Abstract
Refractive error (RE) and myopia are complex polygenic conditions with the majority of genome-wide associated genetic variants in non-exonic regions. Given this, and the onset during childhood, gene-regulation is expected to play an important role in its pathogenesis. This prompted us to explore beyond traditional gene finding approaches. We performed a genetic association study between variants in non-coding RNAs and enhancers, and RE and myopia. We obtained single-nucleotide polymorphisms (SNPs) in microRNA (miRNA) genes, miRNA-binding sites, long non-coding RNAs genes (lncRNAs) and enhancers from publicly available databases: miRNASNPv2, PolymiRTS, VISTA Enhancer Browser, FANTOM5 and lncRNASNP2. We investigated whether SNPs overlapping these elements were associated with RE and myopia leveraged from a large GWAS meta-analysis (N = 160,420). With genetic risk scores (GRSs) per element, we investigated the joint effect of associated variants on RE, axial length (AL)/corneal radius (CR), and AL progression in an independent child cohort, the Generation R Study (N = 3638 children). We constructed a score for biological plausibility per SNP in highly confident miRNA-binding sites and enhancers in chromatin accessible regions. We found that SNPs in two miRNA genes, 14 enhancers and 81 lncRNA genes in chromatin accessible regions and 54 highly confident miRNA-binding sites, were in RE and myopia-associated loci. GRSs from SNPs in enhancers were significantly associated with RE, AL/CR and AL progression. GRSs from lncRNAs were significantly associated with all AL/CR and AL progression. GRSs from miRNAs were not associated with any ocular biometric measurement. GRSs from miRNA-binding sites showed suggestive but inconsistent significance. We prioritized candidate miRNA binding sites and candidate enhancers for future functional validation. Pathways of target and host genes of highly ranked variants included eye development (BMP4, MPPED2), neurogenesis (DDIT4, NTM), extracellular matrix (ANTXR2, BMP3), photoreceptor metabolism (DNAJB12), photoreceptor morphogenesis (CHDR1), neural signaling (VIPR2) and TGF-beta signaling (ANAPC16). This is the first large-scale study of non-coding RNAs and enhancers for RE and myopia. Enhancers and lncRNAs could be of large importance as they are associated with childhood myopia. We provide a confident blueprint for future functional validation by prioritizing candidate miRNA binding sites and candidate enhancers.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00439-024-02721-x.
Background
Myopia, or nearsightedness, is a refractive error (RE) which causes blurred distance vision due to excessive eye elongation, where the focal point of an image is projected in front instead of on the retina. Although this condition can be corrected by prescription glasses, its comorbidities can lead to visual impairment. Each additional diopter [D] of myopia is associated with an increased risk of 57% for myopic maculopathy, 20% for open angle glaucoma, 21% for cataract, and 30% for retinal detachment (Bullimore et al. 2021). Myopia is now recognized as a serious health issue due to the worldwide rise in its prevalence (Morgan et al. 2012, 2018; Modjtahedi et al. 2018; Holden et al. 2016; Williams et al. 2015). There is an urgency for preventive and therapeutic measures, especially for high myopia, which is defined as RE ≤ −6D and/or ≥ 26 mm axial length (AL).
RE and myopia are complex genetic traits associated with hundreds of genetic variants, a strong influence of lifestyle factors, and their interplay (Pärssinen et al. 2014; Verhoeven et al. 2013). Over the last few years, it has become clear that common genetic variants associated with complex traits are preferentially located in regulatory domains (Schaub et al. 2012; Maurano et al. 2012). Similarly, the majority of associated loci of the genome-wide association study (GWAS) meta-analysis on RE and myopia (Tedja et al. 2018) were non-exonic (73% intergenic or intronic, 19% variants overlapping with noncoding RNAs, 4% genetic variants residing in 3′ or 5′ untranslated regions (UTR) and 4% exonic variants (Tedja et al. 2018; Teperino et al. 2013). The knowledge of the role of non-coding RNAs and enhancers during development is growing, and with that growing towards potential therapeutic targets. However, insights into the role of non-coding RNAs and enhancers in the development of myopia are lacking.
For this study, we focused on the non-coding elements microRNAs and microRNA binding sites, long non-coding RNAs, and enhancers. MicroRNAs (miRNAs) are small non-coding RNAs which regulate gene expression post-transcriptionally. In their mature form, they are 19–24 nucleotides in length and one miRNA can regulate multiple target genes (Bartel 2009). They normally function by sequence-specific post-transcriptional gene silencing by binding to 3’ UTRs of target mRNAs, so-called miRNA-binding sites. Studies based on deciphering the role of miRNAs in myopia in humans are limited and often rely on candidate gene-based approaches rather than a comprehensive genome-wide approach. Only two studies reported a genome-wide scan for miRNA-related genetic variants for eye diseases in humans (Ghanbari et al. 2017a, b). These studies focused on glaucoma and age-related macular degeneration (AMD) and revealed associations with various miRNAs and numerous miRNA-binding sites. For myopia, a large-scale genome-wide miRNA expression profiling study has only been performed in mice. This study by Tkatchenko et al. identified 53 differentially expressed miRNAs in the retina, but not in the sclera after 10 days of visual form deprivation (Tkatchenko et al. 2016).
The human genome contains thousands of enhancer regions (Pennacchio et al. 2013). These are cis-regulatory DNA sequences of typically a few hundred base pairs in length, acting as binding sites for sequence-specific transcription factors. They are able to enhance (i.e. upregulate) the transcription of a target gene up to a 100- fold up to 1 million base pairs away from its transcription start site (Shlyueva et al. 2014; Panigrahi and O’Malley 2021; Gasperini et al. 2020), thereby playing a key role in mediating spatiotemporal gene expression. The first study investigating the role of enhancers in myopia was initially intended as a candidate-gene study, where the authors compared KCNQ5 polymorphisms in highly myopic cases and emmetropic controls in Han Chinese (Liao et al. 2017). They revealed associations of KCNQ5 polymorphisms with two intronic genetic variants (rs7744813 and rs9342979), located within a region enriched for the H3K4me1 and H3K27ac histone modifications, which are associated with putative enhancers.
Long non-coding RNAs (lncRNAs) are RNA species that are more than 200 nucleotides in length which do not have protein-coding capacities (Derrien et al. 2012). LncRNAs are able to interact with DNA, RNA and proteins and are implicated in a wide range of biological processes. For example, they play a role in modulating chromatin structure and function, influencing transcription of neighboring and distant genes, influencing RNA splicing, stability and translation, and influencing the formation and regulation of organelles and nuclear condensates. Many lncRNAs have tissue and cell-specific expressions, which change in conditions of normal development as well as pathology (Ulitsky and Bartel 2013; Zhang et al. 2019). Recently, a hypothesis based on in silico analyses was proposed for myopia-related lncRNAs and was of general note, suggesting they fold into complex spatial structures acting as a skeleton for transcription factor targeted binding, thereby co-regulating gene expression (Wang et al. 2021). Despite some interesting leads, the exact role of lncRNAs in myopia is yet to be elucidated.
The aim of this study is to identify RE and myopia-related non-coding genomic sequences including miRNAs, miRNA binding sites, enhancers, and lncRNAs by leveraging data from a large GWAS on RE and myopia. Our results will provide further insights into the complex molecular mechanisms of myopia.
Methods
Genome-wide association studies on RE and myopia
To examine the association of non-coding RNAs and enhancers with RE and myopia, we used summary statistics from the recent GWAS on refractive error and myopia performed by the Consortium for Refractive Error and Myopia (CREAM) (Tedja et al. 2018). In summary, CREAM conducted genome-wide association meta-analyses on RE (44,192 European and 11,935 Asian individuals from CREAM) and age of diagnosis of myopia (104,293 European individuals from 23andMe) imputed to the 1000 Genomes (1000G) reference panel (Auton et al. 2015). The association of ~ 11 million SNPs (minor allele frequency (MAF) > 0.01 and imputation quality > 0.3) was tested for RE and myopia. Detailed methods and summary statistics are described and published elsewhere (Tedja et al. 2018).
Association analysis on genetic variants located in non-coding RNAs and regulatory elements: miRNAs, miRNA-binding sites, enhancers and non-coding RNAs
MiRNAs and miRNA binding sites were assessed following the methodology described earlier by Ghanbari et al. (2017a, 2017b). In summary, we retrieved miRNA-encoding sequences from miRNASNP v2 (Gong et al. 2015a) (N = 1,151 MiRNAs) and genetic variants in miRNA binding sites within the 3’UTR of genes from PolymiRTS 3.0 (Bhattacharya et al. 2014) (N = 18,678 miRNA binding sites). In addition, we linked the putative miRNAs from the PolymiRTS database to the miRNA binding sites. This database comprises candidate miRNAs per binding site based on a perfect match to the seed nucleotides 2–7 and a perfect match to the 8th nucleotide of miRNA, or an anchor adenosine immediately downstream the 2–7 seed in the target. For the genetic variants in enhancers, we applied a similar strategy.
Of all enhancer databases available, we chose the databases which comprised expression in eye tissue or in cell lines derived from the eye: VISTA Enhancer Browser (Visel et al. 2007) and FANTOM5 (Forrest et al. 2014; Andersson et al. 2014). In the VISTA Enhancer Browser (N = 1,913 enhancers), each genomic sequence was previously tested for enhancer activity using transgenic reporter assays in mice at age E11.5, as read-out by LacZ reporter staining. FANTOM5 is an atlas of active in vivo bidirectionally transcribed enhancers across the human body, based on human primary cells and post-mortem tissues. Enhancer predictions were based on a distinct bidirectional CAGE pattern. We included enhancers with activity in at least 3 ocular tissues (N = 7,944 enhancers). We retrieved variants located in human lncRNA transcripts (N = 138,149 lncRNA regions) using the lncRNASNP2 database (Miao et al. 2018; Gong et al. 2015b).
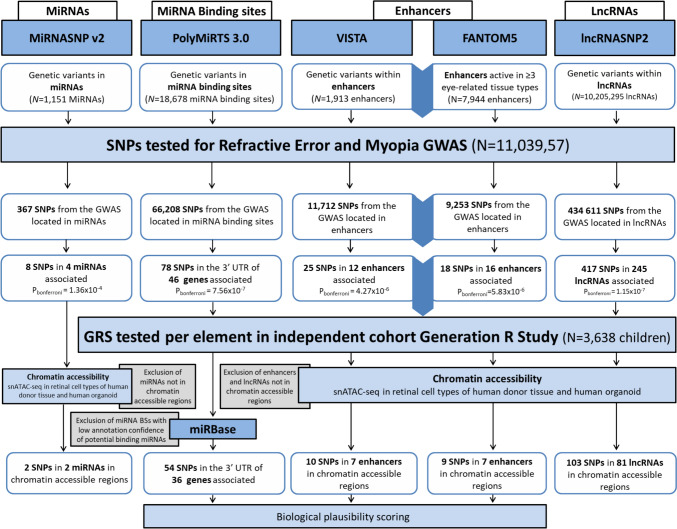
Associations between miRNAs, miRNA binding sites, enhancers and long non-coding RNAs and refractive error and myopia were investigated by performing look-ups of genetic variants (i.e. SNPs) residing in these elements in the summary statistics of the previously mentioned GWAS. Significance was determined by Bonferroni correction for multiple comparisons. Regional association plots showing associations of the non-coding RNAs and enhancers in the GWAS were made by using LocusZoom (http://locuszoom.org/). See Fig. 1 for the methodological overview.
Fig. 1.
Schematic workflow of analyses to identify candidate genetic variants in non-coding RNAs and enhancers per database. SNPs single nucleotide polymorphisms, 3' UTR 3' untranslated region, GRS genetic risk score
Cross-referencing of miRNA target genes and genes associated with refractive error and myopia
Since one miRNA can influence the expression of multiple genes, we extracted all potential target genes predicted from TargetScan v7.2 (Agarwal et al. 2015) and miRDB (Chen and Wang 2020) and identified the presence of previous GWAS candidate genes for refractive error and myopia (i.e. common refractive error genes) and the presence of genes associated with syndromic forms of myopia (Tedja et al. 2019). We only included putative target genes if they were present in both databases and if the target score was > 60 from miRDB database.
Ranking of candidate miRNA binding sites and candidate enhancers
MiRNA binding sites
We included miRNA binding sites with a high confidence score of potential binding miRNAs for the biological plausibility ranking. The criteria for the confidence score were extracted from miRNAs in miRBase (Kozomara et al. 2019) (https://www.mirbase.org/). High confidence was defined as: miRNA with a high annotation confidence, a high number of reads of the mature -3p or -5p miRNA sequence (≥ 10,000), and a high probability to be loaded by RISC. Medium confidence was defined as: miRNA with a high annotation confidence and one of the following: a high number of reads of the mature -3p or -5p miRNA sequence (≥ 10,000), or a high probability to be loaded by RISC. Low confidence was defined as miRNA with a low annotation confidence.
