Abstract
The H2-evolution kinetics play a pivotal role in governing the photocatalytic hydrogen-evolution process. However, achieving precise regulation of the H-adsorption and H-desorption equilibrium (Hads/Hdes) still remains a great challenge. Herein, we propose a fine-tuning d-p hybridization strategy to precisely optimize the Hads/Hdes kinetics in a Ni-Bx modified CdS photocatalyst (Ni-Bx/CdS). X-ray absorption fine-structure spectroscopy and theoretical calculations reveal that increasing B-atom amount in the Ni-Bx cocatalyst gradually strengthens the d-p orbital interaction between Ni3d and B2p, resulting in a consecutive d-band broadening and controllable d-band center on Ni active sites. The above consecutive d-band optimization allows for precise modulation of the Hads/Hdes dynamics in the Ni-Bx/CdS, ultimately demonstrating a remarkable H2-evolution activity of 13.4 mmol g-1 h-1 (AQE = 56.1 %). The femtosecond transient absorption spectroscopy further confirms the rapid electron-transfer dynamics in the Ni-Bx/CdS photocatalyst. This work provides insights into the optimal design of prospective H2-evolution catalysts.
Subject terms: Photocatalysis, Catalytic mechanisms, Physical chemistry
A d-p orbital hybridization strategy is proposed to optimize the d-band center of cocatalyst active sites, achieving a precise H-adsorption/desorption balance for improving photocatalytic H2 evolution kinetics and efficiency.
Introduction
Hydrogen (H2) production over photocatalysts utilizing solar energy is a critical challenge for achieving a carbon-free, sustainable economy1–3. However, bare photocatalysts typically exhibit poor H2-production efficiency due to the significant recombination of photogenerated carriers and sluggish interfacial H2-reduction kinetics4,5. To address these limitations, cocatalysts are commonly employed to accelerate electron-hole separation and provide active sites to boost the interfacial H2-evolution process6,7. Ideally, an outperforming H2-production active site can effectively form an appropriate active site-Had bond, which simultaneously accelerates the proton capture and free H2 molecules release, thereby promoting the overall H2-production process8–11. Unfortunately, most active sites of reported cocatalysts suffer from either too-strong or too-weak active site-Had bonds owing to their unfavorable electron configurations, severely limiting their H2-evolution performance12,13. Therefore, reasonable electronic optimization of the active sites to achieve a balanced H-adsorption/desorption process is a key factor in improving the photocatalytic H2-production performance14–16.
According to the d-band theory, the H-adsorption/desorption dynamics at the active sites are primarily determined by the d-band electronic configuration of transition metal atoms, which governs the active site-Had bond strength and, in turn, controls the H2-evolution process17–19. However, for most transition metals, the d-band electrons possess a typical localized feature, with the majority of d-electrons confined within a narrow energy range, which causes electron inertness and thus inhibits the catalytic performance20. To date, numerous endeavors have been undertaken to adjust the d-band electronic configuration to optimize the catalytic performance of the transition metal active site21–24. For instance, alloying with hetero-transition metal atoms can modulate the d-band configuration of the active site via d–d orbital hybridization25–27. However, such d–d orbital hybridization normally yields low modulation efficiency due to the similarities in d-orbital configurations and electronic properties among transition metal elements (Fig. 1a). To further adjust the d-band electronic configuration, the d–p orbital regulation strategy has been developed28–31, which can more effectively adjust the d-band configuration of transition metal sites through enhanced orbital interactions (Fig. 1b(1)). In this case, the introduced p-orbital increases the overlapped region and effectively broadens the distributed bandwidth of the d-band, causing the formation of a wider electronic d-band energy range (Fig. 1b(2)). As a result, the strong d–p orbital hybridization effect has been widely reported to improve the catalytic performance of the transition metal active sites. For example, the catalytic activity of Nb atoms can be effectively improved via the d–p orbital hybridization within the Nb-N4 active moiety32. Similarly, the introduction of P atoms into Os can significantly boost the water-splitting activity of the Os atoms via d–p hybridization in the osmium phosphide catalyst33. However, the aforementioned d–p regulation strategies typically focus on coordination-number-limited single atoms or composition-fixed metal compound systems34, which exhibits isolated modulation of the d-band configuration, thereby limiting the flexibility in adjusting H2-evolution dynamics. Therefore, it is conceivable that a consecutive d–p modulation strategy could precisely optimize the d-band orbital configuration of transition metal atoms, thereby achieving the optimal catalytic H2-evolution dynamics.
Fig. 1. The strategy of fine-tuning d–p hybridization in Ni–Bx cocatalyst for the suitable Hads/Hdes kinetics.
a Schematic diagram illustrating the low-efficiency d–d orbital regulation. b Schematic diagram illustrating the broadened d-band via high-efficiency d–p orbital regulation. c Schematic diagram for the consecutive Ni d-band broadening to downshift d-band center in composition-adjustable Ni–Bx. d Schematic illustrating the tunable hydrogen-adsorption dynamic to reach the suitable Hads/Hdes balance.
Compared with traditional p-block atoms (O, N, S, etc.), the boron (B) atom possesses a typical electron-deficient feature, enabling it to form metalloid bonds with transition metal atoms and produce composition-tunable metal boride alloys35–39. In this study, a consecutive d–p hybridization strategy is pioneered to modulate the d-band configuration of the Ni active site in composition-adjustable Ni–Bx (Fig. 1c), aiming to precisely optimize the Hads/Hdes dynamic balance at Ni active sites to promote the H2-evolution performance of Ni–Bx cocatalyst (Fig. 1d). Herein, the Ni–Bx cocatalyst with tunable B/Ni ratios is skillfully fabricated on the cadmium sulfide (CdS) surface to produce Ni–Bx/CdS photocatalysts via a mild photo-triggered self-catalytic route. Systematic studies reveal that increasing B content in Ni–Bx cocatalyst can consecutively increase the d-band bandwidth of Ni, thereby controllably downshifting the d-band center of Ni active site to weaken the catalytic Ni–Had bonds, accordingly boosting the H2 desorption to improve the catalytic H2-evolution activity. As a result, the optimal Ni–Bx/CdS photocatalyst displayed an extraordinary photocatalytic H2-evolution performance of 13.4 mmol g−1 h−1 along with abundant viewable hydrogen bubbles, which is 5.5 times higher than that of Ni/CdS photocatalyst (2.4 mmol g−1 h−1), and its H2-evolution activity also surpassed that of the benchmark Pt/CdS and other state-of-the-art photocatalysts (Supplementary Table 1). This work determined a consecutive d–p modulation strategy to precisely optimize the dynamic Hads/Hdes balance of active sites for hydrogen evolution, offering insights for the design of prospective catalysts.
