Abstract
1. Injecting twelve mouse minimum lethal doses of tetanus toxin into one hippocampus of a rat leads to the development of chronic epileptic foci in both hippocampi. These generate intermittent epileptic discharges for 6-8 weeks. Here we compare GABAergic inhibition, 10-18 days after injection, in slices prepared from the injected and contralateral hippocampi (respectively the primary and the secondary or 'mirror' foci), using both neurochemical and electrophysiological methods. 2. Epileptic activity was recorded from slices of both hippocampi from all tetanus toxin-injected rats. Evoked epileptic discharges were similar on the two sides, but spontaneous epileptic discharges were more common contralaterally. 3. Ca(2+)-dependent, K(+)-stimulated (synaptic) release of radiolabelled GABA was depressed in slices from the injected hippocampus, compared with vehicle-injected controls. In contrast, slices from the contralateral hippocampus had normal levels of Ca(2+)-dependent, K(+)-stimulated GABA release, even though adjacent slices were epileptogenic. 4. Intracellular recordings revealed that both fast and slow stimulus-evoked inhibitory postsynaptic potentials (IPSPs) were abolished in CA3 pyramidal cells in the primary focus. In the secondary focus, however, fast IPSPs were seen in seven of twenty-five cells, and slow IPSPs were seen in all cells if the stimulus was strong enough. 5. Monosynaptic IPSPs were isolated pharmacologically by blocking glutamatergic excitatory postsynaptic potentials (EPSPs) with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and D(-)-2-amino-5-phosphopentanoic acid (AP-5). No monosynaptic IPSPs were uncovered in cells from the primary focus at any stimulus strength. Monosynaptic IPSPs were evoked in all cells from both the secondary focus and control slices. The estimated conductances of monosynaptic fast IPSPs were similar in cells from the secondary focus and from the controls, although the former required twice the stimulus strength. 