Next, we assigned points (pt) to each of the genetic variants in miRNA binding sites based on several criteria for biological plausibility estimations. The criteria were grouped into characteristics of the genetic variant, of the target gene and of the miRNA binding site. Genetic variant characteristics: GWAS association strength (genetic variant is a top SNP in its locus of previous mentioned GWAS (2pt) or in LD > 0.8 with a top SNP (1pt)), evidence for eQTL of a genetic variant on a host gene derived from Haploreg v4.1 (Ward and Kellis 2012) (eQTL in ≥ 10 tissues (2pt), eQTL in 1–10 tissues (1pt), no evidence for eQTL (0pt)) and linkage disequilibrium (LD) pattern derived from Haploreg v4.1 (no or few synonymous proxies (2pt), several synonymous proxies (< 50), but no non-synonymous (pt1), several proxies including nonsynonymous (0pt)). Host gene characteristics: expression of gene in ocular cell type (Cowan et al. 2020) (1pt), gene associated with human ocular phenotype derived from extensive literature search and derived from GWAS catalog (Buniello et al. 2019) (1pt). MiRNA characteristics: miRNA(s) associated with myopia; data derived from Tkatchenko et al. (2016) (1pt) and miRNA(s) associated with ocular phenotype using HMDD v3.00 (Huang et al. 2019) (1pt).
Enhancers
We performed a similar ranking system for the enhancers per database (VISTA Enhancer Browser and FANTOM), based on characteristics of the genetic variant, of the putative target gene and of the putative enhancer. The genetic variant and gene trait points were assigned as described for the miRNA binding sites. Enhancer characteristics were as follows: known enriched histone marks (chromatin state 7_enh corresponding to enhancer; core 15-state model, enriched H3K4me1 and H3K27ac histone marks) derived from Haploreg v4.1 (> 10 tissues (2 pt), 1–10 tissues (1 pt), none known (0 pt)) and expression derived from VISTA Enhancer Browser (1 p) or expression score derived from FANTOM 5 (> 10 cell lines (2 pt), 6–10 cell lines (1 pt), 3–5 cell lines (0 pt)). We prioritized genetic variants in candidate enhancers located per database which were in chromatin accessible regions.
Long non-coding RNAs
To identify putative relationships between lncRNAs with disorders, we used the disease associations from the lncRNASNP2 database (Miao et al. 2018). The relationship is tested indirectly by modeling miRNAs and their disease association from the HMDD database in the software TAM (Lu et al. 2010). The disease-associated miRNA set was derived from the HMDD database (Huang et al. 2019). We were unable to construct a ranking system due to the diversity and uncertainty of lncRNA function. No publicly available data exists on lncRNAs associated with myopia. As there are hints of interplay between miRNAs, miRNA binding sites, enhancers and lncRNAs, we checked for an overlap of genetic variants of these non-coding RNAs/regulatory elements. In addition, we checked for an overlap in putative target genes between these regulatory elements.
Expression analysis of miRNAs, miRNA binding sites and enhancers in ocular tissue
To check whether miRNAs in our collection are expressed in ocular tissues, two online databases were screened: miRetina (Karali et al. 2016) and HMDD (Huang et al. 2019).
For the expression of miRNA binding sites and enhancers, we utilized data of previous analyzed single-cell RNA expression on human retinal tissue per retinal cell type in the peripheral neural retina and choroid obtained from multi-organ donors (n = 74,558 peripheral retinal cells from n = 6 eyes; from 3 donors aged between 50 and 80 years) (Cowan et al. 2020).
Absolute expression values (i.e. number of transcripts per cell) were L1-normalized. Genes were considered to be expressed if their expression rate was greater than 1 transcript per 50 cells in at least one retinal cell type. In heat maps showing the expression of a set of genes (e.g., host genes of miRNA binding sites, flanking genes of enhancer locations) across cell types, genes were ordered according to their expression (peak expression and center of mass). To test if a set of genes contained a higher percentage of genes which were expressed than expected by chance given the number of genes in the set, , we used a Monte Carlo permutation test which drew genes from the data set times and counted the number of permutations , where the percentage of expressed genes was at least as high as that in the gene set being evaluated. The reported p-value was then .
Genes were considered cell type specific if their expression in at least one retinal cell type was a positive outlier relative to the distribution of their expression across cell types. To identify positive outliers, we z-scored the expression of one gene across cell types using the mean gene expression and an outlier-robust estimate of the standard deviation generated using the median absolute deviation. A gene was considered cell type specific if the z-score exceeded 5.0.
Chromatin accessibility of the candidate non-coding RNAs and enhancers
To investigate the accessibility of chromatin near our candidate non-coding RNAs (miRNAs and lncRNAs) and enhancers, we sought for overlap between them and the single nucleus assay for transposase-accessible chromatin sequencing peaks (snATAC-seq) at the single-cell level across human retinal cell types (cell types of six developmental time points and adult retinal cell tissue; n = 53) and organoid retinal cell types derived from human induced pluripotent stem cells from two individuals. A region of 500 bytes (i.e. 0.5 kb) spanning-window was used to locate ATAC-seq peaks in the region of the candidate non-coding RNAs and enhancers. Information regarding sex and age of the individuals can be found in the gene expression omnibus (GEO: https://www.ncbi.nlm.nih.gov/geo/, series GSE183684). Detailed methods regarding the sn-ATAC-seq experiments are described in Thomas et al. (2022). We compared peak patterns (i.e. which cell types had ATAC-seq peaks) between the human retinal cell types and organoid retinal cell types per genetic variant per category (non-coding RNA and enhancers).
Overlap between candidate non-coding RNAs and enhancers
To find overlap between candidate enhancers, microRNA binding sites and lncRNAs, we used two strategies. We looked for annotation overlap between target genes of the candidate enhancers and microRNA binding sites, and for positional regional overlap (< 0.5 kb distance) between these non-coding elements.
Linking non-coding RNAs and enhancers to refractive error endophenotypes: genetic risk scores in Generation R
To link the genetic impact of non-coding RNAs and enhancers with its clinical phenotype and to replicate our findings in an individual cohort, we constructed GRSs per non-coding RNA/enhancer in a population-based study in children, Generation R. Generation R is a prospective cohort of 9778 pregnant women and their children, who were born between April 2002 and January 2006 in Rotterdam, The Netherlands. The exact methodology of the Generation R Study has been described elsewhere (Kooijman et al. 2017; Kruithof et al. 2014). The study protocol was approved by the Medical Ethical Committee of Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands (MEC 217.595/2002/20), and written informed consent was obtained for all participants. Of the initial cohort, 6690 (68%), 5862 (60%) and 4929 (50%) children visited the research center at the ages of 6, 9, and 13 years, and 7231 children went to at least one visit. Genetic data were available for 4507 of them, and 3638 children had at least one ocular biometric trait available.
AL and corneal curvature (K1 and K2) were measured by Zeiss IOLMaster 500 at 6 and 9 years of age, and by Zeiss IOLMaster 700 at 13 years of age (Carl Zeiss Meditec, Jena, Germany). Corneal Radius (CR) in millimeters was calculated by the following formula: 337.5/(the average of K1 and K2), where 337.5 is the hypothetical refractive index of the cornea. AL/CR ratio was calculated by dividing AL by CR. Axial elongation in mm/year was calculated by subtracting AL of age 6 from age 9 and age 9 from age 13, divided by the age difference between the visits. Automated cycloplegic refractive error measurements were performed at age 13 years. Two drops (three in case of dark irises) of cyclopentolate (1%) within a 5 min interval were dispensed, and refractive error measurements were performed at least 30 min thereafter, when pupil diameter was ≥ 6 mm. RE was calculated as the sum of the full spherical value and half of the cylindrical value.
Genotyping and quality control were performed as described in Medina-Gomez et al. (2015). In summary, blood samples were taken from cord blood at birth or venipuncture at the age of 6 years at the research center. Genotyping was performed with the Illumina HumanHap 610 or 660 Quad Chips (at birth or age of 6 years, respectively). Quality control (QC) procedures were performed using PLINK (Purcell et al. 2007). The following filters were performed: marker call rate < 0.2– < 0.05, MAF ≥ 1%, deviation from Hardy–Weinberg equilibrium with P < 10–6. Additional QC steps included checks for excess heterozygosity, sex mismatch, relatedness and missing data.
For each participant, four GRSs were computed by a summation of the multiplication of beta-coefficient from the meta-analysis and the allele (A1; Allele 1) dosage of the genetic variants for miRNAs, miRNAs binding sites, enhancers and lincRNAs separately:
Linear regression analyses were performed with the four GRSs as determinants on four ocular biometric traits: AL elongation in mm/year between age 6 and 9 (n = 3004) and AL elongation in mm/year between age 9 and 13 (n = 2705), AL/CR ratio at age 13 (n = 3047) and RE at age 13 (n = 2150). All regression models were adjusted for age, sex, and the first ten principal components of the full cohort. Sensitivity analyses were performed in European children only, adjusted for age, sex and the first ten European-specific principal components. We set the significance threshold for these analyses to a false discovery rate adjusted significance threshold (Benjamini & Hochberg 1995). The analyses were performed in R version 3.6.1 (https://cran.r-project.org/) and Rstudio version 1.1.456 (http://www.rstudio.com/).
Ethics statement
All human research was approved by the relevant institutional review boards and conducted according to the Declaration of Helsinki. Detailed ethics statement on the GWAS meta-analysis of CREAM is provided elsewhere (Tedja et al. 2018).
Results
First, we performed genome-wide look-up analyses for genomic variants overlapping miRNAs and their binding sites, enhancers and lncRNAs in our previously published GWAS data (Tedja et al. 2018). The non-coding RNAs and enhancers were derived from publicly available databases (miRNASNP v2 (Gong et al. 2015a), PolymiRTS 3.0 (Bhattacharya et al. 2014), VISTA Enhancer Browser (Visel et al. 2007), FANTOM5 (Lizio et al. 2015) and lncRNASNP2 (Miao et al. 2018)). Results per element are provided below. A full overview of the workflow with results presented in this paper is depicted in Fig. 1.
Genome-wide scans in RE and myopia associated genetic variants
Genetic variants in miRNA genomic regions
We examined the association of 367 SNPs overlapping with miRNA gene loci with RE and myopia. Of these variants, 258 were in pre-miRNA loci, 73 in mature miRNA sequences, and 36 in miRNA seed regions. We found eight significantly associated genetic variants clustering in three miRNA loci (PBonferroni = 0.05/367 = 1.36E-04). The miRNAs known to be transcribed from these loci were miR-3117-3p, miR-4804-5p, miR-499a-3p and miR-499b-5p (Supplementary Table 1.1). These eight genetic variants were located in the seed region of the abovementioned miRNAs. Of all four miRNAs, genetic variants in miR-4804-5p and miR-499b-5p were predicted to have the largest energy changes (rs266435 = −6.5 ΔΔG [kcal/mol] and rs3746444 = 4.3 ΔΔG, respectively), suggesting that the variants may affect miRNA biogenesis and production, in addition to target recognition. None of the genetic variants associated with miRNAs showed evidence of eQTL in the retina (EyeGEx data available on GTEx).
Using TargetScan7.2 (Wang et al. 2021) and MiRDB (Chen and Wang 2020), we found 28 myopia-related genes among the potential targets for these four miRNAs (Supplementary Table 1.2, Supplementary Table 1.3). The closest annotated genes were not ranked as putative target genes. Several genes had high target scores (n = 10, score > 80, score 99: TCF7L2 and NRIP1), suggesting a high probability of interaction of the miRNAs with these genes.
Genetic variants in miRNA-binding site encoding regions
We examined the association of 66,208 SNPs located within 18,678 miRNA-binding site encoding regions with RE and myopia. We found 78 variants significantly associated with refractive error and myopia (PBonferroni = 0.05/66,208 = 7.55E-07). These variants were located in the 3’UTRs of 46 genes, harboring potential binding sites for 348 miRNAs (Supplementary Table 2.2, Supplementary Table 2.3).
Notably, 12 genes harboring significantly associated miRNA binding sites had not been linked to refractive error or myopia by previous GWAS and animal studies. Six of these genes had been linked in GWAS studies to cholesterol levels (Supplementary Table 2.2, ‘cholesterol levels’ in column ‘Gene associated with refractive error’), four to intelligence phenotypes (Supplementary Table 2.2, ‘educational attainment, intelligence or math ability’ in column ‘Gene associated with refractive error’). Of these genes, CILP2 was associated to both cholesterol and intelligence. Interestingly, ZCAN3 and ZCAN9 were linked to sleep quality and duration and MMP24 with suntan and pigmentation (Supplementary Table 2.2, column ‘Gene associated with refractive error’) (Buniello et al. 2019).
We examined expression of the genes hosting the miRNA binding sites in retinal cell type classes from six human eyes (Cowan et al. 2020). These genes showed significantly higher expression than expected by chance (probability 82.6% (38/46) vs 35.7% (20,386/57,183), the latter represents the unconditional probability of gene expression in retina (PMC permutation test = 9.99E−6, n = 100,000 permutations). Moreover, expression of 12 genes harboring miRNA-binding sites were cell type-specific (Fig. 2A). Some of these genes seem to have a clear role in the retina-to-sclera signaling pathway of myopiagenesis, such as GNGT2 and CDHR1 in cone photoreceptors and BMP2, TSPAN10 and RGR in retinal pigment epithelium. While the role of other genes was less clear, such as ALLP in monocytes and NCAN in erythrocytes.
Fig. 2.
