Abstract
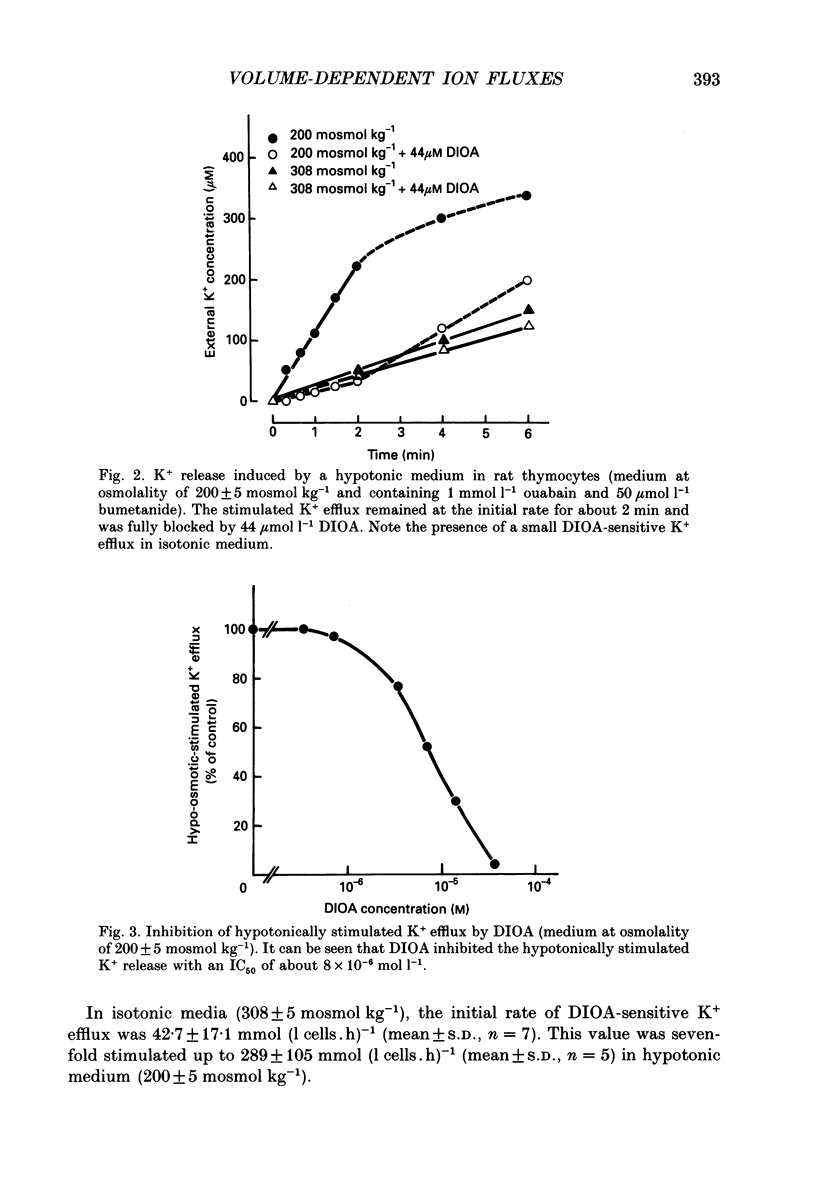
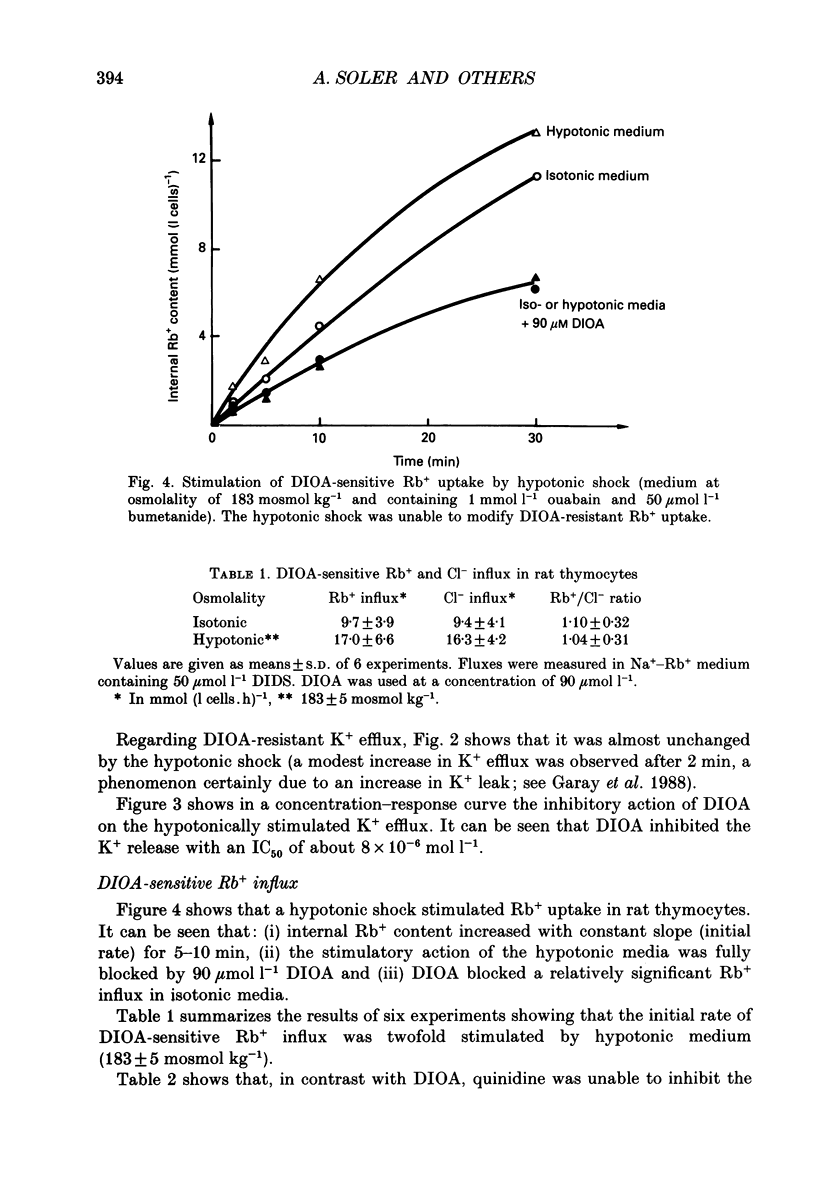
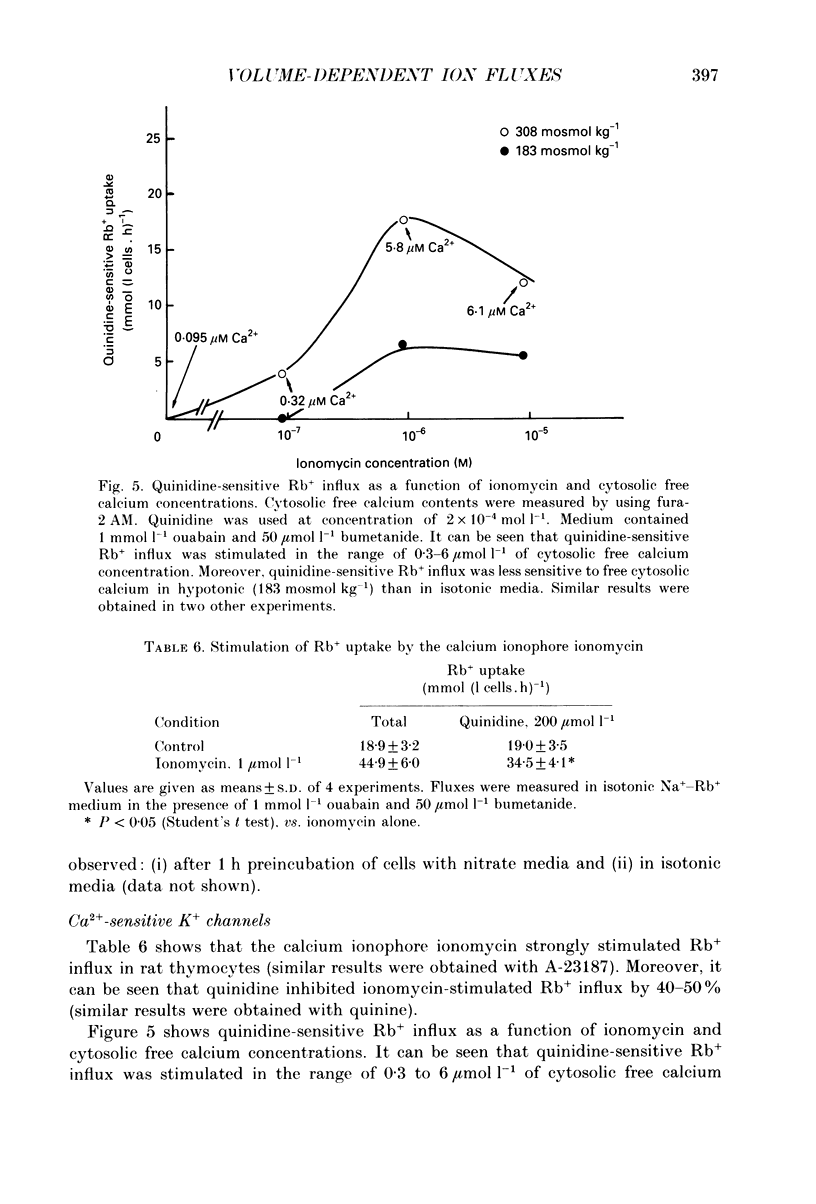
1. Hypotonic stress unmasked inward and outward K+ and Cl- movements in rat thymocytes. This KCl flux stimulation was reduced by DIOA (dihydroindenyl-oxy-alkanoic acid), but not by DIDS (4,4'-diisothiocyanostilbene-2,2'-disulphonate), quinidine, DPAC 144 (5-nitro-2-(2-phenylethyl-amino)-benzoic acid), bumetanide or ouabain. 2. In isotonic media (308 +/- 5 mosmol kg-1), the cells exhibited the following DIOA-sensitive fluxes: (i) a K+ efflux of 42.7 +/- 17.1 mmol (l cells.h)-1 (mean +/- S.D., n = 7), (ii) a Cl- efflux of 68 +/- 21 mmol (l cells.h)-1 (n = 3), (iii) a Rb+ influx of 9.7 +/- 3.9 mmol (l cells.h)-1 (n = 6) and (iv) a Cl- influx of 9.4 +/- 4.1 mmol (l cells.h)-1 (n = 6). 3. Hypotonic shock (183-200 mosmol kg-1) induced a sevenfold stimulation of DIOA-sensitive K+ and Cl- effluxes and a twofold stimulation of DIOA-sensitive Rb+ and Cl- influxes (with a Rb+ to Cl- stoichiometry of 1.04 +/- 0.31; mean +/- S.D., n = 6). 4. The DIOA-sensitive membrane carrier catalysed net outward KCl extrusion (the outward/inward flux ratio was 5-7 in isotonic media and 20 in hypotonic media at 189 mosmol kg-1). Inhibition of DIOA-sensitive 36Cl- efflux by cell K+ depletion suggested coupling of outward K+ and Cl- fluxes. Conversely, inward K+ and Cl- fluxes were found to be uncoupled in NO3- media and in K(+)-free media. 5. The results clearly show that rat thymocyte membranes possess a 1:1 K(+)-Cl- co-transport system which is strongly activated by hypotonic shock and catalyses net KCl extrusion.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrazola A., Rota R., Hannaert P., Soler A., Garay R. P. Cell volume regulation in rat thymocytes. J Physiol. 