3). Surprisingly, although a larger proportion of physicians chose to use NB to treat patients with concomitant cor pulmonale, NB therapy had a positive effect on survival in those who did not have. Subsequently, we conducted survival analysis for patients with concomitant cor pulmonale and found that treatment with SCs, but not NB, during hospitalization could remarkably reduce long-term mortality during follow-up (P = 0.010) (Supplementary Figure S1).
Fig. 3.
Forest plot of Subgroup analysis for mortality within six months of follow-up. Hazard ratio (HR) were adjusted for age, baseline post-bronchodilator FEV1/FVC, frequency of AEs in the previous year, respiratory failure, cor pulmonale, mMRC, CAT and inhaler therapy after discharge. No steroids, patients without corticosteroid therapy; NB, patients with nebulized budesonide therapy; HR, hazard ratio; GOLD, Global Initiative for Chronic Obstructive Lung Disease; AE, acute exacerbation; BMI, body mass index; EOS, eosinophil count
Nebulized Budesonide with Cumulative Dose Less than 60 mg Might be Preferred
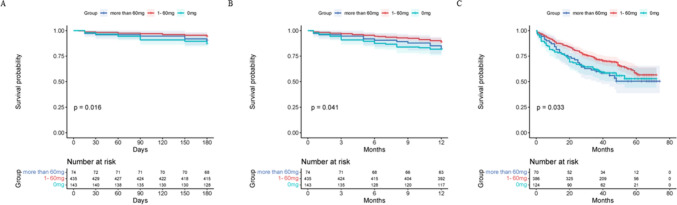
Subsequently, the total dosage of budesonide was stratified by a gradient of 20 mg: 0 mg, 1–20 mg, 21–40 mg, 41–60 mg, 61–80 mg, 81–100 mg, and > 100 mg. The mortality of the 1–20 mg, 21–40 mg and 41–60 mg groups were significantly reduced within six months of follow-up compared to that of the 0 mg group (P = 0.025, P = 0.037, and P = 0.047, respectively) (Fig. 4). We then used 60 mg as the cutoff point to stratify the population into three groups (0 mg, 1–60 mg, > 60 mg). The clinical admission conditions of patients who received nebulized budesonide were significantly worse than those who did not receive nebulization, while there was no difference between the 1–60 mg group and > 60 mg groups in these aspects (Supplementary Table S1). We further compared the effects of budesonide dosage during hospitalization on mortality among the 0 mg, 1–60 mg and > 60 mg groups and found that patients who received 1–60 mg budesonide had the best sustained survival prognosis during the entire follow-up (1–60 mg vs. 0 mg, P = 0.033, 1–60 mg vs. 60 mg, P = 0.049, respectively) (Fig. 5), which was still significant (adjusted HR 0.39; 95% CI 0.17–0.92) over six months of follow-up after adjustment.
Fig. 4.
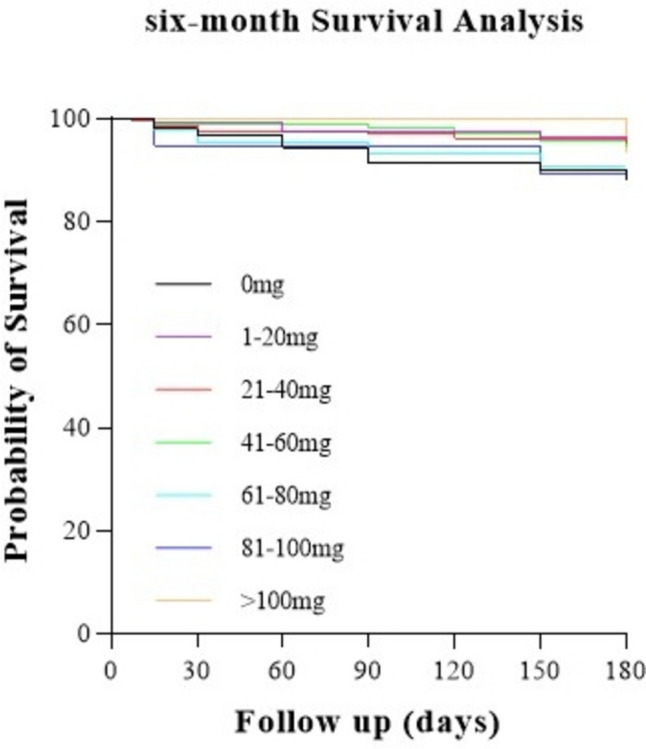
Kaplan–Meier survival curves for the mortality within six months of follow-up in AECOPD patients, according to the total dosage of budesonide. 1–20 mg vs. 0 mg, P = 0.025, 21–40 mg vs. 0 mg, P = 0.037, 41–60 mg vs. 0 mg, P = 0.047, comparison between the other every two groups, P > 0.05
Fig. 5.