Results and discussion
Synthetic strategy and structural characterizations of Ni–Bx/CdS photocatalyst
The composition-adjustable Ni–Bx nanoparticles are meticulously deposited on the CdS surface using a facile light-induced self-catalytic approach (Fig. 2a). Firstly, bare CdS particles are homogeneously dispersed into a mixed solution of nickel acetate (Ni(CH3COO)2) and dimethylamine borane (Me2NH·BH3) to obtain a stable orange suspension (Fig. 2a(1)). Upon exposure to visible light for ~2 min, the CdS semiconductor can be easily excited to produce light-excited electrons, which can successfully migrate to the CdS surface to trigger the reaction between Ni2+ and Me2NH·BH3 to generate the Ni–Bx seeds (Fig. 2a(2)). After the light is turned off, the above Ni–Bx seeds can spontaneously induce the following catalytic reaction of Ni2+ and Me2NH·BH340, ultimately leading to the in situ formation of Ni–Bx nanoparticles on CdS surface to fabricate the Ni–Bx/CdS photocatalyst (Fig. 2a(3)). The above electron-induced self-catalytic formation mechanism of Ni–Bx nanoparticles is further demonstrated via the supporting experiments (Supplementary Fig. 1), in which trace NaBH4 is added as an electron provider into the mixed solution of Ni2+ and Me2NH·BH3 to produce the Ni–Bx seeds. These seeds then catalyze the spontaneous formation of black Ni–Bx nanoparticles41. Furthermore, based on the color changes observed (Supplementary Fig. 2), the orange CdS suspension gradually turns deep dark during the self-catalytic process, suggesting the efficacious formation of black Ni–Bx nanoparticles on CdS surface. In addition, according to the color changes (Supplementary Fig. 3), the Ni–Bx cocatalyst can also be effectively fabricated on other host photocatalysts (the typical TiO2 and g-C3N4 semiconductors) via the present synthetic method. Therefore, it can be rationally concluded that the Ni–Bx/CdS photocatalyst is successfully synthesized through the above photoinduced self-catalytic synthesis route.
Fig. 2. Synthetic strategy and morphology characterization.
a Schematic diagram illustrating the light-induced self-catalytic formation of Ni–Bx cocatalysts on CdS to fabricate Ni–Bx/CdS photocatalyst. b TEM, c–e HRTEM, f HAADF-STEM, and EDS mapping images of Ni–Bx/CdS.
Transmission electron microscopy (TEM) is employed to directly examine the microstructure of the Ni–Bx/CdS photocatalyst, and the resulting images are displayed in Fig. 2b–f. The images clearly show that numerous dark Ni–Bx nanoparticles (5–10 nm) are uniformly distributed on the CdS surface (Fig. 2b), exhibiting a disordered atomic arrangement (Fig. 2c–e), which is consistent with the results of fast Fourier transform and X-ray diffraction (XRD) results of pure Ni–Bx (Supplementary Figs. 4 and 5). Additionally, the successful construction of Ni–Bx/CdS can further be demonstrated by the EDS mapping (Fig. 2f), where the Ni and B signals overlap and are evenly distributed on the CdS surface, providing compelling evidence for the achievement of the Ni–Bx/CdS photocatalyst. Furthermore, based on the inductively coupled plasma optical emission spectroscopy (ICP-OES) results (Supplementary Table 2), it is shown that the composition-tunable Ni–Bx cocatalysts (with the atomic B/Ni ratio range from 0.15 to 0.89) can be successfully fabricated by adjusting the amount of Me2NH·BH3 boron source. Moreover, according to the UV-vis spectra results (Supplementary Fig. 6), the light absorption from 520 to 800 nm of Ni–Bx/CdS is significantly greater than that of CdS and Ni/CdS samples, with the absorption intensity increasing as the B content rises, reaffirming the successful loading of the composition-adjustable Ni–Bx cocatalyst42,43. In addition, XRD, RAMAN, IR, and field emission scanning electron microscopy (FESEM) results (Supplementary Figs. 7–10) demonstrate that the Ni–Bx/CdS displays a similar internal crystalline structure and apparent morphology to CdS and Ni/CdS, indicating that the photoinduced synthetic strategy has minimal impact on the host CdS44–46. Therefore, the above results collectively demonstrate the effective fabrication of the composition-adjustable Ni–Bx cocatalyst on CdS through a mild photoinduced self-catalytic route for synthesizing the Ni–Bx/CdS photocatalyst.