Candidate gene expression in peripheral retinal cell types. A Normalized expression of candidate miRNA binding regions of host gene (rows) within retinal cell types (columns). Color heat map on the right of the graph depicts the level of min–max normalized expression per gene across each retinal cell type. The asterisks depict candidate host genes showing cell-type specific expression. Please note that ALKAL2 is an alias for FAM150B. B and C Normalized expression of candidate genes flanking enhancer regions (rows; B VISTA database, C FANTOM5 database) within retinal cell types (columns). The colors depict the level of min–max normalized expression per gene across each retinal cell type, similarly to the color heat map shown in A. The asterisks depict candidate flanking genes showing cell-type specific expression
Genetic variants in enhancer encoding regions
We examined the association of 11,712 SNPs in 1,806 putative enhancers from the VISTA Enhancer Browser and 9253 SNPs in 4601 putative enhancers from the FANTOM5 database with RE and myopia. We found 25 genetic variants significantly associated with RE and myopia (PBonferroni = 0.05/11,712 = 4.27E−06) in 13 putative enhancers from the VISTA Enhancer Browser, that were linked to 16 flanking genes, and 18 genetic variants significantly associated (PBonferroni = 0.05/9,253 = 5.40E−06) in 16 putative enhancers linked to 17 flanking genes from FANTOM5 (Supplementary Table 3 and Supplementary Table 4). None of the associated putative enhancers from the VISTA Enhancer Browser overlapped with the candidates found in FANTOM5.
When comparing expression of these flanking genes to transcriptomes of the human peripheral retina (Cowan et al. 2020), we found retinal expression of 86.7% (13/15) of flanking VISTA Enhancer Browser genes and 82.4% of flanking FANTOM5 genes (14/17). This rate was significantly higher than that expected by chance 35.7% (20,386/57,183) (VISTA Enhancer Browser, PMC permutation = 9.97E−6; FANTOM5, P MC permutation = 2.00E−5; n = 100,000 permutations). The pattern of gene expression across cell types was also evaluated. Of the genes expressed in the peripheral retina, 23.1% (3/13) of genes associated with VISTA Enhancer Browser’s putative enhancers were cell type specific (ADAMTSL1 in fibroblasts i.e. an important player in extracellular matrix synthesis, TEX41 in choroidal melanocytes and RFBOX1 in amacrine cells) and 28.6% (4/14) of genes associated with FANTOM5 putative enhancers (CD34, NR5A2 and FLT1 in vascular endothelium, and BICC1 in fibroblasts) (Fig. 2B,C).
Genetic variants in lncRNA genomic regions
We found 434,611 genetic variants from the GWAS residing in loci for lncRNAs. Of these, 417 variants in 245 lncRNA loci were significantly associated (PBonferroni = 0.05/434,611 = 1.15E−07; Table 1, Supplementary Table 5), including 47 variants that are located in multiple lncRNAs. Seven genetic variants located in lncRNA regions were top SNPs of their associated loci in the GWAS (Table 2). Using the lncRNASNP2 database (Miao et al. 2018), which includes associations of lncRNAs with ocular diseases, we found three variants associated with retinal neovascularization: NONHSAT192268.1 (P = 0.03; rs9726), NONHSAT158292.1 (P = 0.04; rs3998462) and NONHSAT201383.1 (P = 0.048; rs7692381).
Table 1.
Top 15 genetic variants in candidate lncRNA regions associated with refractive error and myopia
| SNP | A1/A2 | Freq A1 | Beta | P-value GWAS | Chr:pos | LncRNA |
|---|---|---|---|---|---|---|
| rs688220 | a/g | 0.440 | −0.057 | 1.80E−58 | 15:34,998,875 | NONHSAT170622.1 |
| rs619788 | a/c | 0.439 | −0.057 | 6.75E−57 | 15:34,995,106 | NONHSAT170622.1 |
| rs10468072 | t/c | 0.433 | −0.056 | 2.00E−56 | 15:34,995,619 | NONHSAT170622.1 |
| rs10468073 | t/c | 0.433 | −0.056 | 2.49E−56 | 15:34,995,634 | NONHSAT170622.1 |
| rs580839 | a/g | 0.438 | −0.053 | 8.94E−50 | 15:34,998,829 | NONHSAT170622.1 |
| rs12437515 | t/c | 0.567 | 0.053 | 4.14E−49 | 15:34,998,734 | NONHSAT170622.1 |
| rs636751 | a/c | 0.637 | 0.051 | 1.33E−44 | 15:35,012,584 | NONHSAT041601.2 |
| rs12675344 | c/g | 0.589 | −0.041 | 6.76E−30 | 8:60,146,268 | NONHSAT215847.1 |
| rs10957083 | a/t | 0.412 | 0.041 | 7.92E−30 | 8:60,147,766 | NONHSAT215847.1 |
| rs10107159 | t/c | 0.412 | 0.041 | 8.82E−30 | 8:60,148,394 | NONHSAT215847.1 |
SNP Single Nucleotide Polymorphism rs-id, A1/A2 Allele1/Allele2, Freq A1 allele frequency of A1, Beta beta of A1, P-value P-value reported in GWAS of refractive error and myopia, Chr:pos chromosome: position (hg19), LncRNA long non-coding RNA ID
Table 2.
Genetic variants associated in candidate lncRNA regions with refractive error and myopia with topSNP signal
| SNP | Chr:pos | Annotated gene | A1/A2 | Freq A1 | Beta | P-value GWAS | LncRNA |
|---|---|---|---|---|---|---|---|
| rs11145465 | 9:71,766,593 | TJP2 | a/c | 0.212 | −0.0844 | 1.35E−21 | NONHSAT131771.2 |
| rs7692381 | 4:81,903,049 | C4orf22,BMP3 | a/g | 0.763 | 0.07805 | 5.55E−21 | NONHSAT201383.1 |
| rs10003846 | 4:81,923,677 | C4orf22,BMP3 | t/g | 0.117 | −0.0982 | 8.64E−18 | NONHSAT201387.1 |
| rs2276560 | 2:233,450,919 | EIF4E2,EFHD1 | t/c | 0.756 | 0.07125 | 9.85E−17 |
NONHSAT077417.2, NONHSAT077418.2 |
| rs2225986 | 1:200,311,910 | LINC00862 | a/t | 0.381 | −0.0579 | 1.68E−15 | NONHSAT152730.1 |
| rs17400325 | 2:178,565,913 | PDE11A | t/c | 0.951 | 0.13181 | 1.07E−14 | NONHSAT075727.2, NONHSAT183166.1 |
| rs7747 | 4:80,827,062 | ANTXR2 | t/c | 0.202 | 0.06385 | 2.05E−12 | NONHSAT097124.2 |
SNP Single Nucleotide Polymorphism rs-id, A1/A2 Allele1/Allele2, Freq A1 allele frequency of A1, Beta beta of A1, P-value P-value reported in GWAS of refractive error and myopia, Chr:pos chromosome: position (hg19), LncRNA long non-coding RNA ID
Overlap between candidate non-coding RNAs and enhancers
We investigated the overlap between associated non-coding RNA genomic regions and enhancers. The three candidate miRNAs did not overlap with the 348 miRNAs linked to the candidate miRNA-binding sites (Supplementary Table 1.1 and Supplementary Table 2.2). Enhancer hs1980 near TCF7L2 (Supplementary Table 3, rs7903146; Supplementary Table 1.3, locus #3,) may potentially share the same target with miRNA 499a-3p, which has this gene as the putative target gene. Figure 3 depicts the overlap between the candidate non-coding RNAs and enhancers. Interestingly, we found two SNPs overlapping an enhancer, lncRNA and miRNA binding site. They are located in an enhancer region flanking DDIT4, in a lncRNA region and in the miRNA binding site of DDIT4. First, rs6480615 is in enhancer region chr10:74,056,833–74058104, in the lncRNA region which transcribes NONHSAT158293.1, and near miRNA binding site SNPs rs8316 and rs1053639 (< 0.5 kb, r2 = 0.48 and 0.39, respectively). Notably, rs1053639 (marked bold in Fig. 3) is in high linkage disequilibrium (LD) with two other enhancer candidates near DDIT4: hs1834 and chr10:74,020,239–74021717, (< 0.5 kb, r2 = 0.77 and 0.81, respectively). Second, rs2394861 is in enhancer region chr10:74,078,612–74078870, in the lncRNA region, which transcribes NONHSAT155637.1, and also near miRNA binding site SNPs rs8316 and rs1053639 (< 0.5 kb, r2 = 0.51 and 0.28, respectively).
Fig. 3.
Venn diagram and corresponding table depicting the overlap between candidate non-coding regulatory elements. A rs6480615 and rs2394861 lie in a lncRNA region, are also in an enhancer region of DDIT4, and are also in the vicinity of the miRNA binding site in DDIT4. B overlap between enhancer regions and miRNA binding sites. Notably, rs1053639 (marked bold, DDIT4) is in high LD with enhancers (rs11000235, rs10762503). C Enhancer regions coinciding in lncRNA regions (the same SNPs are found in both regulatory databases). D MiRNA binding site regions coinciding in LncRNA regions. Note that the hs-enhancers are from VISTA Enhancer Browser and the chr:pos enhancer regions are from FANTOM5. Grey SNPs are not in chromatin accessible regions
We found one SNP, rs1340044, in the overlapping regions of enhancer hs2537 and in lncRNAs NONHSAT130308.2 and NONHSAT220540.1. Five enhancers were found flanking lncRNAs and RNA genes (chr1:199,730,970–199731311, near LINC01221 and NR5A2; chr1:200,325,072–200325315 near LINC00862; hs1241 near LINC00989; hs534 near TEX41 [alias LINC00953], hs1672 near LOC100996251; Supplementary Table 3 and Supplementary Table 4). Furthermore, we found overlap between four SNPs in lncRNA regions and in miRNA-binding sites. Two SNPs (rs17216041 and rs56278919) are in the 3’UTR of HAPLN4 and reside in the lncRNA NONHSAT063038.2. Rs3828329 is located in the 3’UTR of FAM150B (alias ALKAL2) and is also a lncRNA region of NONHSAT183959.1 and NONHSAT183960.1, and rs756997 resides in 3’UTR of MAU2 and is also a lncRNA region of NONHSAT180241.1 and NONHSAT180243.1 (Supplementary Table 2.2 and Supplementary Table 5). Finally, we observed an overlap between miRNA binding sites and enhancer flanking genes. Two SNPs (rs2296283, and rs17086617) reside in the 3’UTR of FLT1 and one SNP, rs8002446, resides in an enhancer flanking FLT1 (Supplementary Table 2.2 and Supplementary Table 4).
Genetic risk scores per element in the Dutch population-based child cohort Generation R Study in association with ocular biometric traits
Myopia development occurs during (early) childhood, with potential comorbidities later in life. Therefore, we tested the associated variants in non-coding RNA genes and candidate enhancers in an independent population-based child cohort (Table 3). We tested the associations of the genomic elements from which the studied non-coding RNAs were transcribed or that represent the enhancers with ocular biometry in the Generation R Study. To do this, we performed linear regressions to examine associations of GRS constructed from miRNAs, miRNA binding sites, lncRNAs and enhancers with AL elongation (mm/yr) between 6 and 9 years, AL elongation between 9–13 years, AL/CR ratio at 13 years, and RE at 13 years. GRS were calculated based on the sum of the product between the effect of the variants and allele dosage per RNA gene category/enhancer.
Table 3.
Associations of genetic risk scores in the Dutch population-based child cohort Generation R Study and ocular biometric traits
| Trait | Beta (95% CI) | P | FDR adjusted P |
|---|---|---|---|
| MiRNAs (Ntested = 8) | |||
| AL 6–9 yrs | −0.0005 (−0.004–0.003) | 0.76 | 0.83 |
| AL 9–13 yrs | −0.0003 (−0.003–0.002) | 0.83 | 0.83 |
| AL/CR 13 yrs | −0.003 (−0.007–0.001) | 0.17 | 0.56 |
| RE 13 yrs | 0.0436 (−0.036–0.123) | 0.28 | 0.56 |
| MiRNA BSs (Ntested = 78) | |||
| AL 6–9 yrs | −0.0004 (−0.004–0.003) | 0.82 | 0.82 |
| AL 9–13 yrs | −0.002 (−0.005–0.001) | 0.16 | 0.52 |
| AL/CR 13 yrs | −0.002 (−0.006–0.002) | 0.28 | 0.52 |
| RE 13 yrs | 0.036 (−0.046–0.117) | 0.39 | 0.52 |
| Enhancers (Ntested = 43) | |||
| AL 6–9 yrs | −0.002 (−0.006–0.001) | 0.13 | 0.13 |
| AL 9–13 yrs | −0.003 (−0.006–−0.001) | 0.01 | 0.02* |
| AL/CR 13 yrs | −0.005 (−0.009–−0.001) | 8.08E−03 | 0.02* |
| RE 13 yrs | 0.085 (0.008–0.162) | 0.03 | 0.04* |
| LincRNAs (Ntested = 245) | |||
| AL 6–9 yrs | −0.0040 (−0.007–−0.001) | 1.80E−02 | 0.024* |
| AL 9–13 yrs | −0.0048 (−0.008–−0.002) | 5.92E−04 | 0.14E−03* |
| AL/CR 13 yrs | −0.007 (−0.011–−0.003) | 7.47E−04 | 0.14E−03* |
| RE 13 yrs | 0.081 (−0.001–0.164) | 0.053 | 0.053 |
Ntested number of genetic variants tested per regulatory element, 95% CI 95% confidence interval, AL 6–9 yrs axial length elongation between 6 and 9 years of age, AL 9–13 yrs axial length elongation between 9 and 13 years of age, AL/CR 13 yrs axial length/corneal radius at 13 years of age, RE 13 yrs refractive error at 13 years of age, P P-value, FDR adjusted P false discovery rate adjusted P-value
The GRS of enhancer candidates was significantly associated with AL elongation between 9 and 13 years, AL/CR at 13 years and RE at 13 years (P < 0.05), but not with AL elongation between 6 and 9 years. The GRS of lncRNAs was significantly associated with AL progression between 6 and 9 years, 9–13 years and AL/CR at 13 years and borderline significant with RE (FDR adjusted P = 0.053). Interestingly, the mean effects of the GRS of enhancer candidates and lncRNAs (see ‘Beta’s’ in Table 3) tended to have a protective effect on myopia development (i.e. a small negative effect on AL elongation and positive effect on SER). The GRSs of miRNA-binding sites was not significantly associated with any biometric trait in the full cohort adjusting for 10 principal components of the full cohort. In the sensitivity analysis comprising children of European ancestry only and adjusted for 10 European-specific principal components, the GRSs of miRNA-binding sites was significantly associated with AL progression between 9 and 13 years, but not anymore after adjusting for FDR. We found no association between the GRS of miRNAs and any of the ocular biometry parameters (Table 3, Supplementary Table 6 and Supplementary Table 7).