Kaplan–Meier survival curves for the impact of steroids therapy on six-month (A), one-year (B) and long-term (C) mortality in AECOPD patients with cor pulmonale. A: 0 mg vs. 1–60 mg, P = 0.004, comparison between the other every two groups, P > 0.05; B: 0 mg vs. 1–60 mg, P = 0.020, comparison between the other every two groups, P > 0.05; C: 0 mg vs. 1–60 mg, P = 0.033, more than 60 mg vs. 1–60 mg, P = 0.049, comparison between the other every two groups, P > 0.05
Discussion
To our knowledge, this is the first real-world study to explore the impact of steroid therapy during hospitalization on mortality over a long-term follow-up in China. In this study, we revealed that the administration of nebulized budesonide during hospitalization was an independent protective factor against six-month mortality in patients with AECOPD. Subgroup analysis showed that certain patients had a more significant survival benefit after NB therapy. Additionally, less than 60 mg of NB may be suitable for hospitalized AECOPD patients.
The exploration of using corticosteroids, whether systemic corticosteroids or nebulized budesonide, for the treatment of AECOPD has been ongoing for many years [15, 18, 19]. The positive efficacy of both in improving FEV1 and blood gas and reducing the risk of treatment failure is certain. However, there is a relative lack of research on the impact of mortality, especially on survival benefits, during long-term follow-up. This study provided some clinical evidence for this topic. The results suggest that SCs may have a positive impact on survival in the early stage after discharge rather than the later stage, which is consistent with previous studies suggesting that the positive effects of steroid therapy during hospitalization on survival gradually diminished over time [6, 20]. Notably, NB not only plays a good surrogate role in the treatment of acute exacerbations, but univariate survival analysis found that it reduces long-term mortality, which is a surprising and further positive result for the role of NB. After balancing the confounding factors, it was still associated with a significant reduction in six-month mortality.
The primary consideration when administering steroid therapy is balancing the risk of adverse events with the benefits [21, 22]. COPD is more common in older adults, making the use of steroids a topic of debate.. Nevertheless, findings of this study indicate that patients aged 65 and older who experience acute exacerbations of COPD gain more benefits from steroid therapy concerning their survival prognosis. Meanwhile, patients with a lower AE frequency (less than 2 exacerbations per year) are also advised to receive steroid treatment during hospitalization, as it can improve survival outcomes in the long term. In contrast, patients who experience more frequent exacerbations may need more comprehensive management strategies to address the heightened risk of mortality associated with frequent exacerbations [4] rather than relying solely on steroid therapy. While a low BMI and a high smoking history increase the risk of death [23], NB treatment is particularly effective for these individuals.
The results of some of the subgroup analyses were unexpected. Previous studies have shown that steroid therapy during the stable stage is more effective in COPD patients with blood eos > 300 cells/µL [24], but results of the present study indicate that inpatients with eos < 200 cells/µL experience greater survival benefits from steroid therapy. Li et al. also demonstrated that inpatients with blood eos < 200 cells/µL more significant improvements throughout follow-up after receiving steroid treatment [25]. A recent clinical study showed that eos < 150 cells/µL was independent predictor for 30-d mortality in hospitalized AECOPD patients treated with SCs (combined with inhaled corticosteroids (ICS) or not) [26]. These findings suggested that steroid therapy may target different sensitive populations during the stable and exacerbation stages of COPD. Subgroup analysis also suggested that patients with cor pulmonary disease were more likely to benefit from SCS treatment. This could be because SCs improve lung function and blood gas levels while also reducing pulmonary artery systolic pressure [27].