Efficient photocatalytic performance and its interfacial H2-evolution mechanism
The activities of the composition-tunable Ni–Bx cocatalysts are investigated via the photocatalytic hydrogen evolution tests, as displayed in Fig. 3. The pure CdS exhibits a poor H2-evolution activity of 0.4 mmol g−1 h−1 owing to the heavy recombination of electron-hole pair and inferior interfacial active sites (Fig. 3a(1))47,48. Even after the modification of Ni cocatalyst, the Ni/CdS photocatalyst only shows a slight performance improvement (2.4 mmol g−1 h−1, Fig. 3a(2)), implying the fact that the pristine Ni active sites still provide a limited H2-evolution activity. With the further integration of boron atoms into Ni cocatalyst, the resulting Ni–Bx/CdS photocatalysts exhibit significantly elevated H2-generation rates compared to CdS and Ni/CdS, and possess a volcano-plot trend (Fig. 3a(3–7)). Specifically, the optimum Ni–Bx/CdS sample (Ni–Bx/CdS-50) achieves a remarkable photocatalytic H2-production rate of 13.4 mmol g−1 h−1, which is 29.1 and 5.6 times over the CdS and Ni/CdS, respectively. Furthermore, based on its cycling performance, the Ni–Bx/CdS presents sustained durability with high H2-evolution activity (Fig. 3b) and can be well maintained after the photocatalytic performance testing (Supplementary Fig. 11). Impressively, under simulated sunlight irradiation, continuous viewable H2 bubbles can be observed on the surface of Ni–Bx/CdS sample, reconfirming the high-efficiency H2-evolution activity of Ni–Bx cocatalyst (Fig. 3c, d and Supplementary Movie). In addition, the H2-evolution activity of the present Ni–Bx/CdS surpasses that of the benchmark Pt/CdS (Supplementary Fig. 12) and other state-of-the-art CdS-based photocatalysts (Supplementary Table 1). Therefore, the consecutive integration of boron into Ni to produce Ni–Bx cocatalyst can significantly improve the photocatalytic hydrogen-generation activity of CdS.
Fig. 3. Photocatalytic H2-production activities.
a Photocatalytic H2-evolution activity of various samples: (1) CdS, (2) Ni/CdS, (3) Ni–Bx/CdS-5, (4) Ni–Bx/CdS-20, (5) Ni–Bx/CdS-50, (6) Ni–Bx/CdS-100, (7) Ni–Bx/CdS-200. The error bars (mean ± standard deviation) were calculated based on three independent photocatalytic experiments. b Cycling runs of the photocatalytic H2-evolution activity of the Ni–Bx/CdS. c Visible photocatalytic H2-evolution test of Ni–Bx/CdS. d Photographs for the viewable H2 bubbles on Ni–Bx/CdS.
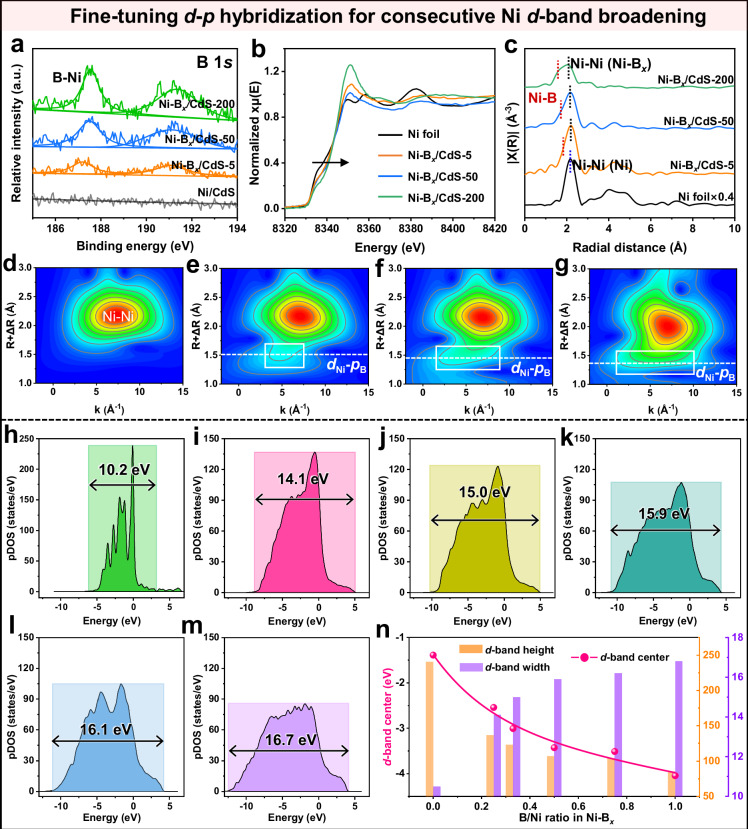
According to the above performance results, the H2-evolution activity of the Ni cocatalyst can be greatly improved by introducing boron, which is primarily ascribed to the fact that increasing boron integration strengthens the Nid–Bp interaction to modulate the d-band configuration of Ni, as demonstrated via the following theoretical and experimental investigations. Based on the X-ray photoelectron spectroscopy (XPS) results of B 1s (Fig. 4a) and Ni 2p (Supplementary Fig. 13), it can be found that with the increase of B-doping amount in the Ni–Bx cocatalyst, the typical Ni peak exhibits a simultaneous shift towards a more negative value, implying a consecutively enhanced electronic interaction between Ni and B in the Ni–Bx cocatalyst. In addition, the above results can also be validated through the Bader charges analysis in Supplementary Fig. 14. As the B-doping amount increases in the Ni–Bx (Supplementary Figs. 15 and 16), the average Bader charge value of Ni atoms increases gradually, while that of boron atoms decreases correspondingly, reconfirming the strengthened Ni3d–B2p electronic interaction. The fine-tuning Ni3d–B2p orbital hybridization in Ni–Bx cocatalyst is further evidenced by the following electronic states and coordination environments analysis using X-ray absorption spectroscopy. As displayed in Fig. 4b, the normalized X-ray absorption near-edge structure (XANES) spectra reveal the Ni adsorption K-edge for various samples. It can be easily observed that with the increase of B-doping amount in Ni–Bx cocatalyst, the Ni adsorption K-edge gradually exhibits a positive shift, demonstrating the consecutively enhanced electronic interaction of Ni and B atoms in Ni–Bx cocatalyst. Moreover, the d–p orbital hybridization in Ni–Bx cocatalyst is investigated via the Ni coordination shell analysis according to the following X-ray absorption fine structure (EXAFS) results. Based on the FT-EXAFS data (Fig. 4c), the Ni foil exhibits the individual dominant peak at ~2.2 Å in R space, suggesting the inherent Ni–Ni coordination in metallic Ni. After the introduction of B into Ni to form Ni–Bx cocatalyst, the peak at ~1.8 Å (representing the distance between first shell layer B and Ni atoms) can be easily observed, corresponding to the presence of Ni–B coordination in Ni–Bx cocatalyst49–51. Importantly, with the increase of the B-doping amount, the Ni–B coordination peaks gradually exhibit a negative shift from ~1.8 to ~1.6 Å, implying the strengthened Nid–Bp orbital interaction in the Ni–Bx cocatalyst. Moreover, wavelet transform-EXAFS is further conducted and the results are shown in Fig. 4d–f. The Ni sample exhibits an isolated signal corresponding to Ni–Ni. With the consecutive introduction of B into Ni, the signal of Ni–B coordination clearly appears and gradually enhances. In addition, with the increase of B-atom amounts in Ni–Bx, the signal of Ni–B shows a negative shift in R space, proving the gradually enhanced interaction between Ni and B in the Ni–Bx cocatalyst. Therefore, the consecutive introduction of B into Ni can effectively enhance the Ni3d–B2p orbital hybridization in Ni–Bx, thereby achieving the consecutive regulation of the Ni active site.