6. Slow IPSPs were found in the secondary focus and in controls, but not in the primary focus. They were sensitive to 3-amino-2-(4-chlorophenyl)-2-hydroxy-propylsulphonic acid (2-OH saclofen). The estimated conductances of slow IPSPs evoked by weak stimuli in the secondary focus were much smaller than in the controls. However, stimuli that could trigger epileptic discharges in the secondary focus, evoked 2-OH saclofen-sensitive slow IPSPs with estimated conductances approaching the controls. This marked increase in the slow IPSP did not occur when EPSPs, and epileptic bursts, were blocked with CNQX and AP-5, suggesting that a strong barrage of excitation is needed to generate full-sized slow IPSPs in the secondary focus.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




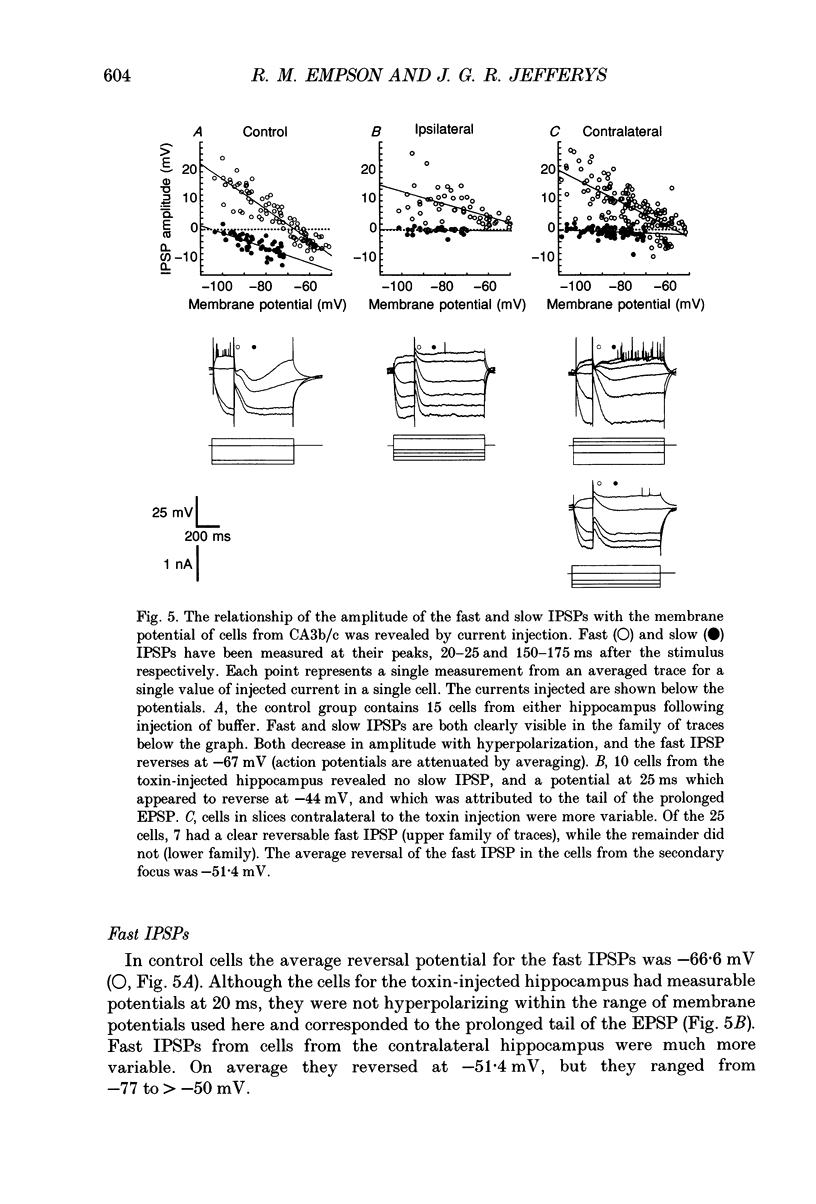







Images in this article
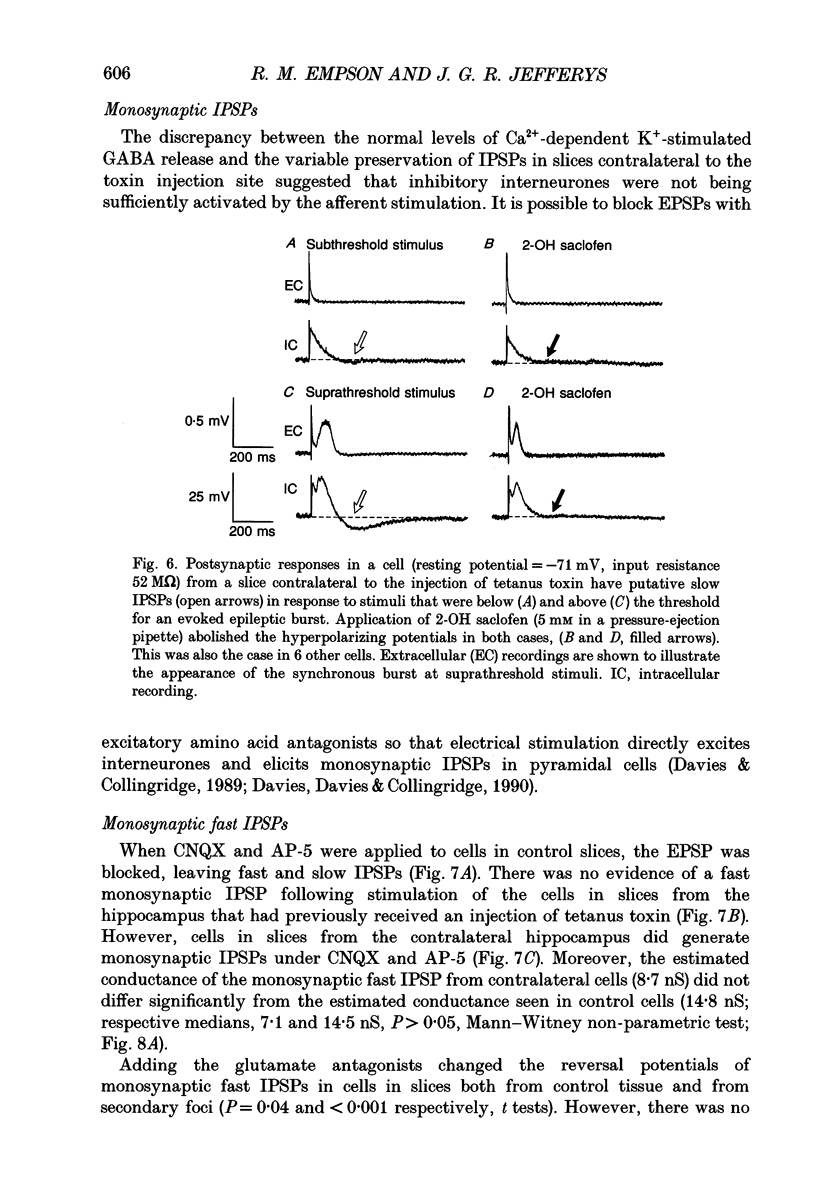
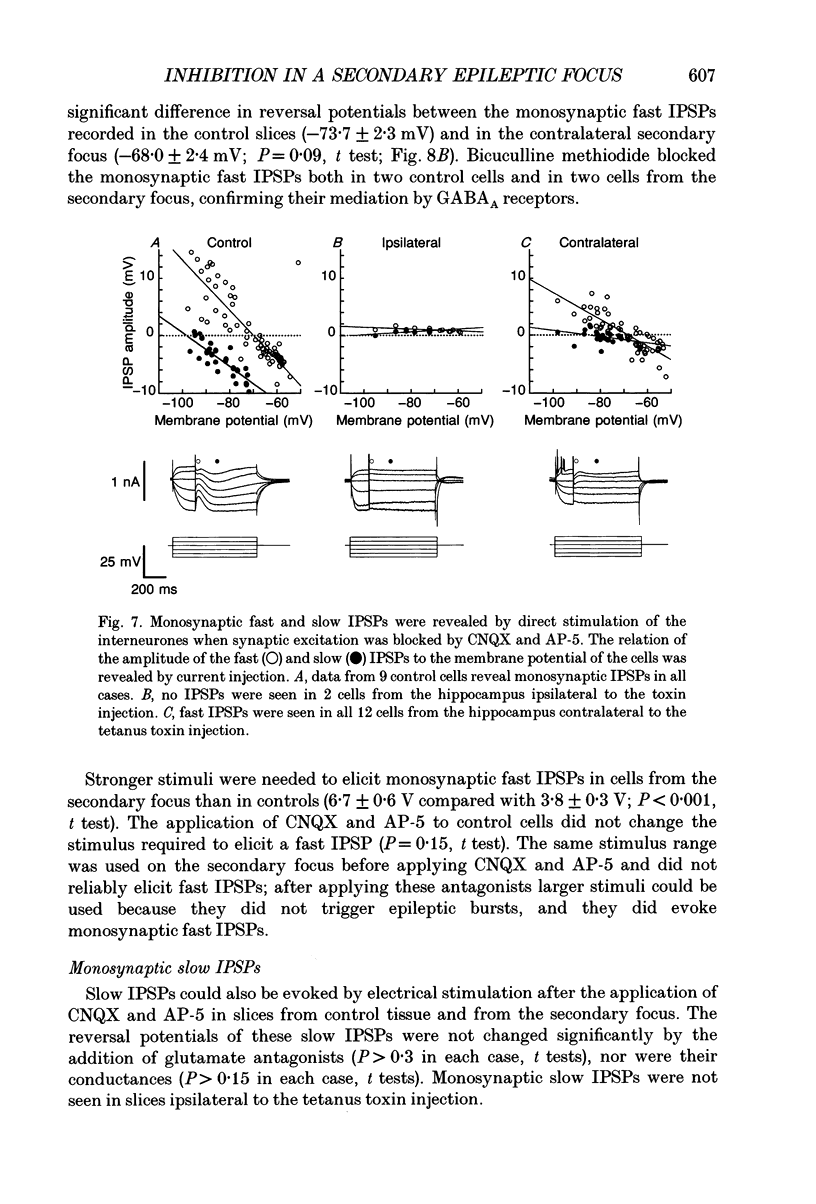
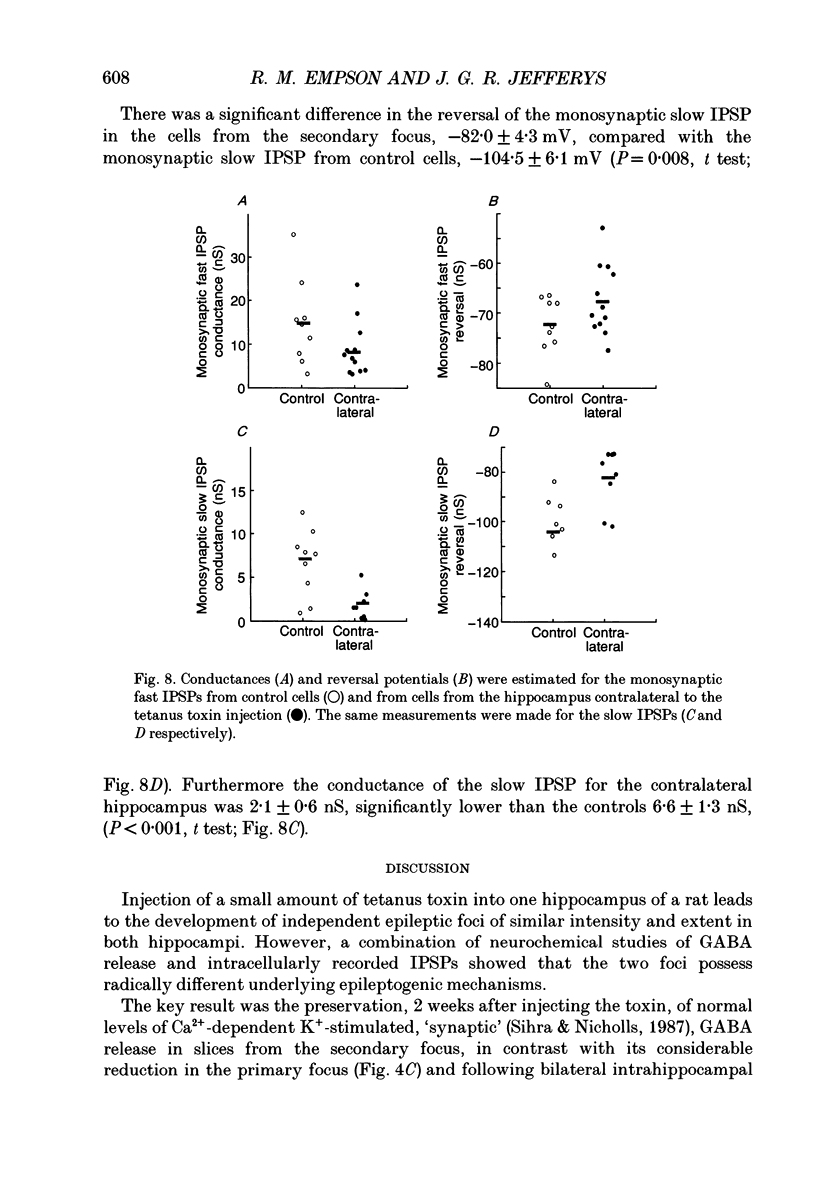
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwood T. J., Wheal H. V. Loss of inhibition in the CA1 region of the kainic acid lesioned hippocampus is not associated with changes in postsynaptic responses to GABA. Brain Res. 1986 Mar 5;367(1-2):390–394. doi: 10.1016/0006-8993(86)91625-2. [DOI] [PubMed] [Google Scholar]
- Babb T. L., Pretorius J. K., Kupfer W. R., Crandall P. H. Glutamate decarboxylase-immunoreactive neurons are preserved in human epileptic hippocampus. J Neurosci. 1989 Jul;9(7):2562–2574. doi: 10.1523/JNEUROSCI.09-07-02562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey G. K., MacDonald R. L., Habig W. H., Hardegree M. C., Nelson P. G. Tetanus toxin: convulsant action on mouse spinal cord neurons in culture. J Neurosci. 1983 Nov;3(11):2310–2323. doi: 10.1523/JNEUROSCI.03-11-02310.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Wendon L. M. A study of the action of tetanus toxin at rat soleus neuromuscular junctions. J Physiol. 