Chromatin accessibility of the candidate non-coding RNAs and enhancers
We explored the presence of accessible chromatin regions in the vicinity of the candidate non-coding RNAs and enhancers. We found evidence for this in several candidate miRNAs, enhancers and lncRNAs (Supplementary Table 8–11) using single nucleus assay for transposase-accessible chromatin sequencing (snATAC-seq) across retinal cell types from human donor and retinal organoids(Thomas et al. 2022). We found chromatin accessibility in the regions of two of the total three loci (66%) comprising two of the total four candidate miRNAs, ten of 25 genetic variants in eight candidate VISTA Enhancer Browser’ enhancers (40%), nine of 18 genetic variants in seven candidate FANTOM5 enhancers (50%) and 103 out of 417 genetic variants in 81 candidate lncRNAs (25%). Rs463250, a candidate genetic variant in the seed region of miR-4804-5p, was found accessible in all tested cell types in human retinal cell types as well as in cell types from ocular organoid cultures (Supplementary Table 8). The majority of chromatin accessible region peaks in the candidate enhancers (63% and 78%, 8 and 9 genetic variants in both human retinal cell type and human organoid retinal cell type in VISTA Enhancer Browser and FANTOM5, respectively; Supplementary Table 9) and lncRNAs (55%, 65 genetic variants in both human retinal cell type and human organoid retinal cell type) corresponded in cell type between human donors and organoids (Supplementary Table 12). Interestingly, the accessibility for some candidate enhancers and lncRNAs was similar in only selected cell types in both human and organoid cell types, e.g. for enhancers rs17491167 in cone and bipolar cells, rs2502120 in bipolar cells, and for lncRNAs rs10763552 in ganglion cells, rs7154598 in early retinal progenitor cells and rs12613585 in bipolar cells. Conversely, other regions appeared to have ubiquitous chromatin accessibility (enhancers: rs6480615, rs6480616, rs12753391 and rs2761882; lncRNAs: rs6480615, Supplementary Table 9–12).
Biological plausibility of myopia-associated miRNA binding sites and enhancers
We did not construct biological plausibility scores for miRNAs due to a lack of association of the GRS of the candidate miRNAs with myopia related biometric traits. Moreover, the level of association was relatively low, there were no convincing peaks in the LocusZoom regional plots for these miRNAs (Supplementary Fig. 1) and no known association with myopia or other ocular phenotype. Based on hints of a potential role for miRNA binding sites, albeit only in the sensitivity analysis for AL, we constructed biological plausibility scores genetic variants in miRNA binding sites. We prioritized the genetic variants in candidate miRNA binding sites with potential binding miRNAs with high confidence annotation scores on miRBase (Table 4, Supplementary Table 2.1–2.3). We scored the genetic variants in candidate enhancers located per database (VISTA Enhancer Browser and FANTOM; Supplementary Table 3 and Supplementary Table 4), giving priority to candidate enhancers in chromatin accessible regions.
Table 4.
Ranking of refractive error and myopia-associated genetic variants in miRNA binding sites with high confidence candidate binding miRNAs
| SNP ID | A1/A2 | Freq A1 | Beta A1 | P-value | Genetic variant | Target gene | MiRNA | Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location (chr:pos) | Strength of GWAS association | LD (R2) | eQTL | LD pattern | Target gene | RNA expression in human ocular cell types | Gene associated with refractive error and myopia |
Gene associated with human ocular phenotype |
A1 miRNA | A2 miRNA | miRNA associated with myopia | miRNA associated with human ocular phenotype | ||||||
| rs13642 | a/t | 0.64 | −0.019 | 3.94E−07 | 11:30,432,220 | – | 0.3875 | ✓ | ✓✓ | MPPED2 | ✓ | ✓ | ✓ |
miR-338-5p miR-16–2-3p miR-195-3p |
NA | ✓ miR-338-5p |
✓ mir-16–2 (retinoblastoma) mir-195 (diabetic retinopathy) |
8 |
| rs63539261 | a/g | 0.24 | 0.027 | 4.61E−10 | 4:80,827,798 | ✓ | 1 | ✓ | ✓✓ | ANTXR2 | ✓ | ✓ | ✓ |
miR-1252-3p miR-3613-3p |
miR-186-5p | – | – | 7 |
| rs79024279 | a/c | 0.11 | −0.033 | 2.32E−08 | 4:81,978,151 | – | 0.1779 | – | ✓✓ | BMP3* | ✓ | ✓ | ✓ |
miR-103b miR-376a-3p miR-376b-3p miR-124-5p |
miR-153-3p | ✓ miR-103 | ✓ miR-124 (retinoblastoma) | 7 |
| 4:80,827,797:A_AGC | i/r | 0.72 | −0.027 | 3.38E−11 | 4:80,827,797 | – | 0.81 | ✓ | ✓✓ | ANTXR2 | ✓ | ✓ | ✓ | miR-330-3p | miR-3613-3p | – | – | 6 |
| rs72870298 | a/g | 0.96 | 0.075 | 5.80E−14 | 4:81,978,176 | – | 0.1243 | – | ✓✓ | BMP3* | ✓ | ✓ | ✓ |
miR-216b-5p miR-4778-3p |
miR-877-3p | ✓ miR-216b-5p | – | 6 |
| rs4933980 | a/t | 0.47 | 0.026 | 3.22E−13 | 10:85,979,232 | ✓ | 0.8001 | ✓ | ✓ | CDHR1* | ✓ | ✓ | ✓ | miR-205-5p | NA | – |
✓ mir-205 (glaucoma) mir-4480 (age-related macular degeneration) |
6 |
| rs2296283 | a/g | 0.53 | 0.022 | 1.21E−09 | 13:28,963,702 | – | 0.6942 | – | ✓✓ | FLT1* | ✓ | ✓ | ✓ |
miR-3135b miR-3940-3p |
miR-128–1-5p miR-128–2-5p |
– | ✓ miR 3135b (age-related macular degeneration) | 6 |
| rs6119447 | a/g | 0.32 | 0.023 | 1.34E−09 | 20:32,668,576 | ✓ | 0.9952 | ✓ | ✓ | RALY | ✓ | ✓ | – |
miR-181a-5p miR-181b-5p miR-181c-5p miR-181d-5p |
NA | – |
✓ miR-181a (lens; trabecular meshwork; corneal fibroblasts) mir-181b (retinoblastoma) mir-181c (proliferative diabetic retinopathy) miR-181a (corneal dystrophy) |
6 |
| rs7922058 | a/g | 0.78 | −0.025 | 3.16E−09 | 10:90,034,039 | – | 0.2494 | ✓ | ✓✓ | RNLS | ✓ | ✓ | – | miR-451a | NA | – | ✓ miR-451a (glaucoma, retinal neovascularization) | 6 |
| rs9886724 | a/g | 0.44 | −0.018 | 4.26E−07 | 9:123,665,019 | – | 0.0021 | ✓✓ | ✓ | TRAF1 | ✓ | ✓ | – |
miR-204-3p miR-4778-5p |
miR-193b-5p miR-152-5p miR-6855-5p |
✓ miR-204 | – | 6 |
| rs6585845 | t/g | 0.49 | −0.029 | 7.49E−16 | 10:85,975,246 | ✓ | 0.9881 | ✓ | – | CDHR1* | ✓ | ✓ | ✓ |
miR-197-3p miR-6511a-3p |
NA | – | – | 5 |
| rs1053639 | a/t | 0.28 | −0.029 | 3.52E−07 | 10:74,035,041 | – | 0.5938 | ✓✓ | ✓ | DDIT4 | ✓ | ✓ | – | NA | miR-140-3p | – | – | 5 |
| rs17086617 | t/c | 0.68 | 0.021 | 4.08E−08 | 13:28,962,686 | – | 0.7321 | – | ✓✓ | FLT1* | ✓ | ✓ | ✓ | miR-153-5p | miR-224-5p | – | – | 5 |
| rs56278919 | a/g | 0.84 | 0.027 | 1.26E−08 | 19:19,367,190 | ✓ | 0.8962 | ✓✓ | – | HAPLN4 | - | – | – |
miR-129–2-3p miR-129–1-3p miR-4687-5p |
miR-328-3p miR-663b |
✓ miR-328 |
✓ mir-663b (pterygium) miR-328 (myopia) |
5 |
| rs12514976 | t/c | 0.79 | 0.022 | 2.04E−07 | 5:132,031,972 | – | 0.0027 | ✓✓ | ✓ | KIF3A | ✓ | – | – | miR-138–2-3p | miR-138–1-3p | – | ✓ miR-138-3p (glaucoma) | 5 |
| rs756997 | c/g | 0.83 | 0.028 | 7.47E−09 | 19:19,467,085 | ✓ | 0.9792 | ✓✓ | – | MAU2 | ✓ | – | ✓ | miR-1296-5p | NA | – | – | 5 |
| 10:86,018,493:C_CA | i/r | 0.18 | −0.028 | 8.20E−09 | 10:86,018,493 | – | 0.126 | – | ✓✓ | RGR* | ✓ | ✓ | ✓ |
miR-2110 miR-3614-5p miR-450a-2-3p |
NA | – | – | 5 |
| rs2010506 | t/c | 0.16 | −0.029 | 4.21E−09 | 19:19,387,356 | ✓ | 0.91 | ✓✓ | – | SUGP1 | ✓ | – | – | miR-183-5p | miR-3177-3p | – | ✓ mir-183 (retinal degeneration; keratitis; retinoblastoma; light-induced retinal injury) | 5 |
| rs868 | a/g | 0.81 | 0.026 | 1.62E−08 | 9:101,911,656 | ✓ | 0.9435 | – | ✓ | TGFBR1 | ✓ | ✓ | ✓ | miR-192-5p | NA | – | – | 5 |
| rs2241003 | c/g | 0.63 | 0.019 | 2.40E−07 | 9:123,666,777 | – | 0.0008 | ✓✓ | – | TRAF1 | ✓ | ✓ | – | NA |
miR-330-5p miR-4687-5p miR-2276-5p |
– | ✓ mir-326 (cataract) | 5 |
| rs7206 | a/g | 0.61 | 0.023 | 5.49E−10 | 6:28,201,138 | ✓ | 0.9416 | ✓✓ | – | ZSCAN9 | ✓ | – | – | NA | miR-424-3p | – | ✓ mir-424 (retinal neovascularization) | 5 |
| rs1049109 | t/c | 0.10 | 0.044 | 4.82E−14 | 2:233,247,027 | – | 0.0145 | ✓ | – | ALPP* | ✓ | ✓ | – | NA |
miR-21-5p miR-590-5p |
– | ✓ mir-21 (uveal melanoma; granular corneal dystrophy) | 4 |
| rs2280278 | c/g | 0.28 | −0.029 | 1.46E−13 | 2:157,441,664 | ✓ | 0.9534 | – | – | GPD2 | ✓ | ✓ | – | NA |
miR-128-3p miR-27a-3p miR-27b-3p miR-216a-3p miR-513a-5p |
– |
✓ miR-216a (inherited retinal disease. 28,704,127) miR-27a (age-related macular degeneration in serum) |
4 |
| rs1064395 | a/g | 0.16 | −0.027 | 1.11E−08 | 19:19,361,735 | ✓ | 0.8763 | ✓✓ | – | NCAN | ✓ | – | – |
miR-509–3-5p miR-509-5p |
NA | – | – | 4 |
The biological plausibility score was calculated for each SNP by combining the results of our predefined criteria (an updated strategy of Ghanbari et al.) as stated below
Genetic variant: location (chr:pos): chromosome: position (hg19); strength of GWAS association: top SNP ✓✓(2), SNP > 0.8 LD with top SNP in locus ✓(1), only passed the threshold (0) (GWAS Tedja et al.); LD (r2): linkage desequillibrium with GWAS top SNP in locus; eQTL data: correlation between SNP and expression of the host gene ✓✓(2), correlation of SNPs with expression of the nearby genes in the locus ✓(1), no correlation (0) (Haploreg v4.1); LD pattern: no or just a few synonymous proxies ✓✓ (2), several synonymous proxies, but no non-synonymous ✓ (1), several proxies including nonsynonymous proxy SNPs (0) (Haploreg v4.1)
Target gene: the genetic variant is located in the 3′ UTR of this target gene: genes with a cell-type specific expression have an asterix (*), expression data derived from Cowan et al. 2020, see next column); RNA expression in human ocular cell types: known RNA expression in human ocular cell types (Fig. 2A; Cowan et al. 2020): ✓ (1) or not known (0); gene associated with refractive error and myopia: known association with refractive error and myopia of gene (GWAS catalog): ✓ (1) or not reported (0); gene associated with human ocular phenotype: known association with human ocular phenotype derived from GWAS catalog, OMIM, Pubmed literature search: ✓ (1) or not associated (0)
MiRNA: A1/A2 mirna: potential binding miRNA to A1/A2 allele. Level of convidence of the potential binding miRNA extracted from miRBase: bold = high confidence annotation and high reads mature miRNA sequence and high probability loaded in RISC: cursive = high confidence annotation and one of the following: high reads mature miRNA sequence or high probability loaded in RISC; MiRNA associated with myopia: known expression association with myopia (Tkatchenko AV et al. 2016 and literature search PubMed): ✓(1) or not known (0); MiRNA associated with human ocular phenotype: known association with human ocular phenotype (PubMed literature search, HMDD v3.0): ✓(1) or not associated (0)
score: sum of the 8 criteria stated above
SNP Single Nucleotide Polymorphism rs-id, A1/A2 Allele1/Allele2, with allele1 = effect allele, Freq A1 frequency of allele1 (i.e. effect allele), Beta A1 effect of allele1, P-value GWAS P-value of SNP in GWAS for refractive error and myopia
We used an updated scoring system based on the strategy of Ghanbari et al. (2017a, b), looking at GWAS association characteristics of the genetic variant (strength of the association in GWAS, eQTL pattern and LD pattern), as well as the characteristics of the target/host gene (expression in human ocular tissue, association with an ocular phenotype, association with myopia), miRNA-binding site specific characteristics (association of potential binding miRNA with myopia, association of potential binding miRNA with ocular phenotype), and enhancer specific characteristics (presence of histone marks in number of tissue types, known expression in embryonic mice, evidence of expression in number of ocular tissues).
