Abstract
1. A neuronal relay for input from group II afferents of hindlimb muscle nerves has been found in the previously little explored sacral segments of the cat spinal cord. 2. Electrical stimulation of group II muscle afferents of a number of nerves evoked negative potentials on the surface (cord dorsum potentials) and population postsynaptic potentials (field potentials) within the sacral segments. The largest potentials were evoked by stimulation of the posterior biceps-semitendinosus and triceps surae nerves which evoke much smaller potentials in other segments. Group II afferents of other nerves, notably those which have their main relay within the middle lumbar segments, were much less effective. 3. The sites at which cord dorsum and field potentials evoked by group II muscle afferents were recorded varied in relation to the external topography of the L7-S2 spinal segments but were consistent in their location relative to the pudendal motor nucleus (Onuf's nucleus). Potentials evoked by group II afferents of the posterior biceps and semitendinosus nerves peaked at a level corresponding to the rostral half of Onuf's nucleus and potentials evoked by afferents of the gastrocnemius nerves peaked just rostral to this nucleus. The largest field potentials (of 0.5-1.0 mV) were recorded within the dorsal horn. Field potentials in the intermediate zone were much smaller (< 0.3 mV) and were seen less frequently. 4. Evidence was obtained that the dorsal horn field potentials are to a great extent evoked monosynaptically by the fast conducting fraction of group II muscle afferents: (i) they were evoked at short latencies (2.4-2.7 ms from the stimulus; 1.3-1.7 ms from group I components of afferent volleys and 0.5-0.7 ms from group II components of these volleys), (ii) the conduction times of impulses in the fastest conducting fraction of group II afferents, between the sacral segments (where these impulses were induced by intraspinal stimuli) and the peripheral nerves, were only about 0.5 ms shorter than the latencies of field potentials recorded at the site of intraspinal stimulation and evoked by stimulation of the same peripheral nerves and, (iii) the field potentials followed repetitive stimuli without temporal facilitation. 5. Negative cord dorsum and field potentials were also evoked by small stretches of the semitendinosus and triceps surae muscles. Although they were smaller than potentials evoked by electrical stimulation of sensory fibres and appeared at longer latencies, their presence is consistent with a contribution of muscle spindle afferents to the actions of group II muscle afferents within the sacral segments.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



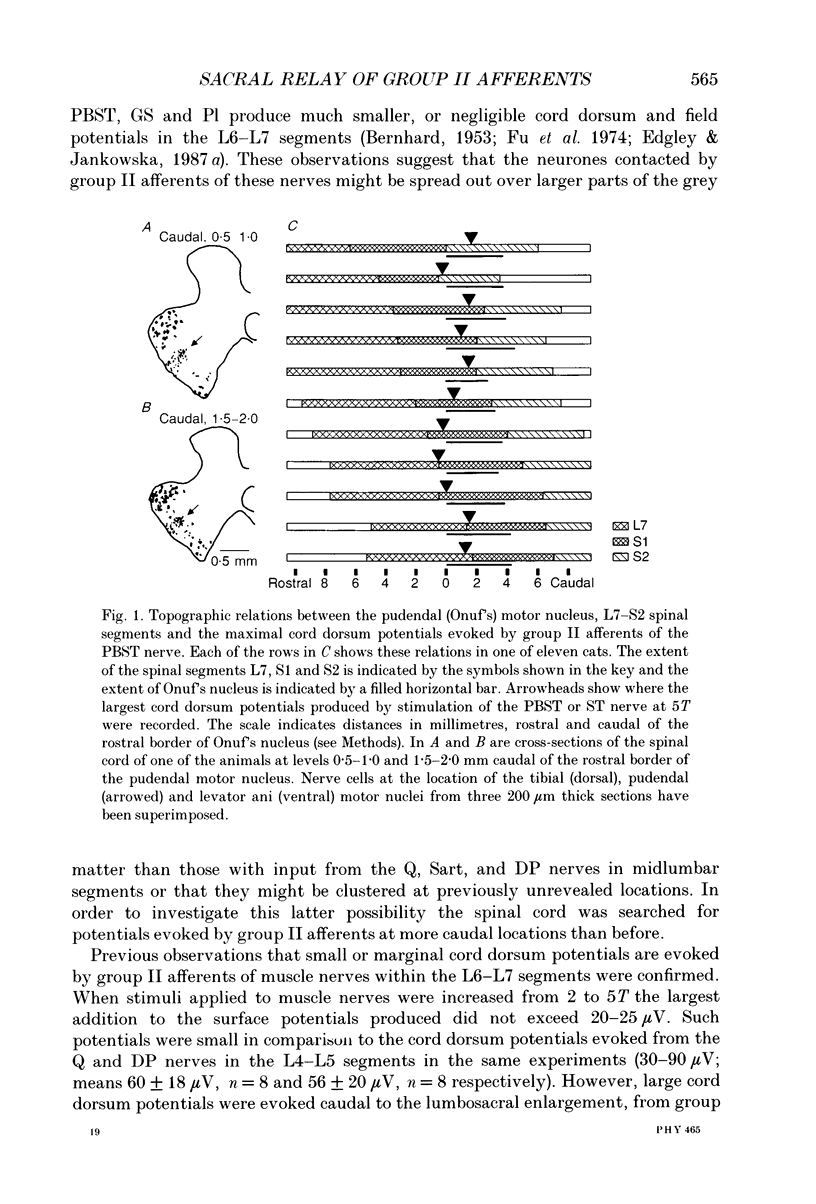
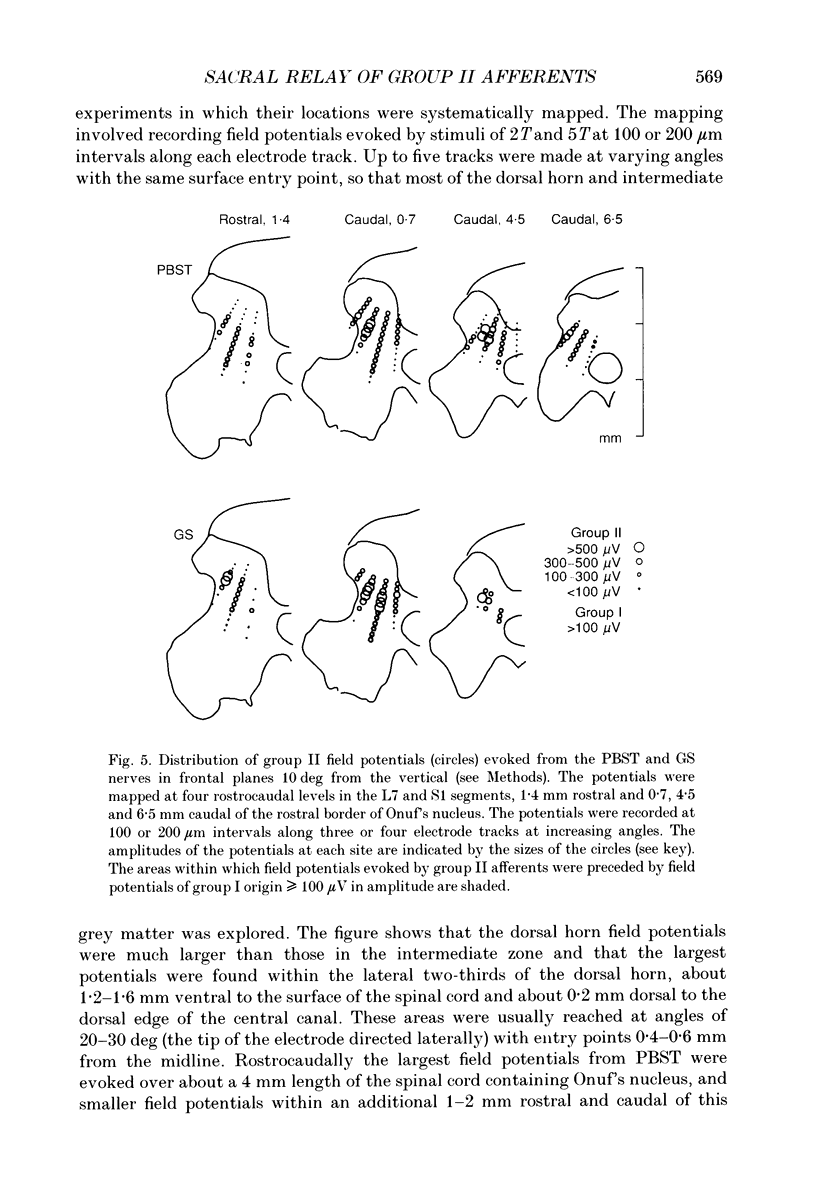

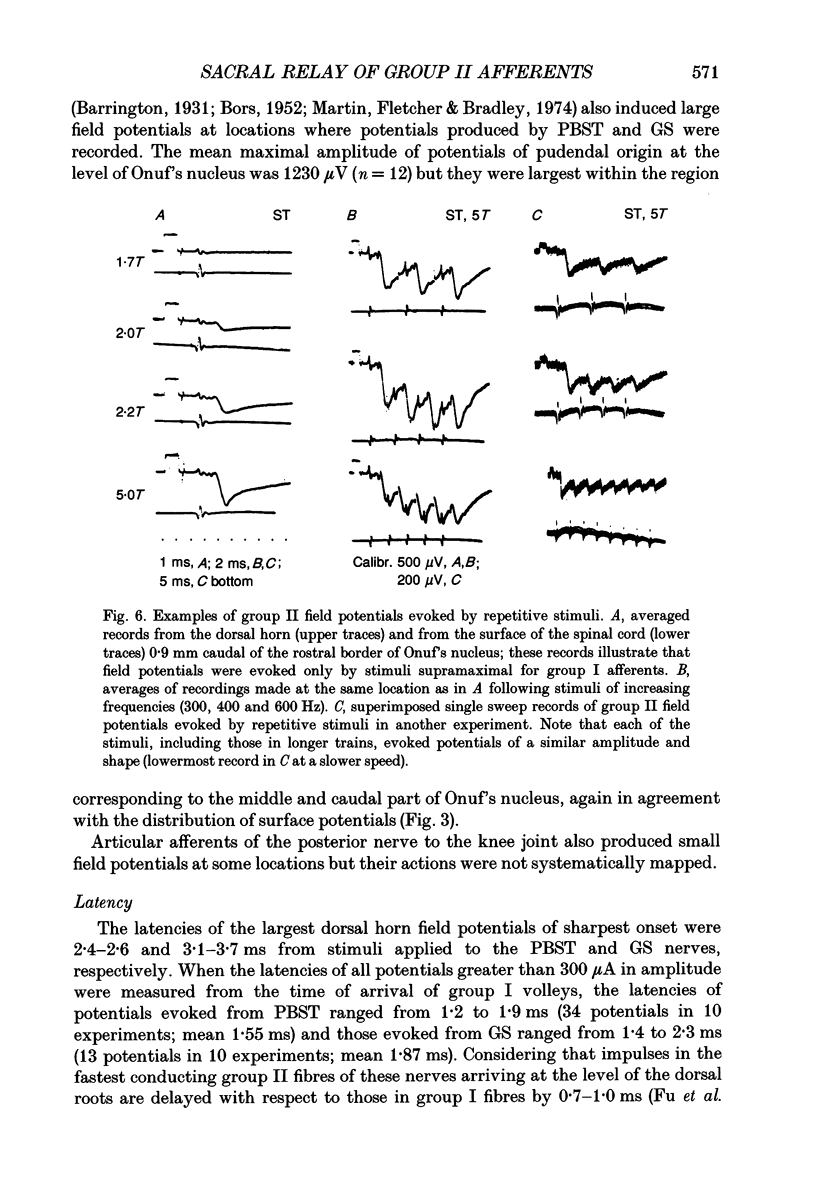

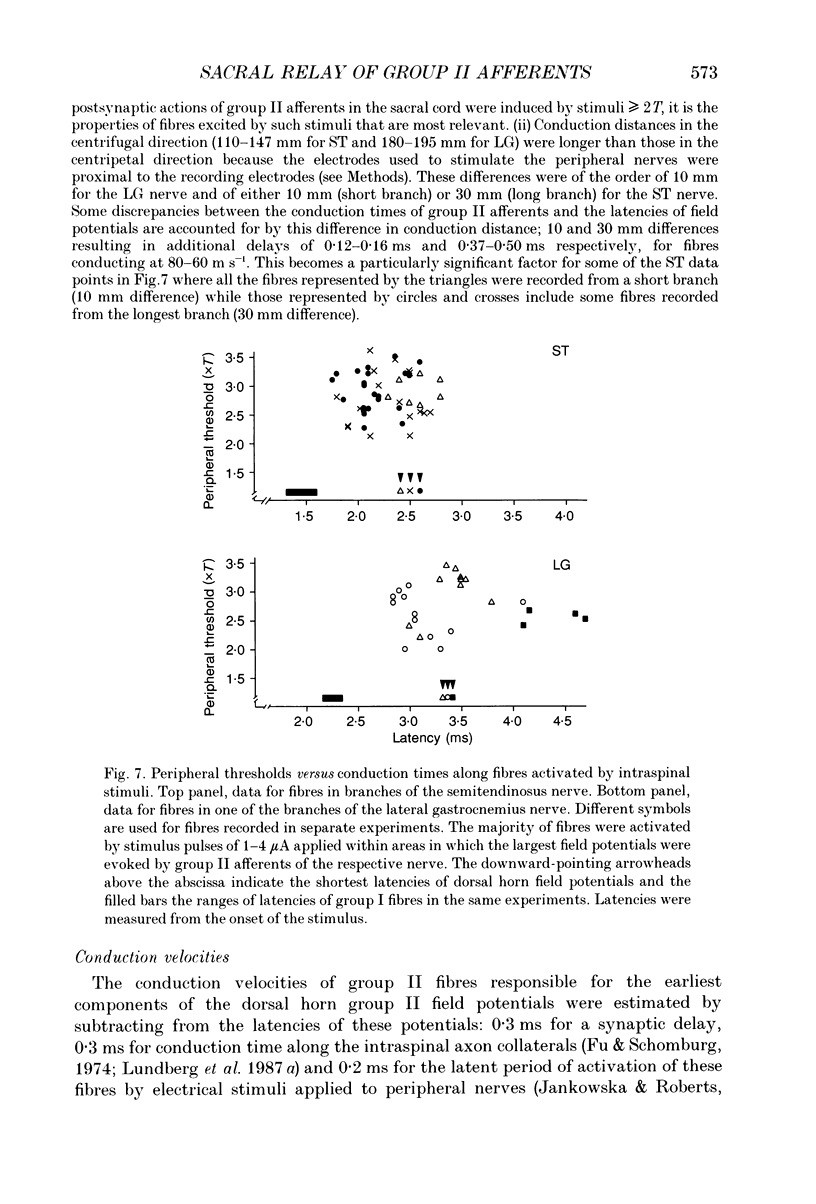







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORS E. Effect of electric stimulation of the pudendal nerves on the vesical neck; its significance for the function of cord bladders: a preliminary report. J Urol. 1952 Jun;67(6):925–935. doi: 10.1016/S0022-5347(17)68436-2. [DOI] [PubMed] [Google Scholar]