1993 Jun;465:403–414. doi: 10.1113/jphysiol.1993.sp019683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz L. R., Orringer E. P. Effects of cetiedil on monovalent cation permeability in the erythrocyte: an explanation for the efficacy of cetiedil in the treatment of sickle cell anemia. Blood Cells. 1982;8(2):283–288. [PubMed] [Google Scholar]
- Brazy P. C., Gunn R. B. Furosemide inhibition of chloride transport in human red blood cells. J Gen Physiol. 1976 Dec;68(6):583–599. doi: 10.1085/jgp.68.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa M., Brugnara C., Cusi D., Tosteson D. C. Modes of operation and variable stoichiometry of the furosemide- sensitive Na and K fluxes in human red cells. J Gen Physiol. 1986 Jan;87(1):113–142. doi: 10.1085/jgp.87.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragoe E. J., Jr Drugs for the treatment of traumatic brain injury. Med Res Rev. 1987 Jul-Sep;7(3):271–305. doi: 10.1002/med.2610070302. [DOI] [PubMed] [Google Scholar]
- Dunham P. B., Ellory J. C. Passive potassium transport in low potassium sheep red cells: dependence upon cell volume and chloride. J Physiol. 1981 Sep;318:511–530. doi: 10.1113/jphysiol.1981.sp013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay R. P., Hannaert P. A., Nazaret C., Cragoe E. J., Jr The significance of the relative effects of loop diuretics and anti-brain edema agents on the Na+,K+,Cl- cotransport system and the Cl-/NaCO3- anion exchanger. Naunyn Schmiedebergs Arch Pharmacol. 1986 Oct;334(2):202–209. doi: 10.1007/BF00505823. [DOI] [PubMed] [Google Scholar]
- Garay R. P., Nazaret C., Hannaert P. A., Cragoe E. J., Jr Demonstration of a [K+,Cl-]-cotransport system in human red cells by its sensitivity to [(dihydroindenyl)oxy]alkanoic acids: regulation of cell swelling and distinction from the bumetanide-sensitive [Na+,K+,Cl-]-cotransport system. Mol Pharmacol. 1988 Jun;33(6):696–701. [PubMed] [Google Scholar]