54 of the 78 genetic variants in miRNA binding sites had highly confident annotated potential binding miRNA binding sites (Table 4). Of these 54, 24 genetic variants in miRNA binding sites in 20 genes had 28 potential binding miRNAs with a high number of experimental mature miRNA sequence reads and a high probability of being loaded in an RNA-induced silencing complex (RISC) (Supplementary Table 2.1). The candidate miRNA binding site in the 3’ UTR of MPPED2 (rs13642) received the highest rank (score = 8pt). Other highly ranked miRNA binding sites were located in the 3’UTR of the genes ANTRX2, BMP3, EFEMP1 and GNGT2. (score = 7pt, latter two in Supplementary Table 2.2).
The highest and second highest ranked genetic variant in a chromatin accessible region from VISTA Enhancer Browser were rs2761882 and rs2855532 in hs2324 with flanking gene BMP4 (score = 8pt and 7pt, respectively; Table 5). The locus of the highest ranked genetic variant is enriched for H3K4me1 and H3K27ac histone marks in six types of tissues. Other high scoring putative enhancers were flanked by genes with known GWAS associations with RE and myopia: RBFOX1 (hs88), VIPR2 (hs1753), HAT1 (hs646). The flanking genes DDIT4 and ANAPC16 (putative enhancer hs1834) are novel associations with RE and myopia.
Table 5.
Ranking of refractive error and myopia-associated genetic variants in candidate putative enhancers (VISTA Enhancer Database) in chromatin accessible regions
| SNP | A1/A2 | Freq A1 | Beta A1 | P-value GWAS | Location (chr: pos) |
VISTA enhancer browser genes | Genetic variant | Gene | Enhancer | Total score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strength of GWAS association | LD | eQTL | LD-pattern (proxies) | RNA expression in human ocular cell types | Gene associated with RE and myopia |
Gene associated with (human) ocular phenotype | Enhancer ID | Histon marks | Expression VISTA enhancer browser | ||||||||
| rs2761882 | t/c | 0.51 | −0.030 | 2.45E−17 | 14:54,420,309 | BMP4 | ✓ | 0.992 | ✓ | ✓✓ | ✓ | ✓ | ✓ | hs2324 | ✓ | – | 8 |
| rs2855532 | a/g | 0.45 | 0.026 | 1.05E−13 | 14:54,419,965 | BMP4 | – | 0.788 | ✓ | ✓✓ | ✓ | ✓ | ✓ | hs2324 | ✓ | – | 7 |
| rs4291918 | c/g | 0.81 | −0.030 | 3.49E−11 | 16:7,419,121 | RBFOX1* | ✓ | 0.854 | – | ✓✓ | ✓ | ✓ | ✓ | hs88 | ✓ | – | 7 |
| rs73169246 | t/c | 0.15 | 0.023 | 2.48E−06 | 7:158,890,593 | VIPR2 | – | 0.004 | ✓ | – | ✓ | ✓ | ✓ | hs1753 | ✓ | ✓ (heart) | 6 |
| rs11000235 | a/g | 0.61 | 0.017 | 1.55E−06 | 10:74,015,776 |
ANAPC16 DDIT4 |
– | 0.533 | ✓✓ | – | ✓ | – | ✓ (DDIT4) | hs1834 | ✓✓ | – | 6 |
| rs62182446 | a/g | 0.18 | 0.034 | 1.49E−13 | 2:172,821,223 | HAT1 | – | 0.660 | ✓ | ✓ | ✓ | ✓ | – | hs646 | ✓ | ✓ (midbrain, forebrain) | 6 |
| rs75192880 | a/g | 0.98 | 0.093 | 2.49E−10 | 7:158,891,125 | VIPR2 | – | 0.746 | – | – | ✓ | ✓ | ✓ | hs1753 | ✓ | ✓ (heart) | 5 |
| rs74356109 | a/g | 0.02 | −0.093 | 2.45E−10 | 7:158,890,890 | VIPR2 | – | 0.746 | – | – | ✓ | ✓ | ✓ | hs1753 | ✓ | ✓ (heart) | 5 |
| rs75958105 | t/c | 0.02 | −0.093 | 2.21E−10 | 7:158,889,485 | VIPR2 | – | 0.746 | – | – | ✓ | ✓ | ✓ | hs1753 | ✓ | ✓ (heart) | 5 |
| rs17491167 | a/g | 0.55 | −0.029 | 3.21E−16 | 2:146,798,739 |
LINC00953 (alias TEX41)* PABPC1P2 |
✓ | 0.948 | – | – | ✓ (LINC00953) | ✓ | ✓ | hs534 | ✓ | – | 5 |
The biological plausibility score was calculated for each SNP by combining the results of our predefined criteria (an updated strategy of Ghanbari et al.) as stated below
Genetic variant: strength of GWAS association: top SNP ✓✓(2), SNP > 0.8 LD with top SNP in locus ✓(1), only passed the threshold (0) (GWAS Tedja et al.); LD (r2): linkage desequillibrium with GWAS top SNP in locus; eQTL data: correlation between SNP and expression of the host gene ✓✓(2), correlation of SNPs with expression of the nearby genes in the locus ✓(1), no correlation (0) (Haploreg v4.1); LD pattern: no or just a few synonymous proxies ✓✓ (2), several synonymous proxies, but no non-synonymous ✓ (1), several proxies including nonsynonymous proxy SNPs (0) (Haploreg v4.1).
Gene: RNA expression in human ocular cell types: known RNA expression in human ocular cell types (Fig. 2A; Cowan et al. 2020): ✓ (1) or not known (0); gene associated with RE and myopia: known association with refractive error and myopia of gene (GWAS catalog): ✓ (1) or not reported (0); gene associated with human ocular phenotype: known association with human ocular phenotype derived from GWAS catalog, OMIM, pubmed literature search: ✓ (1) or not associated (0). Enhancer:
enhancer ID: VISTA enhancer browser ID, ‘hs’ refers to human loci; histon marks: known histone marks (enh_7, H3K4me1 and H3K27ac from Haploreg v4.1): more than 10 cell lines ✓✓(2), 1–10 cell lines ✓(1), none or not available -(0); expression VISTA enhancer browser: evidence for expression of candidate enhancer from in vivo experiments in transgenic embryonic mice (VISTA enhancer browser): yes ✓(1), no—(0); score: sum of the 8 criteria stated above
SNP Single Nucleotide Polymorphism rs-id, A1/A2 Allele1/Allele2, with allele1 = effect Allele, Freq A1 frequency of allele1 (i.e. Effect Allele), Beta A1 beta of A1, P-value GWAS P-value of SNP in GWAS for refractive error and myopia, Location (chr:pos) chromosome: position (hg19), VISTA Enhance Browser Genes flanking genes derived from VISTA enhancer browser, genes with a cell-type specific expression have an asterix (*), expression data derived from Cowan et al. 2020, see column ‘RNA expression in human ocular cell types’)
The highest ranked genetic variant in a putative enhancer from FANTOM5 was rs9415066 (enhancer in chr10:74,091,661–74092150) with flanking gene DNAJB12 (FANTOM5, score = 11pt, Table 6). This genetic variant has a high LD (r2 = 0.86) with a top SNP from the GWAS for RE and myopia. The five highest scoring variants were all linked to flanking gene DNAJB12 and three of these also linked to flanking gene DDIT4. They are associated with three candidate enhancers in chr10:74,091,661–74092150, chr10:74,056,833–74058104 and chr10:74,078,612–74078870. Notably, rs11000235 (VISTA Enhancer Browser analysis), close to ANAPC16 and DDIT4, and rs10762503 (FANTOM5 analysis) with similar flanking genes, are in high LD (r2 = 0.94). Another high-ranking variant in a putative enhancer was in chr11:131,995,649–131,996,509 annotated to flanking gene NTM.
Table 6.
Ranking of refractive error and myopia-associated genetic variants in candidate putative enhancers (FANTOM5) in chromatin accessible regions
| MarkerName | A1/A2 | Freq A1 | Beta A1 | P-value | Location (chr: pos) |
EnhancerID | Closest genes | Genetic variant | Gene | Enhancer | Total score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strength of GWAS association | LD (R2) | eQTL score | LD pattern | RNA expression in human ocular cell types | Gene associated with ocular phenotype | Gene associated with RE or myopia | Histon marks | Expression evidence | |||||||||
| rs9415066 | t/c | 0.394 | −0.019 | 1.34E−07 | 10:74,091,924 | chr10:74,091,661–74092150 | DNAJB12 | ✓ | 0.8631 | ✓✓ | ✓✓ | ✓ | – | ✓ | ✓✓ | ✓✓ | 11 |
| rs6480616 | t/c | 0.369 | −0.020 | 9.67E−08 | 10:74,057,844 | chr10:74,056,833–74058104 | DDIT4;DNAJB12 | – | 0.5961 | ✓✓ | ✓ | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | 10 |
| rs6480615 | a/c | 0.370 | −0.020 | 6.38E−08 | 10:74,057,642 | chr10:74,056,833–74058104 | DDIT4;DNAJB12 | – | 0.5967 | ✓✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓✓ | 9 |
| rs9416016 | t/c | 0.392 | −0.019 | 1.14E−07 | 10:74,091,950 | chr10:74,091,661–74092150 | DNAJB12 | – | 0.786 | ✓✓ | ✓ | ✓ | – | ✓ | ✓✓ | ✓✓ | 9 |
| rs2394861 | t/c | 0.383 | −0.020 | 6.31E−08 | 10:74,078,627 | chr10:74,078,612–74078870 | DDIT4;DNAJB12 | – | 0.627 | ✓✓ | ✓ | ✓ | ✓ | ✓ | ✓✓ | – | 8 |
| rs59238664 | a/g | 0.208 | 0.020 | 4.33E−06 | 11:131,995,914 | chr11:131,995,649–131,996,509 | NTM | – | 0.108 | – | ✓✓ | ✓ | ✓ | ✓ | ✓✓ | ✓ | 8 |
| rs655198 | a/g | 0.441 | 0.017 | 2.57E−06 | 1:42,326,017 | chr1:42,325,828–42,326,208 | HIVEP3 | – | 0.5302 | – | ✓✓ | ✓ | – | ✓ | ✓ | – | 5 |
| rs79535664 | t/c | 0.129 | −0.037 | 6.81E−12 | 10:85,937,758 | chr10:85,937,530–85937952 | C10orf99 | – | 0.06 | – | – | – | ✓ | – | ✓✓ | – | 5 |
| rs2502120 | a/g | 0.173 | −0.021 | 5.08E−06 | 1:199,731,106 | chr1:199,730,970–199731311 | LINC01221;NR5A2* | – | 0.001 | - | ✓ | ✓ | – | – | ✓ | ✓ | 4 |
The biological plausibility score was calculated for each SNP by combining the results of our predefined criteria (an updated strategy of Ghanbari et al.) as stated below
Genetic variant: strength of GWAS association: top SNP ✓✓(2), SNP > 0.8 LD with top SNP in locus ✓(1), only passed the threshold (0) (GWAS Tedja et al.); LD (r2): linkage desequillibrium with GWAS top SNP in locus; eQTL data: correlation between SNP and expression of the host gene ✓✓(2), correlation of SNPs with expression of the nearby genes in the locus ✓(1), no correlation (0) (Haploreg v4.1); LD pattern No or just a few synonymous proxies ✓✓ (2), several synonymous proxies, but no non-synonymous ✓ (1), several proxies including nonsynonymous proxy SNPs (0) (Haploreg v4.1)
Gene: RNA expression in human ocular cell types: RNA expression in human ocular cell types (Fig. 2B; Cowan et al. 2020): ✓(1) or not known-(0); gene associated with RE and myopia: known association with refractive error and myopia of gene (GWAS catalog): ✓ (1) or not reported (0); gene associated with human ocular phenotype: known association with human ocular phenotype derived from GWAS catalog, OMIM, pubmed literature search: ✓ (1) or not associated (0)
Enhancer: histon marks: known histone marks (enh_7, H3K4me1 and H3K27ac from Haploreg v4.1): more than 10 cell lines ✓✓(2), 1–10 cell lines ✓(1), none or not available -(0); expression evidence: evidence for expression of candidate enhancer in human ocular tissue: > 10 cell lines ✓✓(2), 6–10 cell lines ✓(1), 0–5 cell lines—(0) (FANTOM5)
score: sum of the 8 criteria stated above
SNP Single Nucleotide Polymorphism rs-id, A1/A2 Allele1/Allele2, with Allele1 = effect allele, Freq A1 frequency of allele1 (i.e. effect allele), Beta A1 beta of A1, P-value GWAS P-value of SNP in GWAS for refractive error and myopia, Location (chr:pos) chromosome: position (hg19), Enhancer ID enhancer ID from FANTOM5, chromosome:position start–end of region (hg19), Closest Gene closest annotated gene (wANNOVAR), genes with a cell-type specific expression have an asterix (*), expression data derived from Cowan et al. 2020, see column ‘RNA expression in human ocular cell types’)
Discussion
The polygenicity, the developmental trait, and the recent insights into the regulome (Trerotola et al. 2015) compelled us to look further than genes to gain insights into the pathogenesis of RE and myopia. We therefore investigated non-coding RNAs and enhancers in RE and myopia on a genome-wide scale (Ghanbari et al. 2017a, b). We found numerous candidate genetic variants with high confidence residing in non-coding RNAs/enhancers associated with RE and myopia: miRNAs (n = 2 variants in 2 miRNAs), miRNA binding sites (n = 54 variants in 36 miRNA binding sites), putative enhancers (n = 19 variants in 14 enhancer regions) and lncRNAs (n = 103 variants in 81 lncRNA regions). We found a clear overrepresentation of expression of the target and host genes of these non-coding RNAs and enhancers in human retinal cell types.