Fig. 4. Fine-tuning d–p hybridization in Ni–Bx for consecutive Ni d-band broadening.
a High-resolution B 1s XPS spectra. b Normalized XANES spectra. c FT-EXAFS spectra in R space. Wavelet transforms of Ni K-edge EXAFS data of d Ni foil, e Ni–Bx/CdS-5, f Ni–Bx/CdS-50, and g Ni–Bx/CdS-200. Projected DOS of Ni 3d orbital for h Ni, i Ni–B0.26, j Ni–B0.33, k Ni–B0.50, l Ni–B0.75, and m Ni–B1.00. n Calculated d-band heights, d-band widths, and d-band center values of different Ni–Bx.
The fine-tuning Ni3d–B2p orbital hybridization in Ni–Bx can consecutively broaden the d-band width of Ni active site, as further demonstrated by the following density of states (DOS) results (Fig. 4h–m, Supplementary Fig. 17). According to the projected DOS results, it is obvious that the d-band of the pristine Ni shows a highly localized character, where most of the d-electrons are confined to a narrow energy range of −7.2 to 3.1 eV (Fig. 4h, Supplementary Fig. 18(1)). After the consecutive Nid–Bp orbital interaction in Ni–Bx (x = 0.26, 0.33, 0.50, 0.75, and 1.00), all the Ni d-band of different Ni–Bx are significantly broadened (Fig. 4i–m), that is, the more B atoms are introduced, the broader Ni d-band is obtained. Especially, when the atomic B/Ni ratio is raised to 1.00, the distributed Ni d-band can be significantly broadened to a wide energy range of −12.7 to 3.9 eV (Fig. 4m). The d-band broadening degree of Ni–Bx samples is quantified using the d-band full width at half maxima (FWHM), as shown in Supplementary Fig. 19. It can be observed that the FWHM value of the Ni d-band increases progressively with higher B content, again proving the consecutive d-band broadening in Ni–Bx. The above consecutive d-band broadening in Ni–Bx can be assigned to the enhanced Ni3d–B2p orbital hybridization, as demonstrated by the statistical results of Ni–B bond length (Supplementary Fig. 20) according to the theoretical calculation. In this case, the Ni–B bond length of the Ni–Bx structure ranges from 1.8 to 2.6 Å. With increasing B amount in Ni–Bx, the percentage of Ni–B bonds with short length (1.8–2.0 Å) gradually increases (Supplementary Fig. 21), indicating the strengthened Nid–Bp interaction in Ni–Bx via consecutive B introduction, which is in line with the radial distribution function data (Supplementary Fig. 22) of Ni–B bonds in the Ni–Bx models. Consequently, the consecutive d-band broadening inevitably causes the generation of unbalanced electrons above the fermi level, resulting in the downshift of the d-band center (Supplementary Fig. 18(2, 3)). The abovementioned results can be proved by the following d-band center calculations in Fig. 4n. As the B/Ni ratio rises in Ni–Bx, the d-band width increases from 10.2 to 16.6 eV, while the d-band height decreases from 240.6 to 85.4 eV, therefore obtaining the gradually downshifted d-band center from −1.4 to −4.0 eV. Meanwhile, with the consecutive downshift of the d-band center, the electron occupancy number of the Ni3d orbital exhibits a gradual increase as well (Supplementary Fig. 23). Thus, the above results prove that the B introduction can gradually strengthen the Ni3d–B2p orbital interaction to broaden the d-band of Ni, thereby effectively modulating the catalytic d-band center of the Ni–Bx cocatalyst.
The consecutive d-band center modulation in Ni–Bx cocatalyst can precisely optimize the H-adsorption/desorption dynamics on the Ni active site to promote the interfacial H2-evolution reaction (Fig. 5). Herein, the hydrogen-adsorption free energy (ΔGH*) and crystal orbital Hamilton populations (COHP) analyses are conducted to reveal the H2-evolution dynamics according to the surface models of Ni–Bx (Fig. 5a). Theoretically, for the pristine Ni with a high d-band center, the antibonding states formed after the Ni–Had interaction exhibit low electron occupancy (Fig. 5b(left)), resulting in excessively strong H adsorption on Ni, which suppresses H2 desorption. This is further confirmed by the calculated negative ΔGH* value of −0.46 eV for the Ni site in the Ni model (Fig. 5c). Upon incorporating B into Ni to form Ni–Bx, the d-band center of Ni is controllably downshifted, increasing the antibonding orbital electronic occupancy, which destabilizes the Ni–Had bonds and promotes the H2-desorption process (Fig. 5b(right)). Consequently, based on the ΔGH* results in Fig. 5c, the ΔGH* value for the active Ni sites gradually increases and reaches an optimal value of 0.04 eV for Ni–B0.75, optimizing the H-adsorption/desorption balance on the Ni active site and thereby enhancing the H₂-evolution activity. These results are further supported by the COHP (ICOHP) analyses, as displayed in Fig. 5d. It can be observed that with the introduction of B into Ni, the integrated ICOHP value of the Ni–Had bond gradually rises from −2.53 to −1.20, indicating a weakening of H adsorption on the Ni site to promote the H2-desorption process (Fig. 5d and Supplementary Fig. 24). Moreover, the elongated Ni–Had bond length and the increased electron localization function (ELF) on Ni can further prove that the hydrogen-adsorption strength on Ni site further demonstrate that the H-adsorption strength on Ni active site can be effectively weakened through the successive introduction of B into Ni, thus facilitating H2 desorption (Fig. 5e). Therefore, it can be concluded from the above results that increasing B introduction into Ni can effectively strengthen the Nid–Bp orbital hybridization to modulate the catalytic d-band configuration of Ni active site, which is the intrinsic mechanism to optimize the H-adsorption/desorption dynamic on Ni to boost the H2-evolution activity (Fig. 5f).
