Abstract
Purpose
Following the COVID-19 pandemic, group A Streptococcus (GAS) infection has been surging worldwide. We aimed to compare the disease burden between notified cases of streptococcal toxic shock syndrome (STSS) and unreported GAS infections.
Methods
This is a multicentral observational study, retrospectively performed at seven hospitals in Okayama prefecture in Japan from January 2022, to June 2024. Clinical and microbiological data of patients with positive cultures of GAS were collected from the medical records. Primary outcomes were defined as rates of surgical procedures, intensive care unit (ICU) admission, and in-hospital mortality, which were compared among patients with locally-defined STSS, invasive GAS (iGAS), and non-iGAS infection.
Results
GAS was detected in 181 patients, with 154 active cases of GAS infection. The number of patients with GAS infection surged in late 2023. The most common source of infection was skin and soft tissue infections, accounting for 83 cases, including 15 cases of necrotizing fasciitis, and 12 cases (7.8%) were notified to public health authorities as STSS. Among the 25 unreported iGAS cases, 9 (36.0%) underwent surgical intervention, and 4 patients (16.0%) required ICU admission. The mortality rates in the unreported iGAS cases were comparable to those observed in the notified STSS.
Conclusions
We highlighted that the number of iGAS infections was twofold higher than that of notified STSS, with comparable mortality rate between these groups, indicating substantial underestimation of the true burden of iGAS. This epidemiological investigation has significant implications for enhancing infectious disease surveillance frameworks and public health policy development.
Keywords: Epidemiology, Group A Streptococcus, Necrotizing fasciitis, Streptococcal toxic shock syndrome, Surveillance
Introduction
Streptococcal toxic shock syndrome (STSS) is a life-threatening infectious disease characterized by the rapid onset of systemic symptoms and progression to multiple organ failures [1, 2]. Different from the conventional definition of STSS, Japanese surveillance protocols for STSS encompass infections caused not only by group A Streptococcus (GAS, Streptococcus pyogenes), but also by group B, C, and G β-hemolytic streptococci [3]. Notably, mortality rates associated with GAS infections remain substantially high, despite appropriate antimicrobial therapy and surgical interventions. In response to this significant public health concern, comprehensive international surveillance systems for invasive GAS (iGAS) infections have been established globally [4–11].
GAS is classified based on the emm gene sequence, which encodes the M protein, a well-known virulence factor [12]. Recently, the emm1 type (M1) strain was reported to be the most frequently isolated strain from upper respiratory tract specimens in patients with pharyngitis, scarlet fever, and invasive infections in the United Kingdom [13]. Since 2011, a highly pathogenic emm1 type GAS strain, which produces approximately nine times more erythrogenic toxin and exhibits higher transmissibility [14], later termed the “M1UK lineage”, has emerged and become predominant among M1 strains isolated in Europe and Australia [9, 10, 13–15]. This M1UK strain has recently been identified as a cause of STSS in Japan, with reports of its association with respiratory tract infections [16, 17].
In Japan, the number of patients with locally-defined STSS is monitored as a category V notifiable infectious disease under the Infectious Disease Epidemiology Surveillance [18]. Notification is legally required if specific criteria are met, including a state of systemic shock, liver failure, renal failure, acute respiratory distress syndrome, disseminated intravascular coagulation, soft tissue inflammation, generalized erythematous rash, and central nervous system symptoms, and the detection of β-hemolytic streptococci in sterile samples such as blood [19]. Although the result of the STSS surveillance is open to the public [16, 20] updates are infrequent, and detailed clinical information is lacking. Additionally, patients with severe conditions necessitating intensive care unit (ICU) admission or surgical intervention are not captured by the current surveillance system if they fail to fulfill the established diagnostic criteria for STSS. Consequently, the clinical burden of GAS infections may be largely underestimated within the current reporting framework. Therefore, our objective is to elucidate the true impact of GAS infections in Japan, particularly amidst the recent surge of GAS infections.
Methods
Study design and settings
We retrospectively collected the clinical and microbiological data from patients with positive cultures of GAS from the medical and microbiological records at seven hospitals across three municipalities in Okayama Prefecture, Japan (Okayama University Hospital, NHO Okayama Medical Center, Okayama Saiseikai General Hospital, Okayama City Hospital, Tsuyama Chuo Hospital, Okayama Kyoritsu Hospital, Takahashi Central Hospital), between January 1, 2022, and June 30, 2024. Five institutions (Okayama University Hospital, NHO Okayama Medical Center, Okayama Saiseikai General Hospital, Okayama City Hospital, Okayama Kyoritsu Hospital) were located in Okayama City (population approximately 700,000). Tsuyama Chuo Hospital and Takahashi Central Hospital cover Tsuyama City (population approximately 100,000) and Takahashi City (population approximately 29,000), respectively, based on 2020 census data. Among participating institutions, Okayama University Hospital and Tsuyama Chuo Hospital were designated tertiary care hospitals, while the others were secondary acute care hospitals. Ethical approval was obtained from the Institutional Review Board of Okayama University Hospital (No. 2406-015). The requirement for informed consent was waived due to the retrospective nature of the study, which involved the use of fully anonymized, routinely collected data.
