Abstract
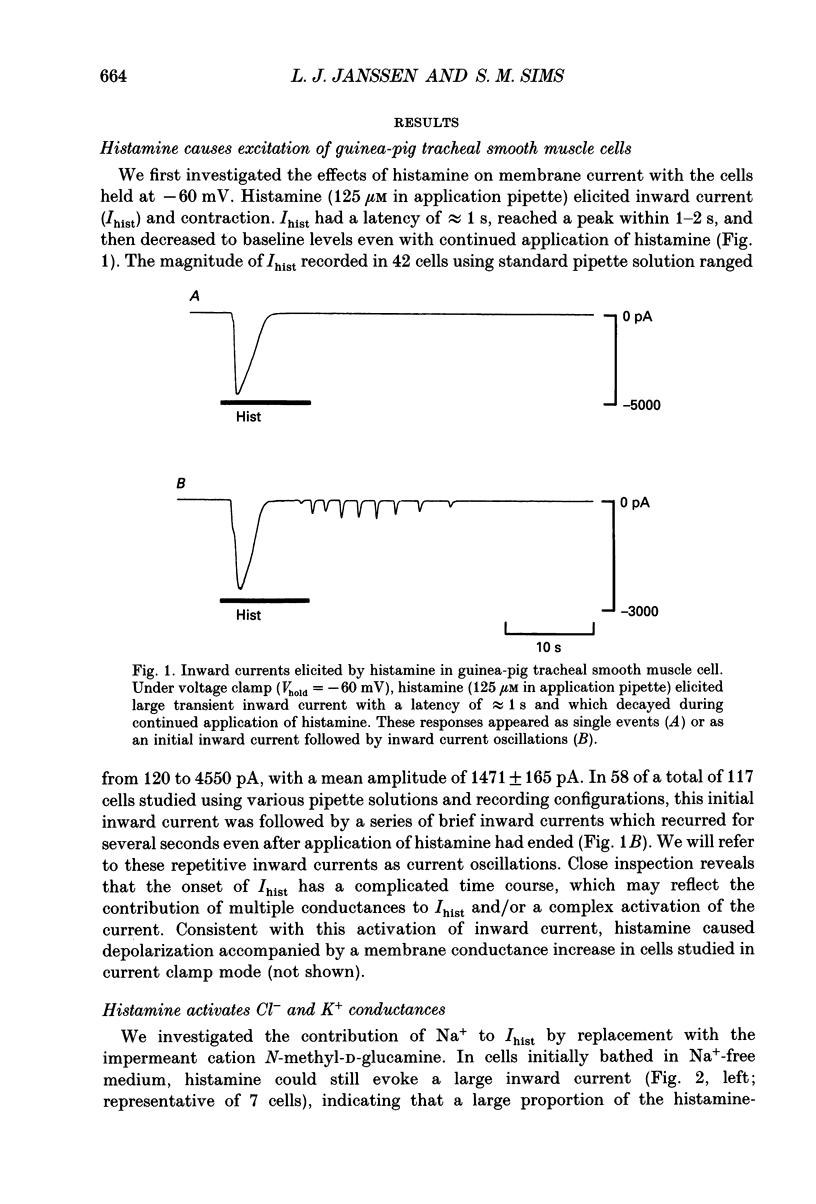
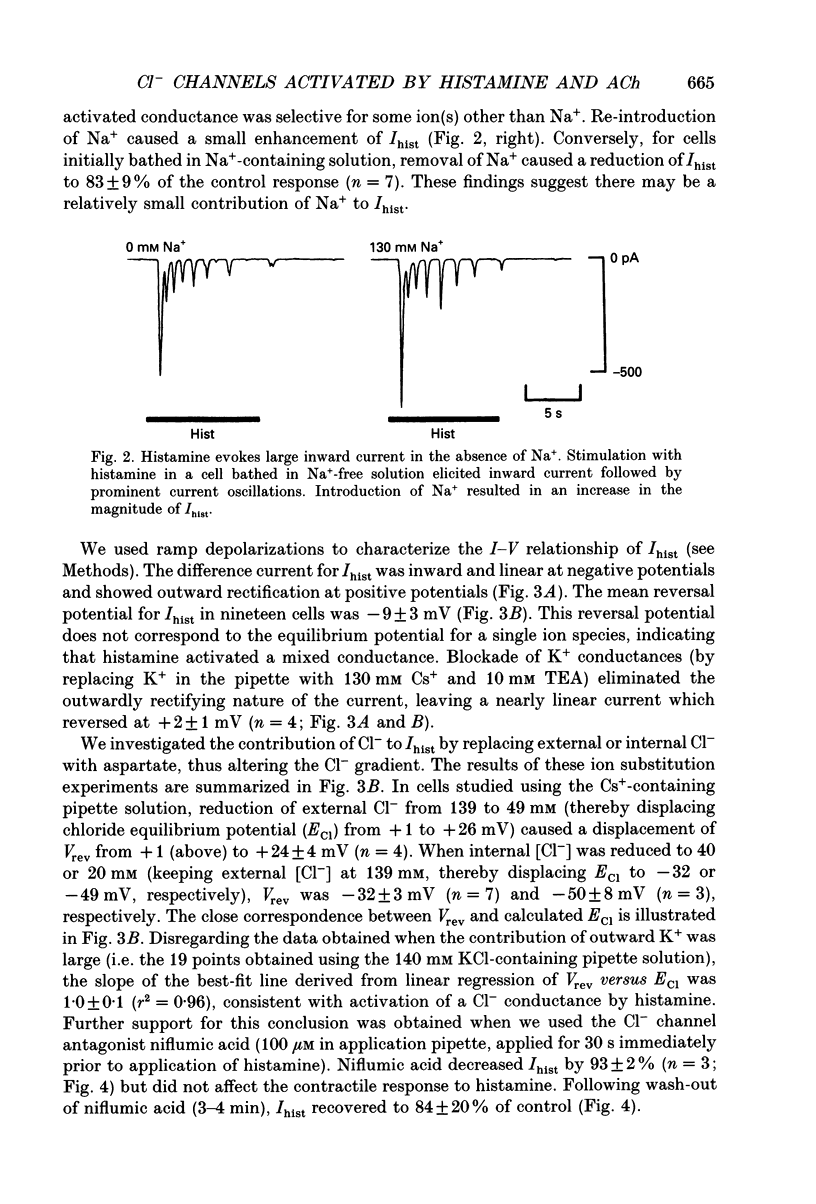
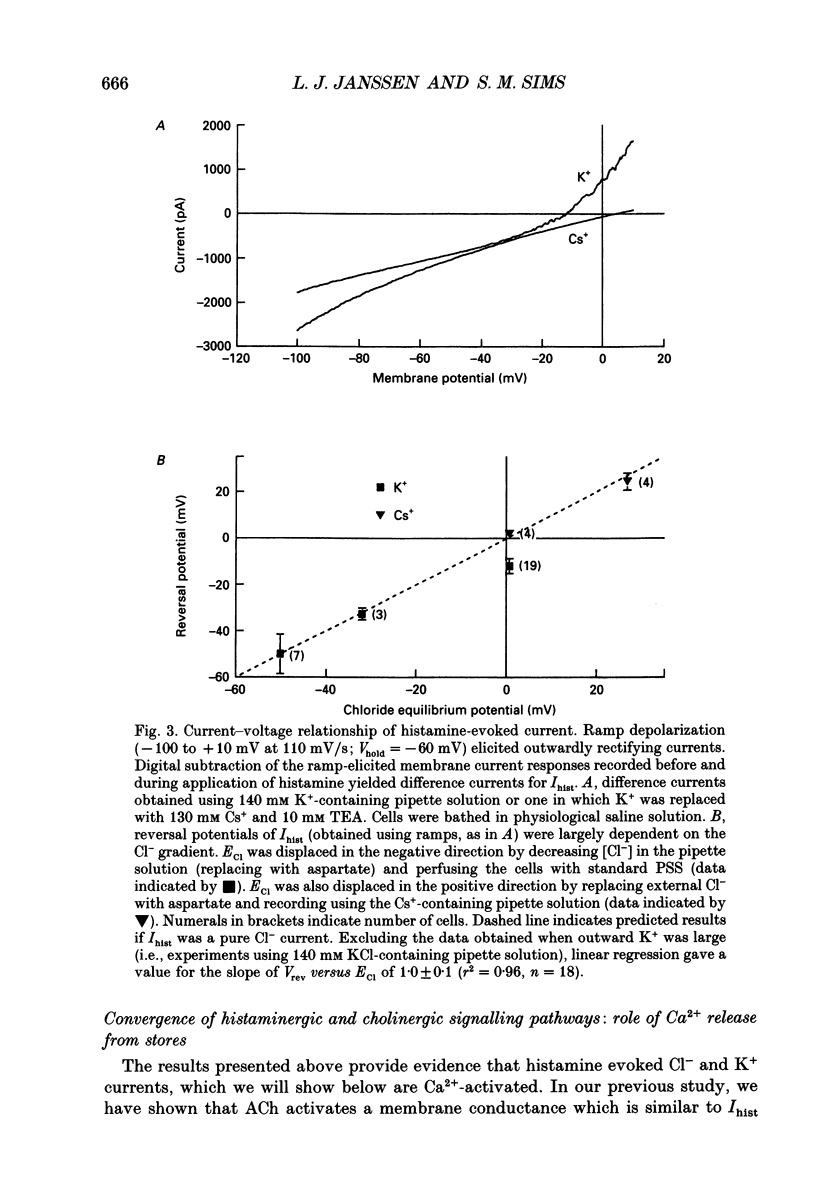
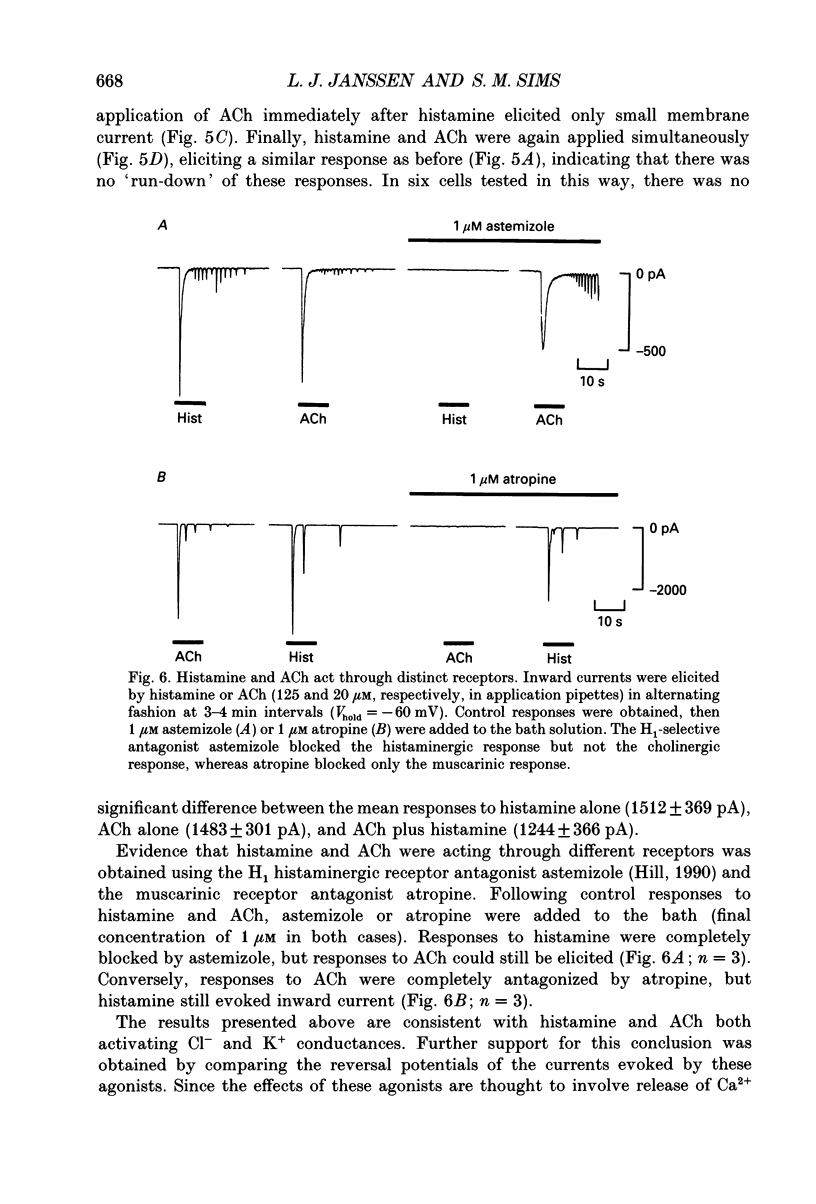
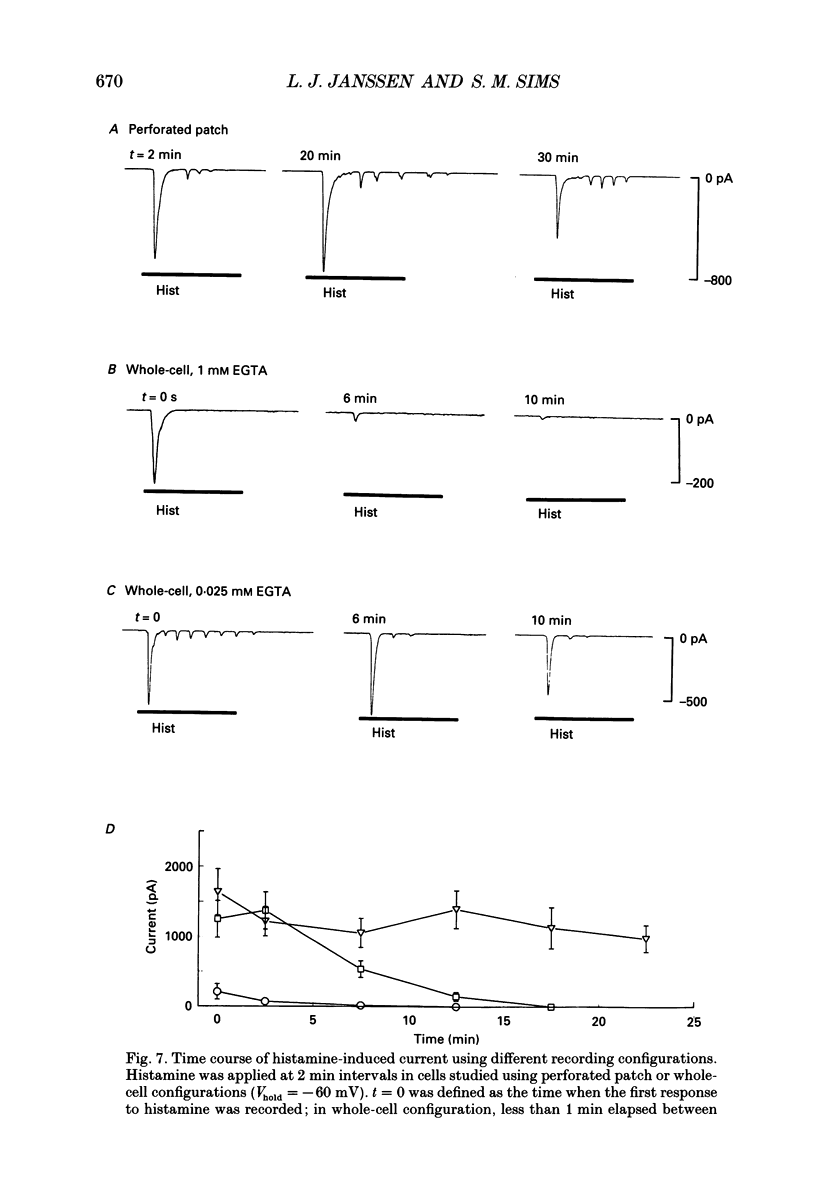
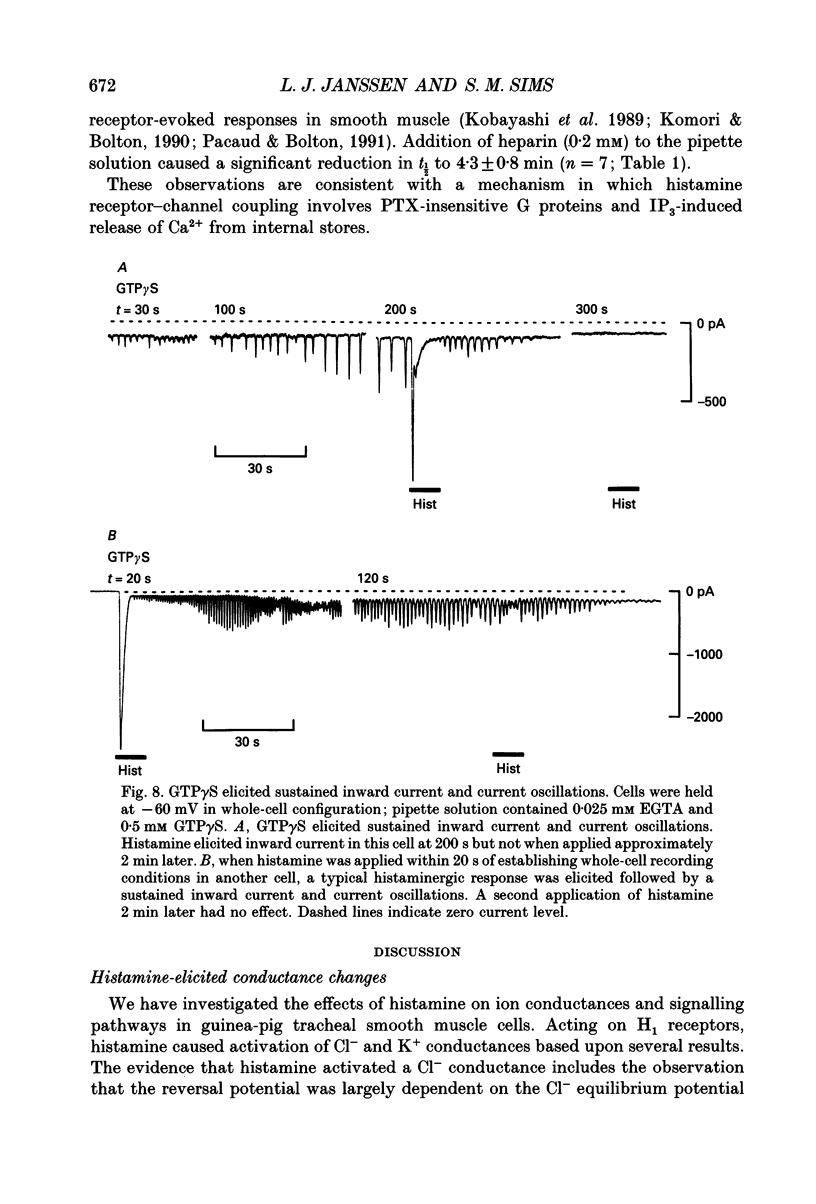
1. We investigated the effects of histamine on membrane currents and contractile state of isolated guinea-pig tracheal myocytes using perforated patch and whole-cell recording techniques. The effects of histamine were compared to those of acetylcholine (ACh) and caffeine. 