The cumulative dose of corticosteroids is often linked to adverse events and increased medical expenses [28]. High-dose NB are associated with a lower failure rate but carry a higher risk of adverse events. The optimal dose and duration for NB treatment remain unclear [8, 12]. In the present study, we analyzed the relationship between the cumulative dose of NB (4–8 mg/day) administered during hospitalization and patients’ outcomes. It was found that patients receiving a total budesonide dose < = 60 mg experienced better long-term survival compared to those not treated with NB. Conversely, patients who received a cumulative dose greater than 60 mg had longer hospital stays. This group may have more complex disease conditions and be less sensitive to treatment. For these patients, prolonged NB treatment did not provide additional benefits. Instead it increased the burden of medical expenses and the risk of adverse events. These results suggest that, monitoring the daily dosage of NB, it is crucial to pay attention to the cumulative dose during hospitalization. If the cumulative dose of NB exceeds 60 mg, it is necessary to carefully consider the continued use of NB treatment.
This study had some limitations. First, due to the limitation of the overall population size, some interesting subgroup analysis results cannot be further analyzed to find possible explanations. For example, whether eosinophils during hospitalization could be used as a predictive factor for the impact of NB treatment on mortality, and what may be the possible reasons for the different conclusions from the guidance of stable stage eosinophils to steroid use. Second, we could not control for the bias caused by different medication preferences due to long enrollment and recall bias during telephone follow-up. Third, as an observational cohort study, our conclusions need to be confirmed or refuted in a large prospective randomized controlled trial.
Conclusion
In summary, our study expanded our understanding of the impact of nebulized budesonide and systemic steroid therapy during hospitalization on long-term survival prognosis in hospitalized AECOPD patients, and identified more recommended steroid regimens for specific populations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all the participants for collecting data for this study.
Author Contributions
Concept and design of the study by ZZh, RXi, and YCh. Data collection and management by ZZh, YCu, RXi, WMe, JWu, JWa, RZh. Statistical analysis using ZZh and XH. Drafting of the manuscript by ZZh and RXi. All authors reviewed and approved the final manuscript. All the authors have read and approved the final manuscript.
Funding
The study was supported by the Natural Science Foundation of Hunan Province (No. 2023JJ30742) and the National Key Clinical Specialty Construction Projects of China.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
This study was approved by the Ethics Committee of the Second Xiangya Hospital of the Central South University (No. ChiCTR-POC-17010431). Written informed consent was obtained from all the participants.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhiqi Zhao and Ruoyan Xiong have been contributed equally to this work.
Contributor Information
Huihui Zeng, Email: bonemarrow@csu.edu.cn.
Yan Chen, Email: chenyan99727@csu.edu.cn.
References
- 1.Diseases GBD, Injuries C (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 396(10258):1204–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou M et al (2019) Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 394(10204):1145–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GOLD, Global Strategy for Prevention, Diagnosis and Management of COPD: 2024 Report. 2024.