Inclusion criteria
We included cases of patients with positive cultures of GAS from any specimen during the study period. Episodes with intervals of three months or more were defined as different cases.
Definitions and study protocol
The patients diagnosed with GAS were classified as either infectious or carrier cases. STSS is a notifiable disease classified as a category V infectious disease that must be reported upon diagnosis [18]. Thus, when patients with this condition, medical practitioners are obligated to inform local public health centers using reporting documents. Since April 2006, all cases of the locally-defined STSS caused by β-hemolytic streptococci are supposed to be reported as category V infectious diseases.
We retrospectively collected the following information from patient medical records: age, sex, time of diagnosis, underlying conditions, specimen type from which GAS was detected, clinical diagnosis, notification status as a category V infectious disease, surgical procedures performed, admission to the ICU, and in-hospital mortality. Age groups were categorized in 10-year increments. To analyze the temporal trend of GAS infections, we divided the study period into six-month intervals: January–June in 2022, July–December in 2022, January–June in 2023, July–December in 2023, and January–June in 2024.
A patient was defined as a GAS carrier if the bacteria were detected at a body site other than the focus of infection or if the attending physician determined that the patient was a carrier. We categorized the infectious foci into six groups: pharyngitis, lower respiratory infections, skin and soft tissue infections (SSTIs), abdominal and pelvic infections, genital infections, and other infections. The infectious foci were determined in most cases by the attending physician based on a match between the site of infection and the site of an obtained specimen. The pharyngitis category encompasses peritonsillar abscess cases confirmed through microbiological cultures from pharyngeal specimens or purulent material obtained from peritonsillar collections. The iGAS infections were defined according to the United States Centers for Disease Control and Prevention (CDC)’s Active Bacterial Core surveillance criteria, comprising cases of STSS, necrotizing fasciitis, and isolation of GAS from normally sterile anatomical sites, including but not limited to blood, cerebrospinal fluid, synovial fluid, peritoneal fluid, osseous tissue, and internal organs [4, 5].We investigated several comorbidities, including hypertension, cardiovascular disease, diabetes mellitus, malignancy, skin disorder, chronic obstructive pulmonary disease or asthma, kidney disorder, liver disorder, mental disorder, and others. Whole-genome sequence analysis of GAS strains isolated from notified cases commenced in January 2024 in Japan, prompted by the increased incidence of GAS infections and the potential emergence of the M1UK in 2023. We also gathered this data from the participating hospital.
Outcome measures and statistical analysis
The primary outcomes assessed included the difference of ICU admission, the need for surgical intervention, notification as a Category V infectious disease, and in-hospital mortality between patients with notified STSS cases and unreported cases. The secondary outcome focused on the difference between SSTI and other cases. Continuous variables were presented as medians with interquartile ranges (IQRs) and evaluated using the Kruskal–Wallis test or Mann–Whitney U test. Categorical variables were reported as numbers and percentages and were assessed using the Chi-square or the Fisher’s exact test. Data analysis was conducted using EZR software, a graphic user interface for the R 3.5.2 software (The R Foundation for Statistical Computing, Vienna, Austria). All reported p values < 0.05 were considered statistically significant.