1984 Mar;348:1–17. doi: 10.1113/jphysiol.1984.sp015095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigalke H., Heller I., Bizzini B., Habermann E. Tetanus toxin and botulinum A toxin inhibit release and uptake of various transmitters, as studied with particulate preparations from rat brain and spinal cord. Naunyn Schmiedebergs Arch Pharmacol. 1981 Jun;316(3):244–251. doi: 10.1007/BF00505657. [DOI] [PubMed] [Google Scholar]
- Bittner M. A., Holz R. W. Effects of tetanus toxin on catecholamine release from intact and digitonin-permeabilized chromaffin cells. J Neurochem. 1988 Aug;51(2):451–456. doi: 10.1111/j.1471-4159.1988.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Brace H. M., Jefferys J. G., Mellanby J. Long-term changes in hippocampal physiology and learning ability of rats after intrahippocampal tetanus toxin. J Physiol. 1985 Nov;368:343–357. doi: 10.1113/jphysiol.1985.sp015861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener K., Amitai Y., Jefferys J. G., Gutnick M. J. Chronic epileptic foci in neocortex: in vivo and in vitro effects of tetanus toxin. Eur J Neurosci. 1991;3(1):47–54. doi: 10.1111/j.1460-9568.1991.tb00810.x. [DOI] [PubMed] [Google Scholar]
- Cain D. P. Long-term potentiation and kindling: how similar are the mechanisms? Trends Neurosci. 1989 Jan;12(1):6–10. doi: 10.1016/0166-2236(89)90146-x. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Thompson P. A., Davies J., Mellanby J. In vitro effect of tetanus toxin on GABA release form rat hippocampal slices. J Neurochem. 1981 Oct;37(4):1039–1041. doi: 10.1111/j.1471-4159.1981.tb04492.x. [DOI] [PubMed] [Google Scholar]
- Cornish S. M., Wheal H. V. Long-term loss of paired pulse inhibition in the kainic acid-lesioned hippocampus of the rat. Neuroscience. 1989;28(3):563–571. doi: 10.1016/0306-4522(89)90005-5. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. N., Collingridge G. L. Role of excitatory amino acid receptors in synaptic transmission in area CA1 of rat hippocampus. Proc R Soc Lond B Biol Sci. 1989 May 22;236(1285):373–384. doi: 10.1098/rspb.1989.0028. [DOI] [PubMed] [Google Scholar]