Fig. 5. Precisely optimized Hads/des dynamics in Ni–Bx cocatalyst.
a Optimized surface structures of Ni and Ni–Bx for DFT calculation. b Diagram illustrating the broadening of d-band to increase the antibonding occupancy to weaken Ni–Had bonds. c Calculated ΔGH* values of multiple structures. d COHP data of Ni–Had bonds. e Electron local function (ELF) result after H adsorption on Ni. f A mechanism for controlling d-band configuration through d–p hybridization to regulate Hads/des kinetics towards enhancing H2-evolution activity.
Directional photoelectron transfer and its dynamics investigation
The directional photoelectron transfer process of Ni–Bx/CdS under light irradiation is investigated using in situ Kelvin probe force microscopy (KPFM) and in situ XPS (Supplementary Figs. 25 and 26). It can be clearly observed from the contact potential difference (CPD) tests (Supplementary Fig. 25) that bare CdS displays a surface potential of 0.24 V52–54. After the modification of Ni–Bx cocatalyst on the CdS surface, a decreased CPD (0.14 V) is observed, indicating the transfer of free electrons from CdS to Ni–Bx, which can be attributed to the higher work function of Ni–Bx compared to CdS (Supplementary Fig. 27). When the Ni–Bx/CdS is irradiated with visible light, an increased surface potential difference (0.21 V) is observed, corresponding to the light-excited photoelectron of CdS, which further induces photoelectron migration to the Ni–Bx cocatalyst, as further evidenced by the in situ irradiated XPS characterization (Supplementary Fig. 26). When the light is turned on, the binding energy of Cd 3d peaks shifts positively to a high value, while that of Ni 2p peaks shift negatively, strongly verifying the photoelectrons transfer from host CdS to Ni–Bx cocatalyst in the Ni–Bx/CdS55.
Femtosecond transient absorption spectroscopy (fs-TAS) is further employed to investigate the photoelectron transfer dynamics of various samples (Fig. 6 and Supplementary Fig. 28). For CdS (Fig. 6a, b), the transient absorption spectrum shows the positive signal (ΔA > 0) at around 480 nm, which mainly belongs to the excited state absorption (ESA), representing the excited-electron transition from low to high energy level in the conduction band (CB)56,57. Furthermore, the negative signal (ΔA < 0) in the range of 550–700 nm can be ascribed to the stimulated emission (SE), corresponding to the electronic emission from CB to the low energy levels58. In addition, a negative ground-state bleach signal at around 470 nm appears within the initial 700 fs (Supplementary Fig. 29)59,60, and then quickly delays or overlaps with the ESA signal in the range of 460–510 nm after 900 fs. After the modification of Ni–Bx cocatalyst on the CdS surface (Fig. 6d, e), the ESA and SE signals of the Ni–Bx/CdS sample are clearly weaker than that of pure CdS photocatalyst, indicating that the pump pulse-excited electrons on CdS surface can effectively migrate to Ni–Bx cocatalyst, resulting in the suppression of the ESA and SE process. The photoelectron transfer from CdS to Ni–Bx cocatalyst is further confirmed by using potassium dichromate as the electron scavenger (Fig. 6g and Supplementary Fig. 30). It is found that, with the addition of Cr2O72−, CdS exhibits very limited ESA and SE signals, implying the fact that the excited electrons on the CB of CdS can be efficiently consumed by Cr2O72− scavenger. Moreover, to quantify the photoelectron transfer efficiency in CdS and Ni–Bx/CdS, the kinetic delay curves of samples for ESA and SE processes are fitted (Fig. 6c, f, h, i). It can be observed that the bare CdS shows a long average lifetime (14.5 ps for the EAS process and 6.4 ps for the SE process), indicating the fact that the excited electrons in the CB of CdS are difficult to be consumed or transferred. With the modification of Ni–Bx cocatalyst, owing to the rapid electron transfer from CdS CB to Ni–Bx, the average lifetimes of excited electrons in both ESA (8.5 ps) and SE (4.7 ps) processes are significantly reduced, strongly suggesting the fast photoelectron transfer in Ni–Bx/CdS. Furthermore, these results are consistent with the findings from photoelectrochemical tests and transient-state photoluminescence (TRPL) spectra (Supplementary Fig. 31). Therefore, the above results forcefully prove that the photoexcited electrons of CdS are efficiently captured by the Ni–Bx cocatalyst, thereby triggering the interfacial directional photoelectron transfer process.
Fig. 6. Photogenerated electron transfer mechanism and dynamics.
Pseudocolor plots, femtosecond transient absorption signals within 100 ps, and corresponding decay curves of CdS (a–c) and Ni–Bx/CdS (d–f) (ESA and SE represent excited state absorption and stimulated emission, respectively). g fs-TAS data of CdS with the addition of Cr2O72−. h, i Diagram illustrating the achievement of expedited interfacial charge transfer in the Ni–Bx/CdS.
In summary, a fine-tuning Ni3d–B2p hybridization strategy is proposed to modulate the d-band configurations of the Ni atoms, thereby precisely optimizing the H-adsorption/desorption dynamics of the Ni active sites in the Ni–Bx cocatalyst. Experimental and theoretical results disclose that the consecutively increasing B introduction in Ni–Bx gradually enhances the Ni3d–B2p orbital hybridization, which can effectively broaden the d-band distribution of Ni to manipulate the d-band center of Ni–Bx cocatalyst. Such results can precisely optimize the H-adsorption/desorption dynamic of the Ni active site to boost the photocatalytic H2-evolution activity of the Ni–Bx/CdS. As expected, the Ni–Bx/CdS photocatalyst shows a high H2-evolution performance of 13.4 mmol g−1 h−1 with continuously viewable H2 bubble release, which is 5.6 times over the Ni/CdS. This study reveals an in-depth mechanism of tunable d-band configurations via modulating d–p electronic interaction, providing insights into the catalyst design and application principles.