Results
Backgrounds and details of the eligible cases
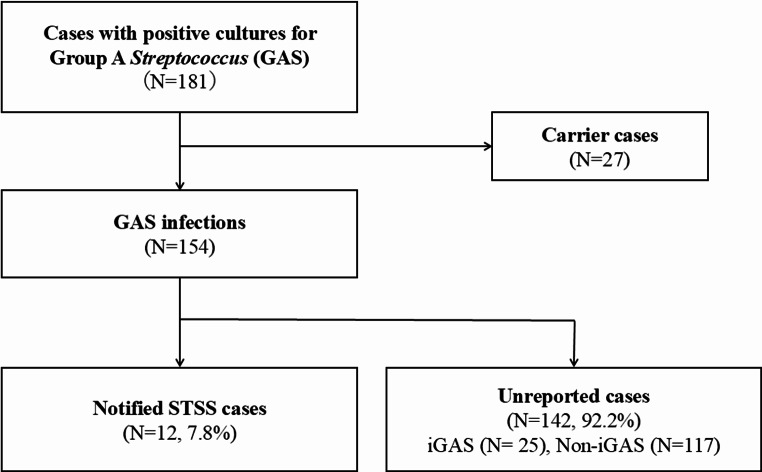
A total of 181 patients had positive cultures for GAS during the study period, with a median age of 43 years [IQR, 19–67] (Table 1). Of the total isolates, 27 represented asymptomatic colonization, while 154 cases were confirmed as active GAS infections. The clinical manifestations comprised 32 cases (17.7%) of pharyngitis, 7 cases (3.9%) of lower respiratory infections, and 83 cases (45.9%) of SSTIs, including 15 cases of necrotizing fasciitis. Among these infectious cases, 37 cases met the diagnostic criteria for iGAS infections. GAS was isolated from 194 specimens collected from these 181 patients, with the most frequent source being pus (41.2%), followed by pharynx (11.3%) and blood (9.8%). Specimens obtained from normally sterile anatomical sites constituted 23 cases (11.9%) of the total isolates, comprising blood cultures, synovial fluid aspirates, and peritoneal fluid specimens. Of the 154 GAS infection cases, 12 cases (7.8%) were notified to public health authorities as STSS cases. The remaining 142 cases (92.2%) were unreported, including 25 iGAS cases (Fig. 1). The number of patients with GAS infection ranged 10–20 cases per half-year from 2022 to the first half of 2023, and increased to 36 cases from July to December 2023 and surged to 76 cases from January to June 2024 (Fig. 2A). Patient ages varied from children to middle-aged population (Fig. 2B).
Table 1.
Clinical backgrounds of patients isolated with GAS and breakdown of GAS-positive specimens
| Number of patients (total) | 181 |
| Age, years (median [IQR]) | 43 [19–67] |
| Sex, male (%) | 110 (60.8) |
| Clinical diagnosis (%) | |
| Carrier state | 27 (14.8) |
| Pharyngitis | 32 (17.7) |
| Lower respiratory infections | 7 (3.9) |
| Skin and soft tissue infections | 83 (45.6) |
| Necrotizing fasciitis | 15 (8.2) |
| Abdominal and pelvic infections | 8 (4.4) |
| Genital infections | 6 (3.3) |
| Other infections* | 18 (9.9) |
| iGAS infections (%) | 37 (20.4) |
| Specimen (%) | |
| Total | 194 |
| Sterile site specimens | 23 (11.9) |
| Blood | 19 (9.8) |
| Synovial fluid | 2 (1.0) |
| Ascites | 2 (1.0) |
| Non-sterile site specimens | 171 (88.1) |
| Pus | 80 (41.2) |
| Pharynx | 22 (11.3) |
| Sputum | 15 (7.7) |
| Skin | 15 (7.7) |
| Eye discharge | 9 (4.6) |
| Vaginal discharge | 8 (4.1) |
| Urine | 6 (3.1) |
| Exudate | 5 (2.6) |
| Others** | 11 (5.7) |
GAS: group A streptococcus, iGAS: invasive GAS, IQR: interquartile range
*Other infections include primary bacteremia (5), ear infections (4), streptococcal toxic shock syndrome (2), conjunctivitis (2), lymphadenitis (2), parotitis (1), poststreptococcal glomerulonephritis (1), and unknown (1)
**Others include ear and nasal discharge (4), tissue (2), wound swabs (2), vulva and uterus (2), and feces (1)
Fig. 1.
Study enrolment flow. GAS: group A streptococcus, iGAS: invasive GAS, STSS: streptococcal toxic shock syndrome. Of the 154 GAS infection cases, 12 cases (7.8%) were notified as STSS cases, whereas the remaining 142 cases (92.2%) did not meet the diagnostic criteria for STSS. Among the unreported cases, invasive cases are 25, 117 are non-invasive cases
Fig. 2.
Number of patients diagnosed with GAS infection, (A) by six-month intervals and (B) by patient age. GAS: group A streptococcus, iGAS: invasive GAS, SSTI: skin and soft tissue infection. Other infections include pharyngitis, respiratory and ear infections, abdominal and pelvic infections, genital infections, parotitis, conjunctivitis, primary bacteremia, and streptococcal toxic shock syndrome
Clinical features and outcomes in GAS infections
Summarization of all cases
Overall details for the GAS infections are presented in Table 2. The median age of 154 patients with GAS infections was 43 years (IQR, 19–67 years), and 60.4% were male. Nearly half (54.5%) of them had no underlying comorbidities. Clinical diagnosis of GAS infection varied, with SSTIs (53.9%), pharyngitis (20.8%), other infections (7.8%), abdominal and pelvic infections (5.2%), lower respiratory infections (4.5%), and genital infections (3.9%). Of all, 19 (12.3%) developed GAS bacteremia, 36 (23.4%) required surgical interventions, 19 (12.3%) were admitted to ICU, and 3 (1.9%) died in the hospitals. The prognosis of two patients was unknown due to transfer to other hospitals. Six cases met the diagnostic criteria for the locally-defined STSS and were notified to the public health authorities in 2024. The M1UK lineage strains were identified in 66.7% (4 out of 6 cases) of these cases, while the remaining cases were attributed to emm81 and emm89 genotypes, with one case each.