- Grinstein S., Dixon S. J. Ion transport, membrane potential, and cytoplasmic pH in lymphocytes: changes during activation. Physiol Rev. 1989 Apr;69(2):417–481. doi: 10.1152/physrev.1989.69.2.417. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Foskett J. K. Ionic mechanisms of cell volume regulation in leukocytes. Annu Rev Physiol. 1990;52:399–414. doi: 10.1146/annurev.ph.52.030190.002151. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A., Sarkadi B., Gelfand E. W. Responses of lymphocytes to anisotonic media: volume-regulating behavior. Am J Physiol. 1984 Mar;246(3 Pt 1):C204–C215. doi: 10.1152/ajpcell.1984.246.3.C204. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O., Lambert I. H. Volume-induced increase of K+ and Cl- permeabilities in Ehrlich ascites tumor cells. Role of internal Ca2+. J Membr Biol. 1984;78(3):211–222. doi: 10.1007/BF01925969. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Hugues M., Romey G., Duval D., Vincent J. P., Lazdunski M. Apamin as a selective blocker of the calcium-dependent potassium channel in neuroblastoma cells: voltage-clamp and biochemical characterization of the toxin receptor. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1308–1312. doi: 10.1073/pnas.79.4.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregenow F. M. Osmoregulatory salt transporting mechanisms: control of cell volume in anisotonic media. Annu Rev Physiol. 1981;43:493–505. doi: 10.1146/annurev.ph.43.030181.002425. [DOI] [PubMed] [Google Scholar]
- Kregenow F. M. The response of duck erythrocytes to nonhemolytic hypotonic media. Evidence for a volume-controlling mechanism. J Gen Physiol. 1971 Oct;58(4):372–395. doi: 10.1085/jgp.58.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf P. K., Adragna N. C., Garay R. P. Activation by N-ethylmaleimide of a latent K+-Cl- flux in human red blood cells. Am J Physiol. 1984 May;246(5 Pt 1):C385–C390. doi: 10.1152/ajpcell.1984.246.5.C385. [DOI] [PubMed] [Google Scholar]