- Franck J. E., Kunkel D. D., Baskin D. G., Schwartzkroin P. A. Inhibition in kainate-lesioned hyperexcitable hippocampi: physiologic, autoradiographic, and immunocytochemical observations. J Neurosci. 1988 Jun;8(6):1991–2002. doi: 10.1523/JNEUROSCI.08-06-01991.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. Electrical changes in the membrane in junctional transmission. Biochim Biophys Acta. 1973 Nov 28;300(3):289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Habermann E., Erdmann G. Pharmacokinetic and histoautoradiographic evidence for the intraaxonal movement of toxin in the pathogenesis of tetanus. Toxicon. 1978;16(6):611–623. doi: 10.1016/0041-0101(78)90189-7. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Bigalke H., Bergey G. K., Neale E. A., Hardegree M. C., Nelson P. G. Tetanus toxin in dissociated spinal cord cultures: long-term characterization of form and action. J Neurochem. 1986 Sep;47(3):930–937. doi: 10.1111/j.1471-4159.1986.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Hawkins C. A., Mellanby J. H. Limbic epilepsy induced by tetanus toxin: a longitudinal electroencephalographic study. Epilepsia. 1987 Jul-Aug;28(4):431–444. doi: 10.1111/j.1528-1157.1987.tb03669.x. [DOI] [PubMed] [Google Scholar]
- Houser C. R. GABA neurons in seizure disorders: a review of immunocytochemical studies. Neurochem Res. 1991 Mar;16(3):295–308. doi: 10.1007/BF00966093. [DOI] [PubMed] [Google Scholar]
- Ishizuka N., Weber J., Amaral D. G. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990 May 22;295(4):580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G. Basic mechanisms of focal epilepsies. Exp Physiol. 1990 Mar;75(2):127–162. doi: 10.1113/expphysiol.1990.sp003390. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G. Chronic epileptic foci in vitro in hippocampal slices from rats with the tetanus toxin epileptic syndrome. J Neurophysiol. 