Table 2.
Comparative analysis of clinical backgrounds, manifestations, and outcomes across the three spectrum of GAS infections: notified STSS cases, unreported iGAS cases, and unreported non-iGAS cases
| Overall | Notified cases | Unreported cases | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| STSS | All GAS cases | iGAS | Non-iGAS | STSS vs. iGAS | STSS vs. Non-iGAS | |||||
| Number of cases (total) | 154 | 12 | 142 | 25 | 117 | |||||
| Age, years (median [IQR]) | 43 [19–67] | 60 [42–78] | 39 [15–63] | 51 [29–73] | 37 [15–59] | 0.63 | < 0.001*** | |||
| Sex, male (%) | 93 (60.4) | 6 (50.0) | 87 (61.3) | 14 (56.0) | 73 (62.4) | 1 | 0.54 | |||
| Underlying clinical conditions (%) | ||||||||||
| Presence of comorbidity | 70 (45.5) | 9 (75.0) | 61 (43.0) | 18 (72.0) | 43 (36.8) | 1 | 0.014*** | |||
| Clinical diagnosis (%) | ||||||||||
| Pharyngitis | 32 (20.8) | 0 (0.0) | 32 (22.5) | 0 (0.0) | 32 (27.4) | - | 0.037*** | |||
| Lower respiratory infections | 7 (4.5) | 0 (0.0) | 7 (4.9) | 0 (0.0) | 7 (6.0) | - | 1 | |||
| Skin and soft tissue infections | 83 (53.9) | 8 (66.7) | 75 (52.8) | 18 (72.0) | 57 (48.7) | 1 | 0.36 | |||
| Necrotizing fasciitis | 15 (9.7) | 4 (33.3) | 11 (7.7) | 11 (44.0) | 0 (0.0) | 0.72 | < 0.001*** | |||
| Abdominal and pelvic infections | 8 (5.2) | 2 (16.7) | 6 (4.2) | 0 (0.0) | 6 (5.1) | 0.099 | 0.16 | |||
| Genital infections | 6 (3.9) | 0 (0.0) | 6 (4.2) | 1 (4.0) | 5 (4.3) | 1 | 1 | |||
| Other infections* | 18 (11.7) | 2** (16.7) | 16 (11.3) | 5 (20.0) | 11 (9.4) | 1 | 0.35 | |||
| iGAS infections | 37 (24.0) | 12 (100) | 25 (17.6) | 25 (100) | 0 (0.0) | - | < 0.001*** | |||
| Complication (%) | ||||||||||
| Bacteremia | 19 (12.3) | 7 (58.3) | 12 (8.5) | 12 (48.0) | 0 (0.0) | 0.73 | < 0.001*** | |||
| Diagnosed year (%) | ||||||||||
| 2022 | 28 (18.2) | 1 (8.3) | 27 (19.0) | 3 (12.0) | 24 (20.5) | 1 | 0.46 | |||
| 2023 | 50 (32.5) | 5 (41.7) | 45 (31.7) | 7 (28.0) | 38 (32.5) | 0.47 | 0.53 | |||
| 2024 (January–June) | 76 (49.4) | 6 (50.0) | 70 (49.3) | 15 (60.0) | 55 (47.0) | 0.73 | 1 | |||
| Outcomes (%) | ||||||||||
| Surgical interventions | 36 (23.4) | 11 (91.7) | 25 (17.6) | 9 (36.0) | 16 (13.7) | 0.0018*** | < 0.001*** | |||
| Intensive care unit | 19 (12.3) | 11 (91.7) | 8 (5.6) | 4 (16.0) | 4 (3.4) | < 0.001*** | < 0.001*** | |||
| In-hospital mortality | 3 (1.9) | 1 (8.3) | 2 (1.4) | 2 (8.0) | 0 (0.0) | 1 | 0.093 | |||
GAS: group A streptococcus, iGAS: invasive GAS, STSS: streptococcal toxic shock syndrome, IQR: interquartile range
*Other infections include ear infections, lymphadenitis, parotitis, conjunctivitis, primary bacteremia, and streptococcal toxic shock syndrome
**Two cases were diagnosed with bacteremia and streptococcal toxic shock syndrome
***Significant differences are observed
Notified STSS cases vs. unreported iGAS cases
Among the unreported cases, iGAS infections accounted for 25 cases (17.6%). No statistically significant differences were observed in age and gender distribution, comorbidity prevalence, clinical diagnoses, annual case distribution, or complications. Although patients with STSS underwent surgical interventions more frequently (91.7% vs. 36.0%; p = 0.0018) and demonstrated higher rates of ICU admission (91.7% versus 16.0%; p < 0.001), in-hospital mortality rates were comparable between STSS cases and unreported iGAS cases (8.3% vs. 8.0%).