- Lauf P. K. K:Cl cotransport: emerging molecular aspects of a ouabain-resistant, volume-responsive transport system in red blood cells. Ren Physiol Biochem. 1988 May-Oct;11(3-5):248–259. doi: 10.1159/000173165. [DOI] [PubMed] [Google Scholar]
- Morlé L., Pothier B., Alloisio N., Féo C., Garay R., Bost M., Delaunay J. Reduction of membrane band 7 and activation of volume stimulated (K+, Cl-)-cotransport in a case of congenital stomatocytosis. Br J Haematol. 1989 Jan;71(1):141–146. doi: 10.1111/j.1365-2141.1989.tb06288.x. [DOI] [PubMed] [Google Scholar]
- Senn N., Garay R. P. Regulation of Na+ and K+ contents in rat thymocytes. Am J Physiol. 1989 Jul;257(1 Pt 1):C12–C18. doi: 10.1152/ajpcell.1989.257.1.C12. [DOI] [PubMed] [Google Scholar]
- Thornhill W. B., Laris P. C. KCl loss and cell shrinkage in the Ehrlich ascites tumor cell induced by hypotonic media, 2-deoxyglucose and propranolol. Biochim Biophys Acta. 1984 Jun 27;773(2):207–218. doi: 10.1016/0005-2736(84)90084-1. [DOI] [PubMed] [Google Scholar]
- Traoré F., Cognard C., Potreau D., Raymond G. The apamin-sensitive potassium current in frog skeletal muscle: its dependence on the extracellular calcium and sensitivity to calcium channel blockers. Pflugers Arch. 1986 Aug;407(2):199–203. doi: 10.1007/BF00580676. [DOI] [PubMed] [Google Scholar]
- Vitoux D., Olivieri O., Garay R. P., Cragoe E. J., Jr, Galacteros F., Beuzard Y. Inhibition of K+ efflux and dehydration of sickle cells by [(dihydroindenyl)oxy]alkanoic acid: an inhibitor of the K+ Cl- cotransport system. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4273–4276. doi: 10.1073/pnas.86.11.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]