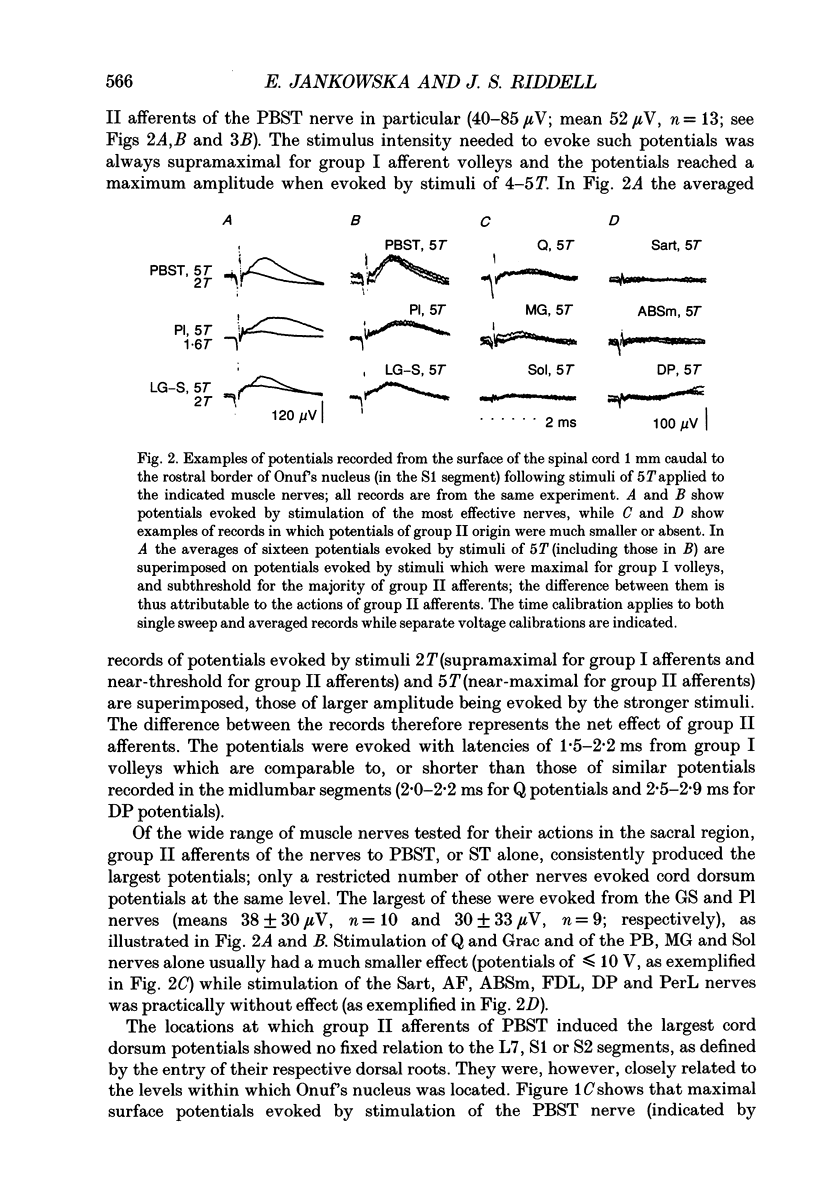
- Beattie M. S., Li Q., Leedy M. G., Bresnahan J. C. Motoneurons innervating the external anal and urethral sphincters of the female cat have different patterns of dendritic arborization. Neurosci Lett. 1990 Mar 26;111(1-2):69–74. doi: 10.1016/0304-3940(90)90346-b. [DOI] [PubMed] [Google Scholar]
- CARPENTER D., LUNDBERG A., NORRSELL U. PRIMARY AFFERENT DEPOLARIZATION EVOKED FROM THE SENSORIMOTOR CORTEX. Acta Physiol Scand. 1963 Sep-Oct;59:126–142. doi: 10.1111/j.1748-1716.1963.tb02729.x. [DOI] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., LANDGREN S. Spinal cord potentials generated by impulses in muscle and cutaneous afferent fibres. J Neurophysiol. 1956 Sep;19(5):452–467. doi: 10.1152/jn.1956.19.5.452. [DOI] [PubMed] [Google Scholar]
- Cavallari P., Edgley S. A., Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol. 1987 Aug;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
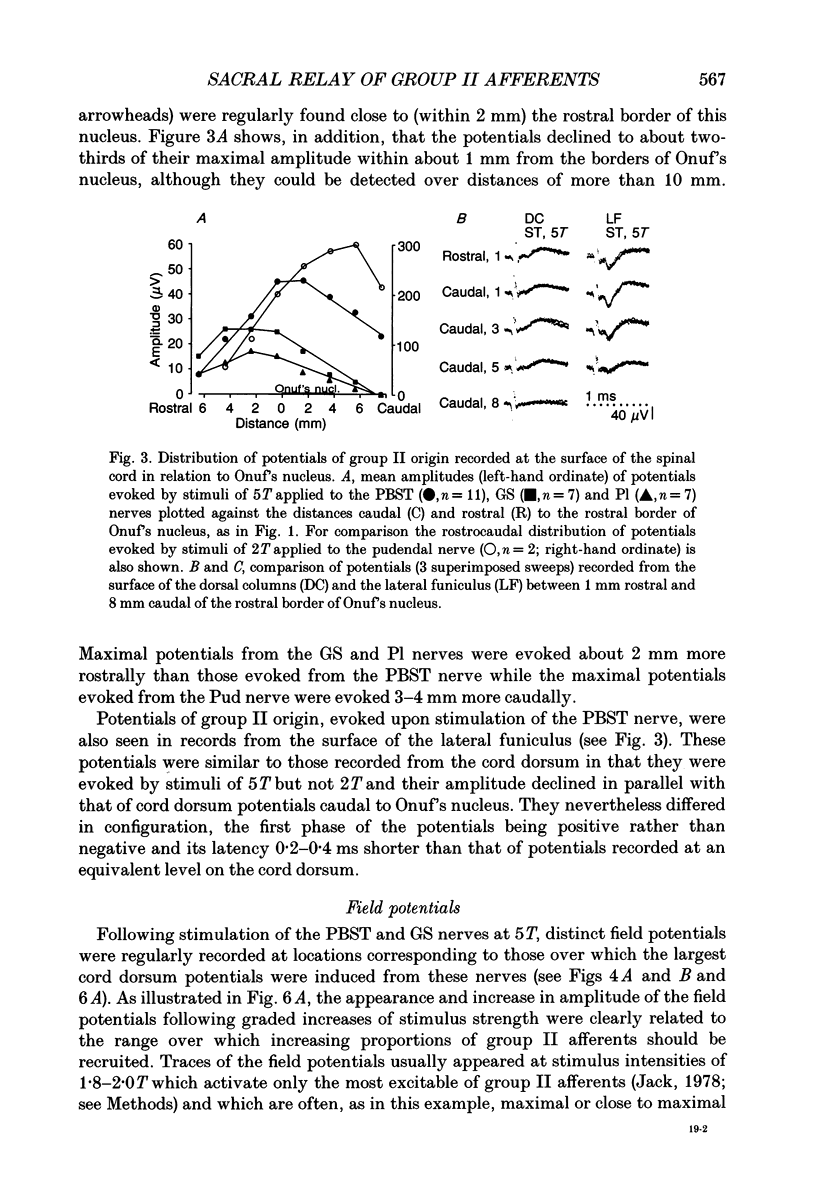
- Cleland C. L., Hayward L., Rymer W. Z. Neural mechanisms underlying the clasp-knife reflex in the cat. II. Stretch-sensitive muscular-free nerve endings. J Neurophysiol. 1990 Oct;64(4):1319–1330. doi: 10.1152/jn.1990.64.4.1319. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
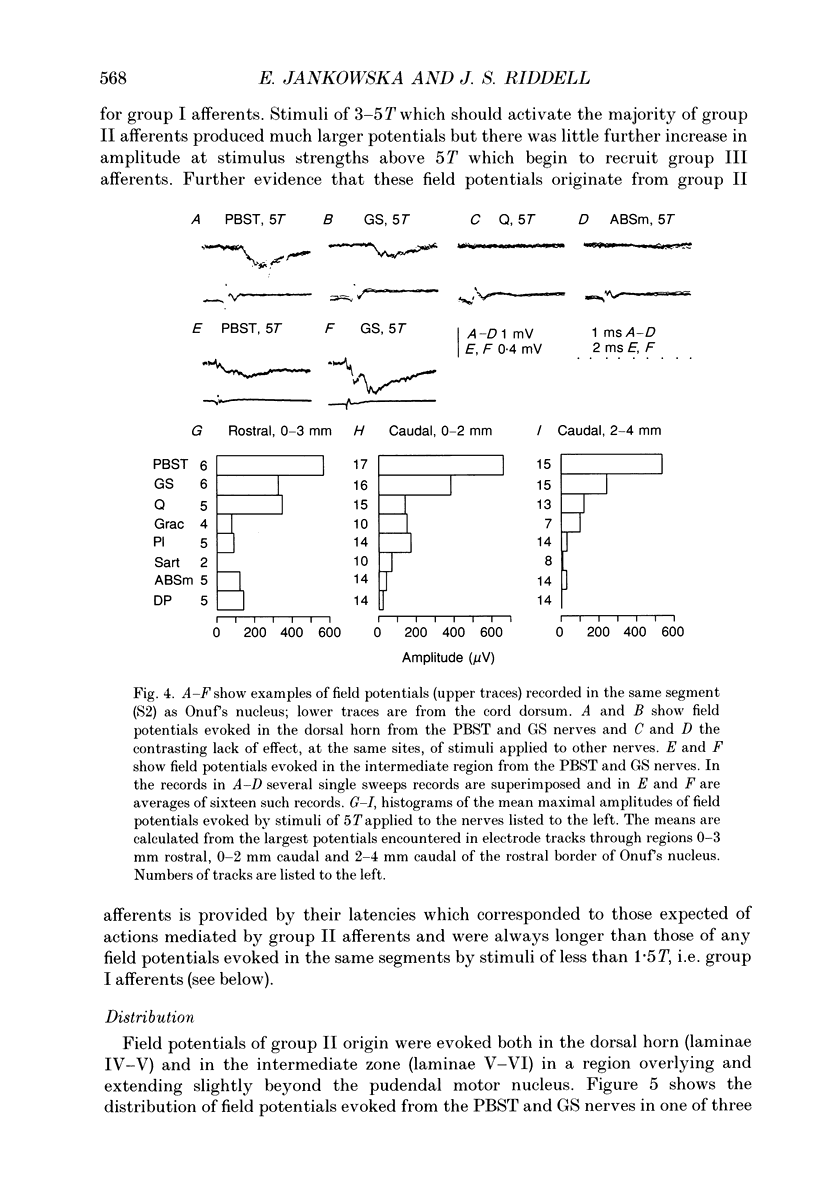