Notified STSS cases vs. unreported non-iGAS cases
Among the unreported cases, non-iGAS infections constituted 117 cases (76.0%) of the total cohort. Patient with the notified STSS cases were significantly older (60 years vs. 37 years; p < 0.001). No significant difference was observed in the proportions of male and female patients. The proportion of patients with underlying diseases was significantly higher among those with the notified STSS cases (75.0% vs. 36.8%; p = 0.038). There were no cases of pharyngitis, lower respiratory infections among the notified STSS cases. Both the numbers of notified STSS cases and unreported non-iGAS cases increased year by year, with no significant difference noted between the two groups. Patients with the notified STSS cases underwent surgical interventions more frequently (91.7% vs. 13.7%; p < 0.001) and had a higher rate of ICU admission (91.7% vs. 3.4%; p < 0.001). Although the difference was not statistically significant, in-hospital mortality was higher among patients with the notified STSS cases (8.3% vs. 0%; p = 0.093).
SSTIs vs. pharyngitis vs. other infections
The clinical characteristics of patients with SSTIs, pharyngitis, and other infections are given in Table 3. SSTIs accounted for 83 cases (53.9%), 32 (20.8%) are pharyngitis, and 39 (25.3%) are other infections. Significant differences were observed in the ages of the three groups (46 years vs. 33 years vs., 47 years) or in the proportions of male patients (65.1% vs. 68.8% vs. 43.6%). However, the prevalence of underlying diseases was significantly higher in SSTI cases for hypertension (p = 0.017), cardiovascular disease (p = 0.017), and skin disorders (p = 0.035). The skin disorders included 12 cases of atopic dermatitis and 2 cases of psoriasis vulgaris. When compared among three GAS infections, there were no significant differences in the proportions of STSS, the need for surgical interventions, and in-hospital mortality. The ICU admission rates of pharyngitis was significantly lower than other groups.
Table 3.
The clinical characteristics of skin and soft tissue infections (SSTIs), pharyngitis, and other infections
| Overall | SSTIs | Pharyngitis | Other infections | p value | |
|---|---|---|---|---|---|
| Number of cases (total) | 154 | 83 (53.9) | 32 (20.8) | 39 (25.3) | |
| Age, years (median [IQR]) | 43 [19–67] | 46 [21–71] | 33 [20–46] | 47 [22–72] | 0.0017** |
| Sex, male (%) | 93 (60.4) | 54 (65.1) | 22 (68.8) | 17 (43.6) | 0.049** |
| Underlying clinical conditions (%) | |||||
| No comorbidity | 84 (54.5) | 39 (47.0) | 28 (87.5) | 17 (43.6) | < 0.001** |
| Hypertension | 15 (9.7) | 13 (15.7) | 0 (0.0) | 2 (5.1) | 0.017** |
| Cardiovascular diseases | 12 (7.8) | 11 (13.3) | 1 (3.1) | 0 (0.0) | 0.017** |
| Diabetes mellitus | 13 (8.4) | 8 (9.6) | 1 (3.1) | 4 (10.3) | 0.50 |
| Malignancy | 9 (5.8) | 5 (6.0) | 0 (0.0) | 4 (10.3) | 0.19 |
| Skin disorders | 14 (9.1) | 12 (14.5) | 0 (0.0) | 2 (5.1) | 0.035** |
| COPD or asthma | 9 (5.8) | 5 (6.0) | 0 (0.0) | 4 (10.3) | 0.19 |
| Kidney disorders | 3 (1.9) | 2 (2.4) | 0 (0.0) | 1 (2.6) | 1 |
| Liver disorders | 5 (3.2) | 1 (1.2) | 1 (3.1) | 3 (7.7) | 0.12 |
| Mental disorders | 6 (3.9) | 3 (3.6) | 1 (3.1) | 2 (5.1) | 0.86 |
| Others* | 13 (8.4) | 8 (9.6) | 1 (3.1) | 4 (10.3) | 0.50 |
| Outcomes (%) | |||||
| Class V notification (STSS) | 12 (7.8) | 8 (9.6) | 0 (0.0) | 4 (10.3) | 0.18 |
| Surgical interventions | 36 (23.4) | 20 (24.1) | 11 (34.4) | 5 (12.8) | 0.11 |
| Intensive care unit | 19 (12.3) | 13 (15.7) | 0 (0.0) | 6 (15.4) | 0.033** |
| In-hospital mortality | 3 (1.9) | 2 (2.4) | 0 (0.0) | 1 (2.6) | 1 |
GAS: group A streptococcus, IQR: interquartile range, COPD: chronic obstructive pulmonary disease, STSS: streptococcal toxic shock syndrome
*Others include Down syndrome, dyslipidemia, hyperuricemia, Hashimoto’s disease, rheumatoid arthritis, chronic sinusitis, uterine fibroids, and endometriosis
**Significant differences are observed among three groups
Discussion