1989 Aug;62(2):458–468. doi: 10.1152/jn.1989.62.2.458. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G., Empson R. M. Development of chronic secondary epileptic foci following intrahippocampal injection of tetanus toxin in the rat. Exp Physiol. 1990 Sep;75(5):733–736. doi: 10.1113/expphysiol.1990.sp003455. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G., Evans B. J., Hughes S. A., Williams S. F. Neuropathology of the chronic epileptic syndrome induced by intrahippocampal tetanus toxin in rat: preservation of pyramidal cells and incidence of dark cells. Neuropathol Appl Neurobiol. 1992 Feb;18(1):53–70. doi: 10.1111/j.1365-2990.1992.tb00764.x. [DOI] [PubMed] [Google Scholar]
- Jordan S. J., Jefferys J. G. Sustained and selective block of IPSPs in brain slices from rats made epileptic by intrahippocampal tetanus toxin. Epilepsy Res. 1992 Apr;11(2):119–129. doi: 10.1016/0920-1211(92)90046-v. [DOI] [PubMed] [Google Scholar]
- Kamphuis W., Gorter J. A., da Silva F. L. A long-lasting decrease in the inhibitory effect of GABA on glutamate responses of hippocampal pyramidal neurons induced by kindling epileptogenesis. Neuroscience. 1991;41(2-3):425–431. doi: 10.1016/0306-4522(91)90338-o. [DOI] [PubMed] [Google Scholar]
- Kamphuis W., Huisman E., Veerman M. J., Lopes da Silva F. H. Development of changes in endogenous GABA release during kindling epileptogenesis in rat hippocampus. Brain Res. 1991 Apr 5;545(1-2):33–40. doi: 10.1016/0006-8993(91)91266-4. [DOI] [PubMed] [Google Scholar]
- Lloyd K. G., Bossi L., Morselli P. L., Munari C., Rougier M., Loiseau H. Alterations of GABA-mediated synaptic transmission in human epilepsy. Adv Neurol. 1986;44:1033–1044. [PubMed] [Google Scholar]
- López-Colomé A. M., Tapia R., Salceda R., Pasantes-Morales H. K+-stimulated release of labeled gamma-aminobutyrate, glycine and taurine in slices of several regions of rat central nervous system. Neuroscience. 1978;3(11):1069–1074. doi: 10.1016/0306-4522(78)90124-0. [DOI] [PubMed] [Google Scholar]