Our GRS analyses showed the strongest association with lncRNAs. They were significantly associated with all AL traits, but borderline significantly with RE. As aforementioned, lncRNAs are thought to have various regulatory functions in the cell, ranging from gene regulation to subcellular organization. Associations of lncRNA with health outcomes are an emerging field. Only one previous study reported an association between these elements and myopia; experiments in guinea pigs revealed that over 300 lncRNAs were differentially expressed between the form-deprivation myopic group and controls, and another 247 lncRNAs were differentially expressed between the lens-induced myopic group and controls (Ulitsky and Bartel 2013).
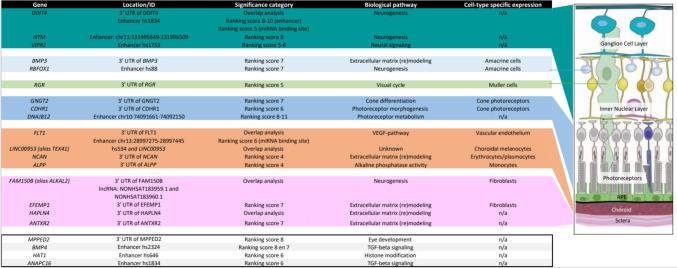
Regarding putative enhancers, evidence for a role for these putative enhancers as a group was provided by the significant association with AL/CR, AL progression and RE in the children. Highly ranked individual candidate variants in enhancers in chromatin accessible regions were associated with eye development (BMP4), neurogenesis (DDIT4, NTM), photoreceptor metabolism (DNAJB12), neural signaling (VIPR2) and TGF-beta signaling (ANAPC16), and all very plausible mechanisms in the retina-to-sclera signaling cascade (Fig. 4).
Fig. 4.
Most notable candidates and their potential corresponding ocular tissue of action. This figure depicts an overview of candidates with the highest ranking (miRNA binding sites and enhancers), notable biological pathways, and cell-type specific expression in human ocular tissue. The location of potential activity of these candidates in the eye is depicted on the right. Of some of the high ranking candidates, the location of potential activity it is still unknown. Note that the hs-enhancers are from VISTA Enhancer Browser and the chr:pos enhancer regions are from FANTOM5. n/a not applicable (no cell-type specific expression found)
BMP4, bone morphogenetic protein 4, is a secreted ligand that binds various TGF-beta receptors, regulating gene expression (Stelzer et al. 2016). Mutations in this gene are associated with microphthalmia, sclerocornea, anterior segment anomalies (i.e. Axenfeld-Rieger malformation and Peter’s anomaly) and, notably, Stickler syndrome which characteristically is associated with high myopia and an increased risk of retinal detachment (Reis et al. 2011; Nixon et al. 2019). It was recently postulated that BMP4 disrupts the barrier integrity of retinal pigment epithelium in light of age-related macular degeneration (Ibrahim et al. 2020). One could postulate and extrapolate this to a role in myopic macular degeneration. NTM, neurotrimin, is a member of a family of immunoglobulin domain-containing glycosyl-phosphatidylinositol (GPI)-anchored cell adhesion molecules (Stelzer et al. 2016). It is known to regulate neurite outgrowth (Gil et al. 1998). In Drosophila, it is shown to regulate photoreceptor axon targeting during development (Oliva et al. 2015). A GWAS association was found between genetic variants annotated to NTM and primary open-angle glaucoma (Ulmer et al. 2012). Murine spatiotemporal expression analyses found NTM to be expressed in the ganglion cell layer, inner nuclear layer and neuroblastic cell layer at E16 (Fearnley et al. 2021). DNAJB12, DnaJ heat shock protein family (Hsp40) member B12, regulates molecular chaperone activity by stimulating ATPase activity (Stelzer et al. 2016). It is involved in the rhodopsin endoplasmic reticulum-associated degradation pathway. Anomalies in this pathway could lead to a lack of degradation of misfolded proteins, which might lead to retinitis pigmentosa (Kennedy et al. 2022). VIPR2, vasoactive intestinal peptide receptor 2, is a small neuropeptide. These Gs-protein-coupled receptors are widely distributed throughout the central nervous system (Stelzer et al. 2016). A form deprivation myopia induction experiment in chicks showed up-regulation of VIPR2 in the retina and choroid in the myopic eyes (Liu et al. 2005). In a case–control study in a Chinese population, genotype imputation and SNP-analysis identified two independent groups of VIPR2 variants associated with high myopia: one group of high-risk SNPs and another group of protective SNPs (Leung et al. 2019). ANAPC16, Anaphase Promoting Complex Subunit 16, is a key regulator in the TGF-beta signaling cascade for lens differentiation (Stelzer et al. 2016; Wu et al. 2007).
As a group, the GRS of miRNA binding sites was not associated with any of the ocular biometric traits. The effects were comparable in both groups (all ethnicities and European only). The association between the GRS of miRNA binding sites and AL progression between 9 and 12 years showed near significance (nominal significance but not anymore after FDR correction), which warrants replication of the associations with GRS and the individual risk variants.
The highest-ranking miRNA binding sites were associated with eye development (MPPED2), extracellular matrix (ANTXR2, BMP3) and photoreceptor morphogenesis (CHDR1). MPPED2 encodes a metallophosphoesterase and has been associated with aniridia through downstream transcriptional effects on PAX6, a master regulator for eye development and an established risk gene for extreme myopia (Stelzer et al. 2016; Balay et al. 2016; Tang et al. 2014; Smirnov et al. 2019). Notably, the 3’UTR sequence with A-allele variant of rs13642 is predicted to be a binding site of three miRNAs: miR-16–2-3p, miR-195-3p and miR-338-5p. These miRNAs are known to be associated with retinoblastoma (Ofir et al. 2011), diabetic retinopathy (Mortuza et al. 2014), and myopia (Tkatchenko et al. 2016; Liu et al. 2022), respectively. MiR-338-5p showed higher expression of the miRNA in myopic murine models compared to controls. ANTXR2, ANTXR cell adhesion molecule 2, is a transmembrane surface protein in collagen IV, VI and laminin, suggesting a role in extracellular matrix adhesion (Stelzer et al. 2016; Bürgi et al. 2017). Variants in this gene cause hyaline fibromatosis syndrome (Dowling et al. 2003). BMP3, Bone Morphogenetic Protein 3, is a secreted ligand of the TGF-beta superfamily of proteins. In relation to refractive error and myopia, it has been associated with retinal detachment in a recent GWAS (Boutin et al. 2020). CHDR1, cadherin related family member 1 (alias RP65), belongs to the cadherin superfamily of calcium-dependent cell adhesion molecules. This protein is a photoreceptor specific cadherin that plays a role in outer segment disc morphogenesis. Mutations in this gene are associated with inherited retinal dystrophies (Stelzer et al. 2016). Retinal dystrophies associated with CDHR1 are clinically heterogeneous (Malechka et al. 2022).
Other noteworthy binding sites were in the 3’UTR of EFEMP1 and GNGT2. EFEMP1, Epidermal-like growth factor containing fibulin extracellular matrix protein 1 (alias FBN3), plays a role in the structural support of tissues (Stelzer et al. 2016). It has been linked to Doyne honeycomb retinal dystrophy and several families with EFEMP1 mutations present with Marfan-like connective tissue disorders and high myopia (Doyne 1899; Stone et al. 1999; Driver et al. 2020; Bizzari et al. 2020; Timpl et al. 2003). Interestingly, a mutation in this gene in murine models showed amplified responses to light in melanopsin-expressing intrinsically photosensitive retinal ganglion cells, resulting in photophobia and circadian rhythm alterations (Thompson et al. 2019). GNGT2, G protein subunit gamma transducin 2, is associated with cone differentiation and phototransduction (Stelzer et al. 2016; Ong et al. 1997). It carries a functional role during embryogenesis in vision and circadian rhythmicity, two enriched pathways associated with myopiagenesis (Lagman et al. 2015; Rodgers et al. 2016). Reports from others on miRNA binding sites in myopia are scarce.
Previously published miRNAs with differential expression were found in our analysis as potential binding miRNAs on associated miRNA binding sites. Two binding sites in BMP3 (miR-103 resulting in higher expression in human fetal sclera compared to adult sclera (Metlapally et al. 2013), and mir-216b-5p resulting in lower expression in myopic murine eyes compared to controls (Tkatchenko et al. 2016), one binding site in MPPED2 (miR-338-5p resulting in higher expression in myopic murine eyes compared to controls) (Tkatchenko et al. 2016) and one binding site in TRAF1 (lower miR-204-5p expression in high myopes compared to controls(Jiang et al. 2024). Up to now, a role of miR-29a and the binding site of miR-328 within 3’ UTR of PAX6 was suggested for extreme myopia (Kennedy et al. 2022; Liu et al. 2005), but our study failed to replicate this finding. However, we indirectly found rs56278919 in the miRNA binding site analysis (ranking score 5), which resides in the 3’UTR of HAPLN4 with miR-318-3p as potential binding miRNA. This gene, Hyaluronan And Proteoglycan Link Protein 4, is predicted to be active in extracellular matrix (Alliance of Genome Resources Consortium 2024).
We observed an intriguing overlap in position of some associated non-coding RNAs/enhancers, implying that these locations are regulatory ‘hotspots’ for myopia. Particularly noteworthy is the overlap of the enhancer region, miRNA binding site, and lncRNA region near protein coding gene DDIT4. DDIT4, DNA damage inducible transcript 4 (alias REDD-1 and RTP801) is an inhibitor in the mTOR pathway and plays a role in neuronal differentiation, migration, and neuronal cell death. Its axon regenerative effects have been investigated in rats using intravitreal injections of small-interfering RNA of RTP801, knocking down its inhibitory effect. In this animal model, it promoted retinal ganglion cell survival after optic nerve head lesion (Morgan-Warren et al. 2016). Furthermore, it is a key regulator of diabetes-induced VEGF synthesis by Müller cells. Elevated expression of this gene in Müller cells is postulated to induce diabetic retinopathy by inducing resistance to insulin action (Stelzer et al. 2016; Moore et al. 2016; Dennis et al. 2015; Miller et al. 2020). It additionally has recently been studied as a potential therapeutic target for downregulation to reduce premature senescence of the RPE in regards to age-related macular degeneration (Chen et al. 2022). Please note that the LD between the genetic variants in the overlap analysis was low, except for rs1053639 which is in a chromatin accessible region. The role of this gene in myopiagenesis is to be elucidated.