- ECCLES J. C., OSCARSSON O., WILLIS W. D. Synaptic action of group I and II afferent fibres of muscle on the cells of the dorsal spinocerebellar tract. J Physiol. 1961 Oct;158:517–543. doi: 10.1113/jphysiol.1961.sp006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987 Apr;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Information processed by dorsal horn spinocerebellar tract neurones in the cat. J Physiol. 1988 Mar;397:81–97. doi: 10.1113/jphysiol.1988.sp016989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R., Tripathi A. Closely coupled excitation of gamma-motoneurones by group III Muscle afferents with low mechanical threshold in the cat. J Physiol. 1982 Oct;331:481–498. doi: 10.1113/jphysiol.1982.sp014385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirchuk B., Hochman S., Shefchyk S. J. An intracellular study of perineal and hindlimb afferent inputs onto sphincter motoneurons in the decerebrate cat. Exp Brain Res. 1992;89(3):511–516. doi: 10.1007/BF00229875. [DOI] [PubMed] [Google Scholar]
- Fern R., Harrison P. J., Riddell J. S. The dorsal column projection of muscle afferent fibres from the cat hindlimb. J Physiol. 1988 Jul;401:97–113. doi: 10.1113/jphysiol.1988.sp017153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E., Jankowska E., Johannisson T., Lipski J. Autogenetic inhibition of motoneurones by impulses in group Ia muscle spindle afferents. J Physiol. 1979 Aug;293:173–195. doi: 10.1113/jphysiol.1979.sp012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T. C., Santini M., Schomburg E. D. Characteristics and distribution of spinal focal synaptic potentials generated by group II muscle afferents. Acta Physiol Scand. 1974 Jul;91(3):298–313. doi: 10.1111/j.1748-1716.1974.tb05686.x. [DOI] [PubMed] [Google Scholar]