We herein described the clinical backgrounds and outcomes of patients diagnosed with GAS infections ranging from January 2022 to June 2024. During the two and half years, we obtained data for 154 GAS infections at seven Japanese hospitals. The number of patients with GAS infection increased starting in July 2023, with 12 notified cases of STSS. The most common sites of infection were SSTIs (83 cases), including 15 cases of necrotizing fasciitis. Clinical outcomes of GAS infections were overall severe, with regard to the requirement for surgical interventions (23.4%), ICU admission (12.3%), and in-hospital mortality (1.9%). The number of unreported iGAS cases was twofold higher than that of the notified STSS cases (25 cases vs. 12 cases), indicating substantial underestimation of the true clinical burden of invasive streptococcal disease within the current surveillance system. Nearly one-fifth of unreported GAS cases underwent surgical interventions, and 5.7% of the cases were managed in the ICU, with two ultimately resulting in in-hospital deaths. These findings underscore the clinical significance of unreported GAS cases that do not meet the STSS notification criteria.
STSS is the most severe form of streptococcal infection, with a mortality rate of 15.7–40.9%, even when appropriate antimicrobial therapy and surgical debridement are given to the patients [1, 21, 22]. It is commonly complicated by bacteremia and necrotizing fasciitis, necessitating early diagnosis and intensive support for organ failures in the ICU [2, 23]. GAS infections develop STSS more frequently than other streptococci, with an odds ratio of 3.28 (95% confidence interval: 1.21–8.77) [3]. Among patients suffering from iGAS infections, the likelihood of developing STSS ranges from 8 to 14% [24]. Many countries, including the United States and Europe, are investigating the spread and epidemiology of GAS infections through surveillance [4, 5, 7, 9–11]. Our study identified 37 iGAS infections, among which notified STSS cases constituted 32.4%, representing a higher proportion than previously documented in the literature [24]. In our study, 8.4% of patients with GAS infections were notified as Category V infectious diseases under the diagnosis of STSS, with an in-hospital mortality rate of 8.3% in notified STSS and 8.0% in unreported iGAS cases. Although these mortality rates are lower than previously reported [3, 21], our analysis was limited to inpatient mortality at each hospital and could not capture deaths occurring after inter-hospital transfer. Furthermore, the incidence of STSS is underestimated due to the stringent notifying criteria for STSS in Japan; indeed, the frequency of unreported iGAS infections was twofold higher than that of reported STSS cases. Similar to practices in other countries, it may be beneficial to include all the iGAS cases in reporting to prove necessary public health alerts.
The M1UK lineage strains among GAS have attracted worldwide attention due to their outstanding phenotype characterized by increased toxin production and higher transmissibility compared to the conventional M1 strain [13–16, 25]. In England, the prevalence of M1UK lineage increased significantly from 8.3% (3 out of 36 cases) to 84.3% (252 out of 299 cases) between 2011 and 2016 [13], and 91.1–95.7% from 2020 to 2023 [26, 27]. The isolation rate of M1UK lineage strains from iGAS infections was reported to be 29.8% for 2022–2023 in Germany [14], and 10.8% for 2019–2021 in the United States [25]. In Belgium, the total number of iGAS infections in 2022 and 2023 increased by 1.3 to 2-fold compared to 2018 and 2019, with M1UK constituting 71.6% of the emm1 strains associated with bloodstream infection [9]. In Japan, of 377 GAS strains isolated from STSS cases since January 2024, 221 (58.6%) were M1 strains, with 194 (51.5%) classified as M1UK lineage strains [16]. Given the virulence and adaptability of the M1UK strain, there is a pressing concern that the spread of the M1UK lineage strains could lead to a further increase in iGAS infections and STSS in Japan. Clinical and epidemiological information on unreported GAS infections, other than STSS, remains scarce in Japan; however, the spread of M1UK lineage strains will continue to raise concerns as observed in international countries.