- Mellanby J., George G., Robinson A., Thompson P. Epileptiform syndrome in rats produced by injecting tetanus toxin into the hippocampus. J Neurol Neurosurg Psychiatry. 1977 Apr;40(4):404–414. doi: 10.1136/jnnp.40.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby J., Green J. How does tetanus toxin act? Neuroscience. 1981;6(3):281–300. doi: 10.1016/0306-4522(81)90123-8. [DOI] [PubMed] [Google Scholar]
- Michelson H. B., Wong R. K. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1991 Sep 20;253(5026):1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- Miles R. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. J Physiol. 1990 Sep;428:61–77. doi: 10.1113/jphysiol.1990.sp018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Misgeld U. Inhibitory role of dentate hilus neurons in guinea pig hippocampal slice. J Neurophysiol. 1990 Jul;64(1):46–56. doi: 10.1152/jn.1990.64.1.46. [DOI] [PubMed] [Google Scholar]
- Najlerahim A., Williams S. F., Pearson R. C., Jefferys J. G. Increased expression of GAD mRNA during the chronic epileptic syndrome due to intrahippocampal tetanus toxin. Exp Brain Res. 1992;90(2):332–342. doi: 10.1007/BF00227246. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. Release of glutamate, aspartate, and gamma-aminobutyric acid from isolated nerve terminals. J Neurochem. 1989 Feb;52(2):331–341. doi: 10.1111/j.1471-4159.1989.tb09126.x. [DOI] [PubMed] [Google Scholar]
- Penner R., Neher E., Dreyer F. Intracellularly injected tetanus toxin inhibits exocytosis in bovine adrenal chromaffin cells. Nature. 1986 Nov 6;324(6092):76–78. doi: 10.1038/324076a0. [DOI] [PubMed] [Google Scholar]
- Ribak C. E., Joubran C., Kesslak J. P., Bakay R. A. A selective decrease in the number of GABAergic somata occurs in pre-seizing monkeys with alumina gel granuloma. Epilepsy Res. 1989 Sep-Oct;4(2):126–138. doi: 10.1016/0920-1211(89)90017-x. [DOI] [PubMed] [Google Scholar]