- Fu T. C., Schomburg E. D. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiol Scand. 1974 Jul;91(3):314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Fyffe R. E. The morphology of group II muscle afferent fibre collaterals [proceedings]. J Physiol. 1979 Nov;296:39P–40P. [PubMed] [Google Scholar]
- HUNT C. C. Relation of function to diameter in afferent fibers of muscle nerves. J Gen Physiol. 1954 Sep 20;38(1):117–131. doi: 10.1085/jgp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. The effect of stretch receptors from muscle on the discharge of motorneurons. J Physiol. 1952 Jul;117(3):359–379. doi: 10.1113/jphysiol.1952.sp004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jami L., Jankowska E. Further evidence for synaptic actions of muscle spindle secondaries in the middle lumbar segments of the cat spinal cord. J Physiol. 1988 Aug;402:671–686. doi: 10.1113/jphysiol.1988.sp017228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Primary afferent depolarization of central terminals of group II muscle afferents in the cat spinal cord. J Physiol. 1989 Apr;411:71–83. doi: 10.1113/jphysiol.1989.sp017561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoheisel U., Lehmann-Willenbrock E., Mense S. Termination patterns of identified group II and III afferent fibres from deep tissues in the spinal cord of the cat. Neuroscience. 1989;28(2):495–507. doi: 10.1016/0306-4522(89)90195-4. [DOI] [PubMed] [Google Scholar]