The strength of our study lies in its ability to clarify the clinical burden and characteristics of unreported GAS infections in Japan. Our findings demonstrated that the incidence of iGAS infections was twofold higher than that of notified STSS cases, with comparable mortality rates between the distinct cohorts. This observation underscores the urgent necessity to implement a more comprehensive surveillance system that incorporates iGAS cases within the Japanese national infectious disease monitoring framework. Several limitations should be noted as well. First, this is a retrospective study, and thus, clinical diagnosis relies on the discretion of the attending physician. Also, the notification of cases as Category V infectious disease depends on the diagnostic facility and clinical assessment. Consequently, the number of notified STSS cases may be underestimated. Second, information regarding all emm-types including the M1UK lineage strain is limited to a subset of cases notified as STSS in 2024, leaving its broader involvement in other GAS infections unclear. Further in-depth national surveillance of the M1UK lineage strain is anticipated in the future. Thirdly, the case number of GAS pharyngitis is likely substantially underestimated due to the predominant diagnostic reliance on rapid antigen detection testing in Japanese clinical settings. Fourthly, in accordance with the United States CDC criteria for confirmed iGAS cases, pneumonia was not included in the iGAS infection in this study. Fifth, we could not differentiate between community-acquired and healthcare-associated onset of the disease. Finally, the prognosis of the two patients transferred to other hospitals remains unknown, potentially resulting in an underestimation of the outcomes.
In conclusion, we highlighted that unreported iGAS cases involve patients at a twofold higher frequency compared to the notified STSS cases, despite comparable clinical outcomes. The clinical burden of unreported GAS infections warrants attention due to their high rates of surgical interventions, ICU admissions, and in-hospital mortality. Our investigation elucidated the clinical characteristics and outcomes of current GAS infections during a period of rapidly escalating incidence and disease severity. These findings have potentially significant implications for strengthening the national surveillance system for streptococcal infections in Japan.
Acknowledgements
The authors thank the participating hospital staffs for their assistance.
Author contributions
H.H. conceived the study; S.F., T.S., Y.I., Y.T, H.Y., K.F., M.Y., and Y.N. performed data extraction; S.F. drafted the manuscript and performed statistical analyses; H.H. revised the manuscript; H.H. supervised the study. All the authors interpreted the results and approved the submission of the manuscript.
Funding
Open Access funding provided by Okayama University.
This study did not receive any funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
Ethical approval was obtained from the Institutional Review Board of Okayama University Hospital (No. 2406-015).
Consent for publication
The requirement for informed consent was waived because this was a retrospective analysis of routinely collected anonymized data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Defining the group A streptococcal toxic shock syndrome (1993) Rationale and consensus definition. The Working Group on severe streptococcal infections. JAMA. American Medical Association (AMA) 390–391 [PubMed]
- 2.Steer AC, Lamagni T, Curtis N, Carapetis JR (2012) Invasive group a streptococcal disease: epidemiology, pathogenesis and management: epidemiology, pathogenesis and management. Drugs 72:1213–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inada M, Iwamoto N, Nomoto H, Tsuzuki S, Takemoto N, Fuwa N et al (2024) Characteristics of streptococcal toxic shock syndrome caused by different beta-hemolytic streptococci species: a single-center retrospective study. Open Forum Infect Dis 11:ofae486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Active Bacterial Core surveillance (ABCs) 2024 [Internet]. [cited 2024 Oct 1]. https://www.cdc.gov/abcs/index.html
- 5.Miller KM, Lamagni T, Cherian T, Cannon JW, Parks T, Adegbola RA et al (2022) Standardization of epidemiological surveillance of invasive group a streptococcal infections. Open Forum Infect Dis 9:S31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed AS, Subbaram K, Faiz R, Naher ZU, Manandhar PL, Ali S (2024) Increasing reports of streptococcal toxic shock syndrome from Japan. New Microbes New Infect. 60–61:101446 [DOI] [PMC free article] [PubMed]
- 7.Guy R, Henderson KL, Coelho J, Hughes H, Mason EL, Gerver SM et al (2023) Increase in invasive group A streptococcal infection notifications, England, 2022. Euro Surveill [Internet]. [cited 2024 Sep 24];28. https://pubmed.ncbi.nlm.nih.gov/36695450/ [DOI] [PMC free article] [PubMed]