Methods
Preparation of Ni–Bx/CdS photocatalyst
The composition-tunable Ni–Bx/CdS photocatalyst is prepared by a photoinduced self-catalytic strategy. First, 100 mg of commercial CdS was added into 80 mL methanol-water solution (10 vol%) under constant magnetic stirring. Next, 5 mL Ni(CH3COO)2 solution (8.48 mg mL−1) is added into the above suspension by an injector, where the mass ratio of Ni to CdS is controlled to be 1:10. Then, under magnetic stirring, various amount (5, 20, 50, 100, 200 mg) of Me2NH·BH3 (B source) is added to the above system, respectively. Subsequently, the above system was evacuated with N2 for 15 min and then was sealed with a rubber septum. The reaction is triggered via 2-min irradiation by using four LED illuminants (3 W, 420 nm) under stirring. After the light is turned off, the above system is magnetically stirred for 2 h under dark conditions to enable the self-catalytic formation of Ni–Bx nanoparticles on CdS. Finally, the products are filtered, washed, and dried overnight at 60 °C. The obtained photocatalysts are referred to Ni–Bx/CdS–X, where X represents the added Me2NH·BH3 amounts of 5, 20, 50, 100 and 200 mg, respectively. In this regard, the Ni–Bx/CdS-50 sample shows the best performance, so it is named as Ni–Bx/CdS photocatalyst. For comparison, the Ni/CdS photocatalyst is prepared by a 2 h of traditional direct photodeposition approach in the lack of adding Me2NH·BH3 boron source. All the above photocatalysts could be obtained with a yield of over 90%. All the chemicals are of analytical grade (AR) and were used without further purification. CdS (99%) and borane dimethylamine complex (C2H10BN, 96%) were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Nickel acetate tetrahydrate (NiC4H6O4·4H2O, 99%) was purchased from Macklin Biochemical Technology Co., Ltd. (Shanghai, China).
Characterization
The ultraviolet-visible diffuse reflectance spectra (UV-vis DRS) of the samples were obtained on the UV-vis spectrophotometer (UV-2600, Shimadzu, Japan). The crystal phase of various samples was analyzed by X-ray diffractometer (XRD-6100, Shimadzu, Japan). The morphologies of all the as-prepared samples were investigated via FESEM (JMS-7500, JEOL, Japan). The microstructure of the Ni–Bx/CdS was studied by TEM (Titan G2, FEI, USA). The elemental content analysis was implemented using ICP-OES. The Fourier transform infrared spectra were collected on a spectrometer (Nicolet iS50, Thermo Scientific, USA). The XPS of samples was measured on the electron spectrometer (ESCALAB 250Xi, Thermo Fisher, USA) with Al Kα source (1486.6 eV), and all the binding energies were referenced to the adventitious C 1s line at 248.4 eV. X-ray absorption fine structure (XAFS) spectra of the Ni K-edge are obtained in transmission mode on Table XAFS-500. Photo-irradiated KPFM was performed on SPM-9700 (Shimadzu, Japan). The time-resolved photoluminescence (TRPL) spectra were acquired using a fluorescence lifetime spectrophotometer (FLS1000, Edinburgh, UK).
Photocatalytic H2-production test
The photocatalytic H2-evolution activity was assessed in a 100 mL three-neck Pyrex flask, maintained at ambient temperature and atmospheric pressure. For each experiment, 0.05 g of the photocatalyst was added to 80 mL of an aqueous solution containing 10 vol% lactic acid as a hole scavenger. The flask was then purged with high-purity nitrogen gas (99.999%) to eliminate air (15 min). The system was stirred magnetically and exposed to four 3 W LED lamps (420 nm) to conduct photocatalytic H2 production. Under 0.5 h of illumination, the evolved gas (0.4 mL) was analyzed using a Shimadzu GC-2014C gas chromatograph (Japan, N2 as the carrier gas). After the 2 h periodic tests, the photocatalytic H2-evolution activity data of various photocatalysts were obtained.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFB3803600 (J.Y.)), National Natural Science Foundation of China (U23A20102 (Z.Z.), U22A20147 (H.Y.) and 22075220 (H.Y.)) and the Natural Science Foundation of Hubei Province of China (2022CFA001 (H.Y.)). We thank the Faculty of Materials Science and Chemistry, China University of Geosciences (CUG), Wuhan for its TEM facilities and the data analysis of Dr. Mingxing Gong.
Author contributions
H.L. and H.Y. conceived and designed the experiments. H.L. and X.Z. carried out the synthesis, characterizations, and theory calculations of the materials. H.L. carried out the photocatalytic test. H.L., X.Z., Z.Z., J.Z., J.Y. and H.Y. contributed to data analysis. J.Y. and H.Y. supervised the project. H.L. and H.Y. wrote the manuscript. J.Y. and H.Y. revised and reviewed the manuscript. All authors discussed the results and commented on the manuscript.
Peer review
Peer review information
Nature Communications thanks Xinlong Tian, Tae Kyu Kim, Zhenjiang Li, Shizhang Qiao, and Ying Zhou reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The experimental data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiaguo Yu, Email: yujiaguo93@cug.edu.cn.
Huogen Yu, Email: yuhuogen@cug.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-56306-x.