Strengths of our study include an unbiased genome-wide overview of candidate non-coding RNAs and enhancers in relation to myopia, and relying on a multi-omics approach by taking into consideration transcriptomic (RNA-sequencing per retinal cell type) and epigenomic features (chromatin accessibility). The overview of highly confident miRNA binding sites and chromatin accessibility per cell-type for each candidate can help in further prioritizing non-coding RNAs/enhancers and cell types for future in-depth functional experiments. Lastly, the confirmation of the association of lncRNAs and enhancers in a large independent population-based child cohort underlines the importance of these elements in childhood myopia.
Limitations of our study include the assumption that the nearest gene of an enhancer is its target, a choice that was based on the lack of availability of cell-specific enhancer-target interaction databases. Additional computational and experimental approaches are needed to identify the exact target gene(s). Another important issue in our in silico analysis of enhancers is the small number of databases comprising ocular tissue expression. VISTA Enhancer Browser was based on transgenic reporter assays in embryonic mice, while FANTOM5 used postmortem tissues and primary cells from humans. The spatiotemporal differences may explain the lack of commonality in findings from both databases. Another drawback is the prioritization of genes based on previously published data, which may harbor selection bias.
Translational and functional experiments are imperative to further elucidate the role of non-coding RNAs and enhancers in genetically complex diseases such as myopia. Selection of candidates for further study should be done assiduously and can be challenging for refractive error and myopia due to the numerous genetic risk variants, the small effect sizes, and the abundance of potential pathways. Apart from the obvious functional proof-of-principle experiments needed to validate these non-coding RNAs/enhancers in retinal cell types in human donors, human organoids or animal models, future research should focus on obtaining larger high throughput RNA expression libraries (for instance utilizing massively multiplexed droplet RNA sequencing) (Gehring et al. 2020) per retinal cell-type (Cowan et al. 2020; Thomas et al. 2022; Lukowski et al. 2019; Yan et al. 2020) and other ocular structures such as choroid and sclera. As some studies have been investigating the aging of retinal cell types, regarding myopia pathogenesis, one should be aware of the possibility of spatiotemporal differences in these (RNA) expression experiments, taking into account its developmental time-point in life (Huang et al. 2023; Wahle et al. 2023; Schumacker et al. 2020). For lncRNAs, sequencing technologies such as long-read sequencing are emerging, but remain challenging (Chiquitto et al. 2022; Wang et al. 2024). Validation of miRNAs can be performed by luciferase transporter assays and their effects can be analyzed by performing CRISPR/Cas in organoid (Geurts and Clevers 2023) or animal models or by blocking miRNAs using morpholinos in animal models (Flynt et al. 2017). Differential expression experiments should be performed to investigate the effects of miRNAs, miRNA binding sites, enhancers and lncRNAs. The complexity of the multiple functions of lncRNAs, both cis and trans, will grasp the future scientific field for at least the next decade. Initially, one might want to start with proper identification of lncRNAs and their cis-regulatory effects by looking for nearby transcription factors and H3K4me3 histone marks in the appropriate tissue (i.e. retinal cell types, choroid and sclera) (Ferrer and Dimitrova 2024).
Other regulatory elements, such as silencers and insulators, and their association to RE and myopia should be investigated similarly. Experiments focusing on methylation of non-coding RNAs can provide more insight on pathologic pathways in myopiagenesis (Swierkowska et al. 2022).
In conclusion, our study highlights the importance of non-coding RNAs and enhancers for the development of RE and myopia. We provide a confident framework for future functional validation by prioritizing candidate miRNA binding sites and candidate enhancers on a genome-wide scale. A particularly important role appears to be present for enhancers and lncRNAs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Acknowledgements regarding the previously published GWAS on RE and myopia are published elsewhere. We thank Roxanna Haak for her bioinformatic support regarding the VISTA Enhancer Browser. The Generation R study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam, and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR-MDC), Rotterdam. We gratefully acknowledge the contribution of children and parents, general practitioners, hospitals, midwives and pharmacies in Rotterdam. The authors were supported by the following foundations: Netherlands Organization for Scientific Research (NWO; grant 91617076 to V.J.M.V. and grant 91815655 to C.C.W.K.), European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant 648268 and grant 101098324 to C.C.W.K.) and the EMBO Short-Term Fellowship (grant no. 8529 to J.S.J.). The funding organisations had no role in the design or conduct of this research. They provided unrestricted grants. None of the authors have financial disclosures that relate to this manuscript.
Author contributions
M.S.T. performed the main association and ranking analyses with contributions of J.S.J.. M.S.T. and C.C.W.K. drafted the manuscript, and J.S.J., C.E., P.G.H., J.F.F., C.S.C., M.A.M.S., M.G., P.J.v.d.S., S.J.E., T.S.B. and V.J.M.V. critically reviewed the manuscript. M.S.T. and C.A.E. performed data analysis for the genetic risk scores in Generation R; CREAM contributed to GWAS data. T.S.B., P.J.v.d.S consulted M.S.T. on the analyses of the enhancers. S.J.E., M.A.M.S. and M.G. consulted M.S.T. on the analyses of the miRNA and miRNA binding sites. T.J.C. and C.S.C. provided retinal cell expression data, C.S.C. and M.S.T. analyzed the expression experiments, M.A.M.S. contributed to the illustrations. M.S.T., C.C.W.K. and V.J.M.V. conceived and designed the outline of the current report.
Funding
EMBO Short-Term Fellowship, 8529, Netherlands Organization for Scientific Research (NWO), 91815655, 91617076, European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme, 648268
Data availability
All findings on the associations of RNA genomic regions and enhancers are published in this paper and available in the (supplementary) tables. The top SNP summary statistics of the GWAS meta-analysis on RE and myopia have been published elsewhere. Further GWAS summary statistics are be available upon request. Please contact c.c.w.klaver@erasmusmc.nl (CREAM) for more information and to access the data.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agarwal V, Bell GW, Nam J-W, Bartel DP (2015) Predicting effective microRNA target sites in mammalian mRNAs. Elife. 10.7554/eLife.05005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliance of Genome Resources Consortium (2024) Updates to the alliance of genome resources central infrastructure. Genetics. 10.1093/genetics/iyae049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson R, Gebhard C, Miguel-Escalada I, Hoof I, Bornholdt J, Boyd M et al (2014) An atlas of active enhancers across human cell types and tissues. Nature 507:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, 1000 Genomes Project Consortium et al (2015) A global reference for human genetic variation. Nature 526:68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balay L, Totten E, Okada L, Zell S, Ticho B, Israel J et al (2016) A familial pericentric inversion of chromosome 11 associated with a microdeletion of 163 kb and microduplication of 288 kb at 11p13 and 11q22.3 without aniridia or eye anomalies. Am J Med Genet A 170A:202–209 [DOI] [PubMed] [Google Scholar]
- Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Ziebarth JD, Cui Y (2014) PolymiRTS database 3.0: linking polymorphisms in microRNAs and their target sites with human diseases and biological pathways. Nucleic Acids Res 42:D86-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzari S, El-Bazzal L, Nair P, Younan A, Stora S, Mehawej C et al (2020) Recessive marfanoid syndrome with herniation associated with a homozygous mutation in fibulin-3. Eur J Med Genet 63:103869 [DOI] [PubMed] [Google Scholar]
- Boutin TS, Charteris DG, Chandra A, Campbell S, Hayward C, Campbell A et al (2020) Insights into the genetic basis of retinal detachment. Hum Mol Genet 29:689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullimore MA, Ritchey ER, Shah S, Leveziel N, Bourne RRA, Flitcroft DI (2021) The risks and benefits of myopia control. Ophthalmology. 10.1016/j.ophtha.2021.04.032 [DOI] [PubMed] [Google Scholar]
- Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C et al (2019) The NHGRI-EBI GWAS catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res 47:D1005–D1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürgi J, Kunz B, Abrami L, Deuquet J, Piersigilli A, Scholl-Bürgi S et al (2017) CMG2/ANTXR2 regulates extracellular collagen VI which accumulates in hyaline fibromatosis syndrome. Nat Commun 8:15861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang X (2020) miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res 48:D127–D131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Xin G, Li S, Dong Y, Yu X, Wan C et al (2022) Berberine-mediated REDD1 down-regulation ameliorates senescence of retinal pigment epithelium by interrupting the ROS-DDR positive feedback loop. Phytomedicine 104:154181 [DOI] [PubMed] [Google Scholar]
- Chiquitto AG, Silva LOL, Oliveira LS, Domingues DS, Paschoal AR (2022) Impact of sequencing technologies on long non-coding RNA computational identification. 10.1101/2022.04.15.488462
- Cowan CS, Renner M, De Gennaro M, Gross-Scherf B, Goldblum D, Hou Y et al (2020) Cell types of the human retina and its organoids at single-cell resolution. Cell 182:1623–40.e34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis MD, Kimball SR, Fort PE, Jefferson LS (2015) Regulated in development and DNA damage 1 is necessary for hyperglycemia-induced vascular endothelial growth factor expression in the retina of diabetic rodents. J Biol Chem 290:3865–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien T, Guigó R, Johnson R (2012) The long non-coding RNAs: a new (P)layer in the “dark matter.” Front Genet. 10.3389/fgene.2011.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling O, Difeo A, Ramirez MC, Tukel T, Narla G, Bonafe L et al (2003) Mutations in capillary morphogenesis gene-2 result in the allelic disorders juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet 73:957–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyne RW (1899) Peculiar condition of choroiditis occurring in several members of the same family. Trans Ophthalmol Soc UK 19:71 [Google Scholar]
- Driver SGW, Jackson MR, Richter K, Tomlinson P, Brockway B, Halliday BJ et al (2020) Biallelic variants in EFEMP1 in a man with a pronounced connective tissue phenotype. Eur J Hum Genet 28:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley S, Raja R, Cloutier J-F (2021) Spatiotemporal expression of IgLON family members in the developing mouse nervous system. Sci Rep 11:19536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer J, Dimitrova N (2024) Transcription regulation by long non-coding RNAs: mechanisms and disease relevance. Nat Rev Mol Cell Biol 25:396–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynt AS, Rao M, Patton JG (2017) Blocking zebrafish microRNAs with morpholinos. Methods Mol Biol 1565:59–78 [DOI] [PubMed] [Google Scholar]
- Forrest ARR, Kawaji H, Rehli M, Baillie JK, de Hoon MJL, FANTOM Consortium and the RIKEN PMI and CLST (DGT) et al (2014) A promoter-level mammalian expression atlas. Nature 507:462–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperini M, Tome JM, Shendure J (2020) Towards a comprehensive catalogue of validated and target-linked human enhancers. Nat Rev Genet 21:292–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring J, Hwee Park J, Chen S, Thomson M, Pachter L (2020) Highly multiplexed single-cell RNA-seq by DNA oligonucleotide tagging of cellular proteins. Nat Biotechnol 38:35–38 [DOI] [PubMed] [Google Scholar]
- Geurts MH, Clevers H (2023) CRISPR engineering in organoids for gene repair and disease modelling. Nat Rev Bioeng 1:32–45 [Google Scholar]
- Ghanbari M, Iglesias AI, Springelkamp H, van Duijn CM, Ikram MA, Dehghan A et al (2017a) A genome-wide scan for microRNA-related genetic variants associated with primary open-angle glaucoma. Invest Ophthalmol vis Sci 58:5368–5377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari M, Erkeland SJ, Xu L, Colijn JM, Franco OH, Dehghan A et al (2017b) Genetic variants in microRNAs and their binding sites within gene 3’UTRs associate with susceptibility to age-related macular degeneration. Hum Mutat 38:827–838 [DOI] [PubMed] [Google Scholar]
- Gil OD, Zanazzi G, Struyk AF, Salzer JL (1998) Neurotrimin mediates bifunctional effects on neurite outgrowth via homophilic and heterophilic interactions. J Neurosci 18:9312–9325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Liu C, Liu W, Wu Y, Ma Z, Chen H et al (2015a) An update of miRNASNP database for better SNP selection by GWAS data, miRNA expression and online tools. Database 2015:bav029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Liu W, Zhang J, Miao X, Guo A-Y (2015b) lncRNASNP: a database of SNPs in lncRNAs and their potential functions in human and mouse. Nucleic Acids Res. 10.1093/nar/gku1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P et al (2016) Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 123:1036–1042 [DOI] [PubMed] [Google Scholar]
- Huang Z, Shi J, Gao Y, Cui C, Zhang S, Li J et al (2019) HMDD v3.0: a database for experimentally supported human microRNA-disease associations. Nucleic Acids Res 47:D1013–D1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Li R, Ye L, Zhang S, Tian H, Du M et al (2023) Deep Sc-RNA sequencing decoding the molecular dynamic architecture of the human retina. Sci China Life Sci 66:496–515 [DOI] [PubMed] [Google Scholar]