- Hongo T., Lundberg A., Phillips C. G., Thompson R. F. The pattern of monosynaptic Ia-connections to hindlimb motor nuclei in the baboon: a comparison with the cat. Proc R Soc Lond B Biol Sci. 1984 May 22;221(1224):261–289. doi: 10.1098/rspb.1984.0034. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38(4):335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E., McCrea D., Mackel R. Pattern of 'non-reciprocal' inhibition of motoneurones by impulses in group Ia muscle spindle afferents in the cat. J Physiol. 1981 Jul;316:393–409. doi: 10.1113/jphysiol.1981.sp013796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Noga B. R. Contralaterally projecting lamina VIII interneurones in middle lumbar segments in the cat. Brain Res. 1990 Dec 10;535(2):327–330. doi: 10.1016/0006-8993(90)91618-q. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Padel Y., Zarzecki P. Crossed disynaptic inhibition of sacral motoneurones. J Physiol. 1978 Dec;285:425–444. doi: 10.1113/jphysiol.1978.sp012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. J Physiol. 1972 May;222(3):597–622. doi: 10.1113/jphysiol.1972.sp009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez I., Rudomin P., Solodkin M. PAD patterns of physiologically identified afferent fibres from the medial gastrocnemius muscle. Exp Brain Res. 1988;71(3):643–657. doi: 10.1007/BF00248758. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from muscle spindle secondary endings of intercostal and triceps surae muscles in the cat. J Physiol. 