References
- 1.Wang, J. et al. Enabling enhanced photocatalytic hydrogen evolution in water by doping Cs2SnBr6 perovskite with Pt. ACS Energy Lett.9, 653–661 (2024). [Google Scholar]
- 2.Wang, X. et al. Sulfone-containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nat. Chem.10, 1180–1189 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Bie, C. et al. A bifunctional CdS/MoO2/MoS2 catalyst enhances photocatalytic H2 evolution and pyruvic acid synthesis. Angew. Chem. Int. Ed.61, e202212045 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Jin, N. et al. Type-I CdS/ZnS core/shell quantum dot-gold heterostructural nanocrystals for enhanced photocatalytic hydrogen generation. J. Am. Chem. Soc.145, 21886–21896 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Zhou, Q. et al. Photocatalytic sacrificial H2 evolution dominated by micropore-confined exciton transfer in hydrogen-bonded organic frameworks. Nat. Catal.6, 574–584 (2023). [Google Scholar]
- 6.Cao, S. et al. Ultrasmall CoP nanoparticles as efficient cocatalysts for photocatalytic formic acid dehydrogenation. Joule2, 549–557 (2018). [Google Scholar]
- 7.Gao, D. et al. Optimizing atomic hydrogen desorption of sulfur-rich NiS1+x cocatalyst for boosting photocatalytic H2 evolution. Adv. Mater.34, 108475 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Pérez, J. et al. Strategies to break linear scaling relationships. Nat. Catal.2, 971–976 (2019). [Google Scholar]
- 9.He, L. et al. Molybdenum carbide-oxide heterostructures: in situ surface reconfiguration toward efficient electrocatalytic hydrogen evolution. Angew. Chem. Int. Ed.59, 3544–3548 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Shah, A. H. et al. The role of alkali metal cations and platinum-surface hydroxyl in the alkaline hydrogen evolution reaction. Nat. Catal.5, 923–933 (2022). [Google Scholar]
- 11.Huang, Z. et al. Edge sites dominate the hydrogen evolution reaction on platinum nanocatalysts. Nat. Catal.7, 678–688 (2024). [Google Scholar]
- 12.Xie, Y. et al. Evidence for an interface of hybrid cocatalysts favoring photocatalytic hydrogen evolution kinetics. ACS Appl. Mater. Interfaces15, 59309–59318 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Wang, M. et al. Self-optimized H-adsorption affinity of CuRu alloy cocatalysts towards efficient photocatalytic H2 evolution. J. Mater. Sci. Technol.174, 168–175 (2024). [Google Scholar]
- 14.Su, H. et al. A 2D bimetallic Ni-Co hydroxide monolayer cocatalyst for boosting photocatalytic H2 evolution. Chem. Commun.58, 6180–6183 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Gao, D. et al. Reversing free-electron transfer of MoS2+x cocatalyst for optimizing antibonding-orbital occupancy enables high photocatalytic H2 evolution. Angew. Chem. Int. Ed.62, e202304559 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Zhao, T. et al. Heterostructured V-doped Ni2P/Ni12P5 electrocatalysts for hydrogen evolution in anion exchange membrane water electrolyzers. Small18, 2204758 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Liu, J. et al. Optimizing hydrogen adsorption by d-d Orbital modulation for efficient hydrogen evolution catalysis. Adv. Energy Mater.12, 2103301 (2022). [Google Scholar]
- 18.Yan, Y. et al. Orienting electron fillings in d orbitals of cobalt single atoms for effective zinc-air battery at a subzero temperature. Adv. Funct. Mater.34, 2316100 (2024). [Google Scholar]
- 19.Wu, X. et al. Tuning the d-band center of Co3O4 via octahedral and tetrahedral codoping for oxygen evolution reaction. ACS Catal.14, 5888–5897 (2024). [Google Scholar]
- 20.Tian, J. et al. Sabatier relations in electrocatalysts based on high-entropy alloys with wide-distributed d-band centers for Li-O2 batteries. Angew. Chem. Int. Ed.62, e202310894 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Gao, D. et al. Tailoring antibonding-orbital occupancy state of selenium in Se-enriched ReSe2+x cocatalyst for exceptional H2 evolution of TiO2 photocatalyst. Adv. Funct. Mater.33, 2209994 (2023). [Google Scholar]
- 22.Liu, H. et al. Tailoring d-band center of high-valent metal-oxo species for pollutant removal via complete polymerization. Nat. Commun.15, 2327 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, C. et al. Octahedral nanocrystals of Ru-doped PtFeNiCuW/CNTs high-entropy alloy: high performance toward pH-universal hydrogen evolution reaction. Adv. Mater.36, 2400433 (2024). [DOI] [PubMed] [Google Scholar]
- 24.Zhang, X. et al. Enhancing photocatalytic H2O2 production with Au co-catalysts through electronic structure modification. Nat. Commun.15, 3212 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, A. et al. Opening the bandgap of metallic half-heuslers via the introduction of d-d orbital interactions. Adv. Sci.10, 2302086 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei, M. et al. High-entropy alloy nanocrystal assembled by nanosheets with d-d electron interaction for hydrogen evolution reaction. Energy Environ. Sci.16, 4009 (2023). [Google Scholar]
- 27.Joshi, U. et al. Ruthenium-tungsten composite catalyst for the efficient and contamination-resistant electrochemical evolution of hydrogen. ACS Appl. Mater. Interfaces10, 6354–6360 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Han, Z. et al. Engineering d-p orbital hybridization in single-atom metal-embedded three-dimensional electrodes for Li-S batteries. Adv. Mater.33, 2105947 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Li, F. et al. Rhodium and carbon sites with strong d-p orbital interaction for efficient bifunctional catalysis. ACS Nano17, 24282–24289 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Zhu, W. et al. Weakened d-p orbital hybridization in in situ reconstructed Ru/β-Co(OH)2 heterointerfaces for accelerated ammonia electrosynthesis from nitrates. Energy Environ. Sci.16, 2483 (2023). [Google Scholar]
- 31.Feng, Y. et al. Alleviating the competitive adsorption of hydrogen and hydroxyl intermediates on Ru by d-p orbital hybridization for hydrogen electrooxidation. Chem. Sci.15, 2123 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, Y. et al. d-p hybridization-induced “trapping-coupling-conversion” enables high-efficiency Nb single-atom catalysis for Li-S batteries. J. Am. Chem. Soc.145, 1728–1739 (2023). [DOI] [PubMed] [Google Scholar]
- 33.Li, Q. et al. Strong d-p orbital hybridization of Os-P via ultrafast microwave plasma assistance for anion exchange membrane electrolysis. Adv. Funct. Mater. 34, 2408517 (2024).