- 4.Suissa S, Dell’Aniello S, Ernst P (2012) Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 67(11):957–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walters JA et al (2014) Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014(9):CD001288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niewoehner DE et al (1999) Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med 340(25):1941–7 [DOI] [PubMed] [Google Scholar]
- 7.McEvoy CE, Niewoehner DE (1997) Adverse effects of corticosteroid therapy for COPD. A Crit Rev Chest 111(3):732–743 [DOI] [PubMed] [Google Scholar]
- 8.Maltais F et al (2002) Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 165(5):698–703 [DOI] [PubMed] [Google Scholar]
- 9.Gunen H et al (2007) The role of nebulised budesonide in the treatment of exacerbations of COPD. Eur Respir J 29(4):660–667 [DOI] [PubMed] [Google Scholar]
- 10.Mirici A, Meral M, Akgun M (2003) Comparison of the efficacy of nebulised budesonide with parenteral corticosteroids in the treatment of acute exacerbations of chronic obstructive pulmonary disease. Clin Drug Investig 23(1):55–62 [DOI] [PubMed] [Google Scholar]
- 11.Ding Z et al (2016) A randomized, controlled multicentric study of inhaled budesonide and intravenous methylprednisolone in the treatment on acute exacerbation of chronic obstructive pulmonary disease. Respir Med 121:39–47 [DOI] [PubMed] [Google Scholar]
- 12.Zhang R et al (2020) Optimization of nebulized budesonide in the treatment of acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 15:409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y et al (2020) Comparison of the clinical outcomes between nebulized and systemic corticosteroids in the treatment of acute exacerbation of COPD in China (CONTAIN Study): a post hoc analysis. Int J Chron Obstruct Pulmon Dis 15:2343–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourbeau J et al (2016) Early COPD exacerbation treatment with combination of ICS and LABA for patients presenting with mild-to-moderate worsening of dyspnea. COPD 13(4):439–447 [DOI] [PubMed] [Google Scholar]
- 15.Wood-Baker R, Walters J, Walters EH (2007) Systemic corticosteroids in chronic obstructive pulmonary disease: an overview of Cochrane systematic reviews. Respir Med 101(3):371–377 [DOI] [PubMed] [Google Scholar]
- 16.(GOLD), G.I.f.C.O.L.D., Global Strategy for Prevention, Diagnosis and Management of COPD: 2016 Report.
- 17.Sin DD et al (2005) Inhaled corticosteroids and mortality in chronic obstructive pulmonary disease. Thorax 60(12):992–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walters JA et al (2014) Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014(9):1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pleasants RA et al (2018) Nebulized corticosteroids in the treatment of COPD exacerbations: systematic review, meta-analysis, and clinical perspective. Respir Care 63(10):1302–1310 [DOI] [PubMed] [Google Scholar]
- 20.Leuppi JD et al (2013) Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 309(21):2223–2231 [DOI] [PubMed] [Google Scholar]
- 21.Groenewegen KH, Schols AM, Wouters EF (2003) Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 124(2):459–467 [DOI] [PubMed] [Google Scholar]
- 22.Alia I et al (2011) Efficacy of corticosteroid therapy in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. Arch Intern Med 171(21):1939–1946 [DOI] [PubMed] [Google Scholar]
- 23.Wada H et al (2021) Low BMI and weight loss aggravate COPD mortality in men, findings from a large prospective cohort: the JACC study. Sci Rep 11(1):1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(GOLD), G.I.f.C.O.L.D. Global Strategy for Prevention, Diagnosis and Management of COPD: 2023 Report. 2023; Available from: https://goldcopd.org/2023-gold-report-2/.
- 25.Li L et al (2021) Personalized variable vs fixed-dose systemic corticosteroid therapy in hospitalized patients with acute exacerbations of COPD: a prospective, multicenter, randomized. Open-Label Clin Trial Chest 160(5):1660–1669 [DOI] [PubMed] [Google Scholar]
- 26.Kleinhendler E et al (2024) Effect of added inhaled corticosteroids to systemic steroids on COPD exacerbation outcomes. Respir Care. 10.4187/respcare.11954 [DOI] [PubMed] [Google Scholar]
- 27.Kunter E et al (2008) Effect of corticosteroids on hemostasis and pulmonary arterial pressure during chronic obstructive pulmonary disease exacerbation. Respiration 75(2):145–154 [DOI] [PubMed] [Google Scholar]
- 28.Zhang J et al (2019) Comparative analysis of medical expenditure with nebulized budesonide versus systemic corticosteroids in hospitalized patients with acute exacerbations of chronic obstructive pulmonary disease in China. Int J Chron Obstruct Pulmon Dis 14:1195–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.