1975 Feb;245(2):64P–66P. [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Comments on reflex actions evoked by electrical stimulation of group II muscle afferents. Brain Res. 1977 Feb 25;122(3):551–555. doi: 10.1016/0006-8993(77)90466-8. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Exp Brain Res. 1987;65(2):271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Reflex pathways from group II muscle afferents. 2. Functional characteristics of reflex pathways to alpha-motoneurones. Exp Brain Res. 1987;65(2):282–293. doi: 10.1007/BF00236300. [DOI] [PubMed] [Google Scholar]
- Mackel R. Segmental and descending control of the external urethral and anal sphincters in the cat. J Physiol. 1979 Sep;294:105–122. doi: 10.1113/jphysiol.1979.sp012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J. B., Fleshman J. W., Sypert G. W. Properties of single-fiber spindle group II EPSPs in triceps surae motoneurons. J Neurophysiol. 1980 Oct;44(4):713–725. doi: 10.1152/jn.1980.44.4.713. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- ROMANES G. J. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951 Apr;94(2):313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- Riddell J. S., Jankowska E., Eide E. Depolarization of group II muscle afferents by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J Physiol. 1993 Feb;461:723–741. doi: 10.1113/jphysiol.1993.sp019538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppolo J. R., Nadelhaft I., de Groat W. C. The organization of pudendal motoneurons and primary afferent projections in the spinal cord of the rhesus monkey revealed by horseradish peroxidase. J Comp Neurol. 1985 Apr 22;234(4):475–488. doi: 10.1002/cne.902340406. [DOI] [PubMed] [Google Scholar]
- Rymer W. Z., Houk J. C., Crago P. E. Mechanisms of the clasp-knife reflex studied in an animal model. Exp Brain Res. 1979 Sep;37(1):93–113. doi: 10.1007/BF01474257. [DOI] [PubMed] [Google Scholar]
- Sato M., Mizuno N., Konishi A. Localization of motoneurons innervating perineal muscles: a HRP study in cat. Brain Res. 1978 Jan 20;140(1):149–154. doi: 10.1016/0006-8993(78)90244-5. [DOI] [PubMed] [Google Scholar]
- Stauffer E. K., Watt D. G., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. J Neurophysiol. 1976 Nov;39(6):1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]
- Stuart D. G., Mosher C. G., Gerlach R. L., Reinking R. M. Selective activation of Ia afferents by transient muscle stretch. Exp Brain Res. 1970 Jun 25;10(5):477–487. doi: 10.1007/BF00234264. [DOI] [PubMed] [Google Scholar]
- Thor K. B., Morgan C., Nadelhaft I., Houston M., De Groat W. C. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989 Oct 8;288(2):263–279. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- Ueyama T., Mizuno N., Nomura S., Konishi A., Itoh K., Arakawa H. Central distribution of afferent and efferent components of the pudendal nerve in cat. J Comp Neurol. 1984 Jan 1;222(1):38–46. doi: 10.1002/cne.902220104. [DOI] [PubMed] [Google Scholar]