- 34.Zhao, J. et al. Tailoring d-p orbital hybridization to decipher the essential effects of heteroatom substitution on redox kinetics. Angew. Chem. Int. Ed.63, e202404968 (2024). [DOI] [PubMed] [Google Scholar]
- 35.Kang, Y. et al. Porous nanoarchitectures of nonprecious metal borides: from controlled synthesis to heterogeneous catalyst applications. ACS Catal.12, 14773–14793 (2022). [Google Scholar]
- 36.Qi, Y. et al. Insights into the activity of nickel boride/nickel heterostructures for efficient methanol electrooxidation. Nat. Commun.13, 4602 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, E. et al. Nonprecious metal borides: emerging electrocatalysts for hydrogen production. Acc. Chem. Res.55, 56–64 (2022). [DOI] [PubMed] [Google Scholar]
- 38.Long, H. et al. Amorphization-induced reverse electron transfer in NiB cocatalyst for boosting photocatalytic H2 production. Appl. Catal. B Environ.340, 123270 (2024). [Google Scholar]
- 39.Park, H. et al. Graphene- and phosphorene-like boron layers with contrasting activities in highly active Mo2B4 for hydrogen evolution. J. Am. Chem. Soc.139, 12915–12918 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Kang, Y. et al. Mesoporous metal-metalloid amorphous alloys: the first synthesis of open 3D mesoporous Ni-B amorphous alloy spheres via a dual chemical reduction method. Small16, 1906707 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Kang, Y. et al. Amorphous alloy architectures in pore walls: mesoporous amorphous NiCoB alloy spheres with controlled compositions via a chemical reduction. ACS Nano14, 17224–17232 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Xiang, X. et al. Ultrafast electron transfer from CdS quantum dots to atomically-dispersed Pt for enhanced H2 evolution and value-added chemical synthesis. Appl. Catal. B Environ.340, 123196 (2024). [Google Scholar]
- 43.Zhang, J. et al. Electron transfer kinetics in CdS/Pt heterojunction photocatalyst during water splitting. Chin. J. Catal.42, 530–2538 (2022). [Google Scholar]
- 44.Wu, F. et al. Enhanced spin-polarized electric field modulating p-band center on Ni-doped CdS for boosting photocatalytic hydrogen evolution. Small20, 2309439 (2024). [DOI] [PubMed] [Google Scholar]
- 45.Xiang, X. et al. Cadmium chalcogenide (CdS, CdSe, CdTe) quantum dots for solar-to-fuel conversion. Adv. Photonics Res.3, 2200065 (2022). [Google Scholar]
- 46.Ge, F. et al. Elucidating facet-dependent photocatalytic activities of metastable CdS and Au@CdS core-shell nanocrystals. ACS Appl. Mater. Interfaces16, 32847–32856 (2024). [DOI] [PubMed] [Google Scholar]
- 47.Boonta, W. et al. Rhenium(I) complex-containing amphiphilic metallopolymer stabilizing CdS quantum dots for synergistically boosting photoreduction of CO2. ACS Catal.18, 12391–12402 (2023). [Google Scholar]
- 48.Yang, Y. et al. Enhanced photocatalytic H2-production activity of CdS nanoflower using single atom Pt and graphene quantum dot as dual cocatalysts. Chin. J. Struct. Chem.41, 2206006–2206014 (2022). [Google Scholar]
- 49.Wang, F. et al. Modulating electronic structure of atomically dispersed nickel sites through boron and nitrogen dual coordination boosts oxygen reduction. Adv. Funct. Mater.33, 2213863 (2023). [Google Scholar]
- 50.Wang, N. et al. Temperature-induced low-coordinate Ni single-atom catalyst for boosted CO2 electroreduction activity. Small19, 2301469 (2023). [DOI] [PubMed] [Google Scholar]
- 51.Wu, J. et al. Composition engineering of amorphous nickel boride nanoarchitectures enabling highly efficient electrosynthesis of hydrogen peroxide. Adv. Mater.34, 2202995 (2022). [DOI] [PubMed] [Google Scholar]
- 52.Zhang, Z. et al. Amplified internal electric field of Cs2CuBr4@WO3-x S-scheme heterojunction for efficient CO2 photoreduction. J. Energy Chem.92, 521–533 (2024). [Google Scholar]
- 53.Biglarbeigi, P. et al. Unraveling spatiotemporal transient dynamics at the nanoscale via wavelet transform-based kelvin probe force microscopy. ACS Nano17, 21506–21517 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, F. et al. In situ metal-oxygen-hydrogen modified B-TiO2@Co2P-X S-scheme heterojunction effectively enhanced charge separation for photo-assisted uranium reduction. Adv. Sci.11, 2305439 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao, S. et al. Insights into photocatalytic mechanism of H2 production integrated with organic transformation over WO3/Zn0.5Cd0.5S S-scheme heterojunction. Adv. Sci.11, 2305439 (2024). [Google Scholar]
- 56.Cheng, C. et al. In-situ formatting donor-acceptor polymer with giant dipole moment and ultrafast exciton separation. Nat. Commun.15, 1313 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng, X. et al. Ultrafast electron transfer at the In2O3/Nb2O5 S-scheme interface for CO2 photoreduction. Nat. Commun.15, 4807 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ostovar, B. et al. The role of the plasmon in interfacial charge transfer. Sci. Adv.10, eadp3353 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu, J. et al. COF/In2S3 S-scheme photocatalyst with enhanced light absorption and H2O2-production activity and fs-TA investigation. Adv. Mater.36, 2400288 (2024). [DOI] [PubMed] [Google Scholar]
- 60.Dana, J. et al. Unusually strong biexciton repulsion detected in quantum confined CsPbBr3 nanocrystals with two and three pulse femtosecond spectroscopy. ACS Nano15, 9039–9047 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The experimental data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.