- Ibrahim AS, Hussein K, Wang F, Wan M, Saad N, Essa M et al (2020) Bone morphogenetic protein (BMP)4 but not BMP2 disrupts the barrier integrity of retinal pigment epithelia and induces their migration: a potential role in neovascular age-related macular degeneration. J Clin Med Res. 10.3390/jcm9072293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Hong N, Guo D, Shen J, Qian X, Dong F (2024) MiR-204-5p may regulate oxidative stress in myopia. Sci Rep 14:9770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karali M, Persico M, Mutarelli M, Carissimo A, Pizzo M, Singh Marwah V et al (2016) High-resolution analysis of the human retina miRNome reveals isomiR variations and novel microRNAs. Nucleic Acids Res 44:1525–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A, Ren HY, Madden VJ, Cyr DM (2022) Lysosome docking to WIPI1 rings and ER-connected phagophores occurs during DNAJB12- and GABARAP-dependent selective autophagy of misfolded P23H-rhodopsin. Mol Biol Cell 33:ar84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IJzendoorn MH et al (2016) The Generation R Study: design and cohort update 2017. Eur J Epidemiol 31:1243–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara A, Birgaoanu M, Griffiths-Jones S (2019) miRBase: from microRNA sequences to function. Nucleic Acids Res 47:D155–D162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof CJ, Kooijman MN, van Duijn CM, Franco OH, de Jongste JC, Klaver CCW et al (2014) The Generation R Study: biobank update 2015. Eur J Epidemiol 29:911–927 [DOI] [PubMed] [Google Scholar]
- Lagman D, Callado-Pérez A, Franzén IE, Larhammar D, Abalo XM (2015) Transducin duplicates in the zebrafish retina and pineal complex: differential specialisation after the teleost tetraploidisation. PLoS ONE 10:e0121330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KH, Luo S, Kwarteng R, Chen S-G, Yap MKH, Huang C-L et al (2019) The myopia susceptibility locus vasoactive intestinal peptide receptor 2 (VIPR2) contains variants with opposite effects. Sci Rep 9:18165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Yap MKH, Leung KH, Kao PYP, Liu LQ, Yip SP (2017) Genetic association study of KCNQ5 polymorphisms with high myopia. Biomed Res Int 2017:3024156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S-Z, Wang H, Jiang J-J, Wang P-B, Wu X-Y, Tan X-P et al (2005) Dynamic expression of VIPR2 in form deprivation myopia. Zhong Nan Da Xue Xue Bao Yi Xue Ban 30:456–459 [PubMed] [Google Scholar]
- Liu S, Chen H, Ma W, Zhong Y, Liang Y, Gu L et al (2022) Non-coding RNAs and related molecules associated with form-deprivation myopia in mice. J Cell Mol Med 26:186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizio M, Harshbarger J, Shimoji H, Severin J, Kasukawa T, Sahin S et al (2015) Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol 16:22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Shi B, Wang J, Cao Q, Cui Q (2010) TAM: a method for enrichment and depletion analysis of a microRNA category in a list of microRNAs. BMC Bioinform. 10.1186/1471-2105-11-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowski SW, Lo CY, Sharov AA, Nguyen Q, Fang L, Hung SS et al (2019) A single-cell transcriptome atlas of the adult human retina. EMBO J 38:e100811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malechka VV, Cukras CA, Chew EY, Sergeev YV, Blain D, Jeffrey BG et al (2022) Clinical phenotypes of CDHR1-associated retinal dystrophies. Genes. 10.3390/genes13050925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H et al (2012) Systematic localization of common disease-associated variation in regulatory DNA. Science 337:1190–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Gomez C, Felix JF, Estrada K, Peters MJ, Herrera L, Kruithof CJ et al (2015) Challenges in conducting genome-wide association studies in highly admixed multi-ethnic populations: the Generation R Study. Eur J Epidemiol 30:317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlapally R, Gonzalez P, Hawthorne FA, Tran-Viet K-N, Wildsoet CF, Young TL (2013) Scleral micro-RNA signatures in adult and fetal eyes. PLoS ONE 8:e78984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y-R, Liu W, Zhang Q, Guo A-Y (2018) lncRNASNP2: an updated database of functional SNPs and mutations in human and mouse lncRNAs. Nucleic Acids Res 46:D276–D280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WP, Sunilkumar S, Giordano JF, Toro AL, Barber AJ, Dennis MD (2020) The stress response protein REDD1 promotes diabetes-induced oxidative stress in the retina by Keap1-independent Nrf2 degradation. J Biol Chem 295:7350–7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modjtahedi BS, Ferris FL 3rd, Hunter DG, Fong DS (2018) Public health burden and potential interventions for myopia. Ophthalmology 125:628–630 [DOI] [PubMed] [Google Scholar]
- Moore JA, Miller WP, Dennis MD (2016) Glucosamine induces REDD1 to suppress insulin action in retinal Müller cells. Cell Signal 28:384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan IG, Ohno-Matsui K, Saw S-M (2012) Myopia. Lancet 379:1739–1748 [DOI] [PubMed] [Google Scholar]
- Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M et al (2018) The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res 62:134–149 [DOI] [PubMed] [Google Scholar]
- Morgan-Warren PJ, O’Neill J, de Cogan F, Spivak I, Ashush H, Kalinski H et al (2016) siRNA-mediated knockdown of the mTOR Inhibitor RTP801 promotes retinal ganglion cell survival and axon elongation by direct and indirect mechanisms. Invest Ophthalmol vis Sci 57:429–443 [DOI] [PubMed] [Google Scholar]
- Mortuza R, Feng B, Chakrabarti S (2014) miR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia 57:1037–1046 [DOI] [PubMed] [Google Scholar]
- Nixon TRW, Richards A, Towns LK, Fuller G, Abbs S, Alexander P et al (2019) Bone morphogenetic protein 4 (BMP4) loss-of-function variant associated with autosomal dominant Stickler syndrome and renal dysplasia. Eur J Hum Genet 27:369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofir M, Hacohen D, Ginsberg D (2011) MiR-15 and miR-16 are direct transcriptional targets of E2F1 that limit E2F-induced proliferation by targeting cyclin E. Mol Cancer Res 9:440–447 [DOI] [PubMed] [Google Scholar]
- Oliva C, Molina-Fernandez C, Maureira M, Candia N, López E, Hassan B et al (2015) Hindsight regulates photoreceptor axon targeting through transcriptional control of jitterbug/filamin and multiple genes involved in axon guidance in Drosophila. Dev Neurobiol 75:1018–1032 [DOI] [PubMed] [Google Scholar]
- Ong OC, Hu K, Rong H, Lee RH, Fung BK (1997) Gene structure and chromosome localization of the G gamma c subunit of human cone G-protein (GNGT2). Genomics 44:101–109 [DOI] [PubMed] [Google Scholar]
- Panigrahi A, O’Malley BW (2021) Mechanisms of enhancer action: the known and the unknown. Genome Biol 22:108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pärssinen O, Kauppinen M, Viljanen A (2014) The progression of myopia from its onset at age 8–12 to adulthood and the influence of heredity and external factors on myopic progression. A 23-year follow-up study. Acta Ophthalmol 92:730–739 [DOI] [PubMed] [Google Scholar]
- Pennacchio LA, Bickmore W, Dean A, Nobrega MA, Bejerano G (2013) Enhancers: five essential questions. Nat Rev Genet 14:288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D et al (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LM, Tyler RC, Schilter KF, Abdul-Rahman O, Innis JW, Kozel BA et al (2011) BMP4 loss-of-function mutations in developmental eye disorders including SHORT syndrome. Hum Genet 130:495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers HM, Belcastro M, Sokolov M, Mathers PH (2016) Embryonic markers of cone differentiation. Mol vis 22:1455–1467 [PMC free article] [PubMed] [Google Scholar]
- Schaub MA, Boyle AP, Kundaje A, Batzoglou S, Snyder M (2012) Linking disease associations with regulatory information in the human genome. Genome Res 22:1748–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacker ST, Coppage KR, Enke RA (2020) RNA sequencing analysis of the human retina and associated ocular tissues. Sci Data 7:199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyueva D, Stampfel G, Stark A (2014) Transcriptional enhancers: from properties to genome-wide predictions. Nat Rev Genet 15:272–286 [DOI] [PubMed] [Google Scholar]
- Smirnov VM, Calvas P, Drumare I, Marks C, Defoort-Dhellemmes S (2019) Extreme myopia in a family with a missense PAX6 mutation: extended phenotype. Ophthalmic Genet 40:64–65 [DOI] [PubMed] [Google Scholar]
- Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S et al (2016) The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinform 54:1.30.1–1.30.33 [DOI] [PubMed] [Google Scholar]
- Stone EM, Lotery AJ, Munier FL, Héon E, Piguet B, Guymer RH et al (1999) A single EFEMP1 mutation associated with both malattia leventinese and doyne honeycomb retinal dystrophy. Nat Genet 22:199–202 [DOI] [PubMed] [Google Scholar]
- Swierkowska J, Vishweswaraiah S, Mrugacz M, Radhakrishna U, Gajecka M (2022) Differential methylation of microRNA encoding genes may contribute to high myopia. Front Genet 13:1089784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SM, Rong SS, Young AL, Tam POS, Pang CP, Chen LJ (2014) PAX6 gene associated with high myopia: a meta-analysis. Optom vis Sci 91:419–429 [DOI] [PubMed] [Google Scholar]
- Tedja MS, Wojciechowski R, Hysi PG, Eriksson N, Furlotte NA, Verhoeven VJM et al (2018) Genome-wide association meta-analysis highlights light-induced signaling as a driver for refractive error. Nat Genet 50:834–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedja MS, Haarman AEG, Meester-Smoor MA, Kaprio J, Mackey DA, Guggenheim JA et al (2019) IMI-myopia genetics report. Invest Ophthalmol vis Sci 60:M89-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teperino R, Lempradl A, Pospisilik JA (2013) Bridging epigenomics and complex disease: the basics. Cell Mol Life Sci 70:1609–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ED, Timms AE, Giles S, Harkins-Perry S, Lyu P, Hoang T et al (2022) Cell-specific cis-regulatory elements and mechanisms of non-coding genetic disease in human retina and retinal organoids. Dev Cell 57:820–36.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S, Blodi FR, Larson DR, Anderson MG, Stasheff SF (2019) The Efemp1R345W macular dystrophy mutation causes amplified circadian and photophobic responses to light in mice. Investig Opthalmol vis Sci. 10.1167/iovs.19-26881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Sasaki T, Kostka G, Chu M-L (2003) Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol 4:479–489 [DOI] [PubMed] [Google Scholar]
- Tkatchenko AV, Luo X, Tkatchenko TV, Vaz C, Tanavde VM, Maurer-Stroh S et al (2016) Large-scale microRNA expression profiling identifies putative retinal miRNA-mRNA signaling pathways underlying form-deprivation myopia in mice. PLoS ONE 11:e0162541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trerotola M, Relli V, Simeone P, Alberti S (2015) Epigenetic inheritance and the missing heritability. Hum Genom 9:17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitsky I, Bartel DP (2013) lincRNAs: genomics, evolution, and mechanisms. Cell. 10.1016/j.cell.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer M, Li J, Yaspan BL, Ozel AB, Richards JE, Moroi SE et al (2012) Genome-wide analysis of central corneal thickness in primary open-angle glaucoma cases in the NEIGHBOR and GLAUGEN consortia. Invest Ophthalmol vis Sci 53:4468–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven VJM, Buitendijk GHS, Rivadeneira F, Uitterlinden AG, Vingerling JR, Consortium for Refractive Error and Myopia (CREAM) et al (2013) Education influences the role of genetics in myopia. Eur J Epidemiol 28:973–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel A, Minovitsky S, Dubchak I, Pennacchio LA (2007) VISTA enhancer browser–a database of tissue-specific human enhancers. Nucleic Acids Res 35:D88-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle P, Brancati G, Harmel C, He Z, Gut G, Del Castillo JS et al (2023) Multimodal spatiotemporal phenotyping of human retinal organoid development. Nat Biotechnol 41:1765–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li J, Wang S, Lu X, Zhang G, Zhuang Y et al (2021) Contribution of structural accessibility to the cooperative relationship of TF-lncRNA in myopia. Brief Bioinform. 10.1093/bib/bbab082 [DOI] [PubMed] [Google Scholar]
- Wang M, Li Y, Wang J, Oh SH, Chen R (2024) Integrating short-read and long-read single-cell RNA sequencing for comprehensive transcriptome profiling in mouse retina. BioRxiv. 10.1101/2024.02.20.58123439829740 [Google Scholar]
- Ward LD, Kellis M (2012) HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 40:D930–D934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KM, Verhoeven VJM, Cumberland P, Bertelsen G, Wolfram C, Buitendijk GHS et al (2015) Prevalence of refractive error in Europe: the European eye epidemiology (E3) consortium. Eur J Epidemiol 30:305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Glickstein S, Liu W, Fujita T, Li W, Yang Q et al (2007) The anaphase-promoting complex coordinates initiation of lens differentiation. Mol Biol Cell 18:1018–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Peng Y-R, van Zyl T, Regev A, Shekhar K, Juric D et al (2020) Cell atlas of the human fovea and peripheral retina. Sci Rep 10:9802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang W, Zhu W, Dong J, Cheng Y, Yin Z et al (2019) Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int J Mol Sci. 10.3390/ijms20225573 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All findings on the associations of RNA genomic regions and enhancers are published in this paper and available in the (supplementary) tables. The top SNP summary statistics of the GWAS meta-analysis on RE and myopia have been published elsewhere. Further GWAS summary statistics are be available upon request. Please contact c.c.w.klaver@erasmusmc.nl (CREAM) for more information and to access the data.