- Schwab M., Agid Y., Glowinski J., Thoenen H. Retrograde axonal transport of 125I-tetanus toxin as a tool for tracing fiber connections in the central nervous system; connections of the rostral part of the rat neostriatum. Brain Res. 1977 May 6;126(2):211–224. doi: 10.1016/0006-8993(77)90722-3. [DOI] [PubMed] [Google Scholar]
- Segal M. A subset of local interneurons generate slow inhibitory postsynaptic potentials in hippocampal neurons. Brain Res. 1990 Mar 12;511(1):163–164. doi: 10.1016/0006-8993(90)90236-5. [DOI] [PubMed] [Google Scholar]
- Sihra T. S., Nicholls D. G. 4-Aminobutyrate can be released exocytotically from guinea-pig cerebral cortical synaptosomes. J Neurochem. 1987 Jul;49(1):261–267. doi: 10.1111/j.1471-4159.1987.tb03424.x. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987 Jan 2;235(4784):73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the "dormant basket cell" hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991 Jan;1(1):41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- Szerb J. C., Ross T. E., Gurevich L. Compartments of labeled and endogenous gamma-aminobutyric acid giving rise to release evoked by potassium or veratridine in rat cortical slices. J Neurochem. 1981 Nov;37(5):1186–1192. doi: 10.1111/j.1471-4159.1981.tb04669.x. [DOI] [PubMed] [Google Scholar]
- Tasker J. G., Dudek F. E. Electrophysiology of GABA-mediated synaptic transmission and possible roles in epilepsy. Neurochem Res. 1991 Mar;16(3):251–262. doi: 10.1007/BF00966088. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Knowles W. D., Miles R., Wong R. K. Models of the cellular mechanism underlying propagation of epileptiform activity in the CA2-CA3 region of the hippocampal slice. Neuroscience. 1987 May;21(2):457–470. doi: 10.1016/0306-4522(87)90135-7. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Traub R. D., Miles R. Cellular basis of neuronal synchrony in epilepsy. Adv Neurol. 1986;44:583–592. [PubMed] [Google Scholar]