- 8.Chen M, Cai J, Davies MR, Li Y, Zhang C, Yao W et al (2020) Increase of emm1 isolates among group a Streptococcus strains causing scarlet fever in Shanghai, China. Int J Infect Dis 98:305–314 [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Ruiz JP, Lin Q, Lammens C, Smeesters PR, van Kleef-van Koeveringe S, Matheeussen V et al (2023) Increase in bloodstream infections caused by emm1 group A Streptococcus correlates with emergence of toxigenic M1UK, Belgium, May 2022 to August 2023. Euro Surveill [Internet]. [cited 2024 Oct 18];28. https://pubmed.ncbi.nlm.nih.gov/37676145/ [DOI] [PMC free article] [PubMed]
- 10.Rümke LW, Davies MA, Vestjens SMT, van der Putten BCL, Bril-Keijzers WCM, van Houten MA et al (2024) Nationwide upsurge in invasive disease in the context of longitudinal surveillance of carriage and invasive Streptococcus pyogenes 2009–2023, the Netherlands: a molecular epidemiological study. J Clin Microbiol 62:e0076624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johannesen TB, Munkstrup C, Edslev SM, Baig S, Nielsen S, Funk T et al Increase in invasive group A streptococcal infections and emergence of novel, rapidly expanding sub-lineage of the virulent Streptococcus pyogenes M1 clone, Denmark, 2023. Euro Surveill [Internet]. 2023 [cited 2024 Oct 19];28. https://pubmed.ncbi.nlm.nih.gov/37382884/ [DOI] [PMC free article] [PubMed]
- 12.Facklam RF, Martin DR, Lovgren M, Johnson DR, Efstratiou A, Thompson TA et al (2002) Extension of the Lancefield classification for group a streptococci by addition of 22 new M protein gene sequence types from clinical isolates: emm103 to emm124. Clin Infect Dis 34:28–38 [DOI] [PubMed] [Google Scholar]
- 13.Lynskey NN, Jauneikaite E, Li HK, Zhi X, Turner CE, Mosavie M et al (2019) Emergence of dominant toxigenic M1T1 Streptococcus pyogenes clone during increased scarlet fever activity in England: a population-based molecular epidemiological study. Lancet Infect Dis 19:1209–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolters M, Berinson B, Degel-Brossmann N, Hoffmann A, Bluszis R, Aepfelbacher M et al (2024) Population of invasive group a streptococci isolates from a German tertiary care center is dominated by the hypertoxigenic virulent M1UK genotype. Infection 52:667–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies MR, Keller N, Brouwer S, Jespersen MG, Cork AJ, Hayes AJ et al (2023) Detection of Streptococcus pyogenes M1UK in Australia and characterization of the mutation driving enhanced expression of superantigen SpeA. Nat Commun 14:1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute of Infectious Diseases Japan [Internet]. [cited 2024 Oct 1]. https://www.niid.go.jp/niid/images/cepr/RA/STSS/240701_NIID_STSS_2_Eng.pdf
- 17.Kawaguchi A, Nagaoka K, Kawasuji H, Kawagishi T, Fuchigami T, Ikeda K et al (2024) COVID-19 complicated with severe M1UK-lineage Streptococcus pyogenes infection in elderly patients: a report of two cases. Int J Infect Dis. 107246 [DOI] [PubMed]
- 18.Ministry of Justice Act on the Prevention of Infectious Diseases and Medical Care for Patients with Infectious Diseases. [cited 2024 Oct 1]. https://www.japaneselawtranslation.go.jp/en/laws/view/2830/en
- 19.Ministry of Health Labour and Welfare [Internet]. [cited 2024 Oct 18]. https://www.mhlw.go.jp/stf/english/index.html
- 20.National Institute of Infectious Diseases [Internet] [cited 2024 Oct 18]. https://www.niid.go.jp/niid/en/
- 21.Ikebe T, Tominaga K, Shima T, Okuno R, Kubota H, Ogata K et al (2015) Increased prevalence of group a streptococcus isolates in streptococcal toxic shock syndrome cases in Japan from 2010 to 2012. Epidemiol Infect 143:864–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parks T, Wilson C, Curtis N, Norrby-Teglund A, Sriskandan S (2018) Polyspecific intravenous immunoglobulin in clindamycin-treated patients with streptococcal toxic shock syndrome: a systematic review and meta-analysis. Clin Infect Dis 67:1434–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz M, Roux X, Huttner B, Pugin J (2018) Streptococcal toxic shock syndrome in the intensive care unit. Ann Intensive Care 8:88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baxter F, McChesney J (2000) Severe group a streptococcal infection and streptococcal toxic shock syndrome. Can J Anaesth 47:1129–1140 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Rivers J, Mathis S, Li Z, Chochua S, Metcalf BJ et al (2023) Expansion of invasive group a Streptococcus M1UK lineage in active bacterial core surveillance, United States, 2019–2021. Emerg Infect Dis 29:2116–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieira A, Wan Y, Ryan Y, Li HK, Guy RL, Papangeli M et al (2024) Rapid expansion and international spread of M1UK in the post-pandemic UK upsurge of Streptococcus pyogenes. Nat Commun 15:3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhi X, Li HK, Li H, Loboda Z, Charles S, Vieira A et al (2023) Emerging invasive group a Streptococcus M1UK lineage detected by allele-specific PCR, England, 2020. Emerg Infect Dis 29:1007–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.