2. During voltage clamp (Vhold = -60 mV), histamine elicited contraction and an inward current (Ihist) which was often followed by current oscillations. Ihist had a reversal potential (Vrev) of -9 +/- 3 mV. 3. Ihist was dependent on the Cl- gradient and was antagonized by the Cl- channel blocker niflumic acid. Vrev was more positive (+2 +/- 1 mV) when K(+)-selective currents were blocked by Cs+ and TEA. When all external Na+ was replaced with N-methyl-D-glucamine, there was a small reduction in the amplitude of Ihist. 4. The histamine-induced current was similar to that elicited by ACh and by caffeine with respect to time course, amplitude, and current-voltage relationship. Responses to histamine and to ACh were non-additive, consistent with a convergence of histaminergic and cholinergic signalling pathways. Ihist was antagonized by the H1 histaminergic receptor antagonist astemizole, but not by atropine. 5. When recorded using the perforated patch configuration, Ihist could be elicited repeatedly for more than 30 min. When cells were studied in the whole-cell configuration using a pipette solution containing 0.025 mM EGTA, the amplitude of Ihist was initially the same as that obtained using perforated patch but then decreased; the time required for the responses to decrease to 50% (t1/2) was 8.2 +/- 1.0 min. When 1 mM EGTA was included in the pipette solution (whole-cell configuration), the initial response to histamine was significantly decreased in size and t1/2 was reduced to 3.3 +/- 0.7 min. 6. The characteristics of the signalling pathway were examined in cells studied using the whole-cell configuration with 0.025 mM EGTA in the recording pipette. Heparin significantly reduced t1/2 to 4.3 +/- 0.8 min. GTP gamma S elicited inward current and oscillations; both effects were enhanced by histamine. GTP gamma S also reduced t1/2 to 1.4 +/- 0.1 min. Pertussis toxin did not alter the amplitude or time course of Ihist. 7. We conclude that in guinea-pig tracheal myocytes, binding of histamine to H1 receptors leads to release of Ca2+ from intracellular stores and subsequent activation of Cl- and K+ conductances as well as contraction. Furthermore, we demonstrate that ACh elicits similar physiological responses due to a convergence of the histaminergic and muscarinic signalling pathways.
Full text
PDF


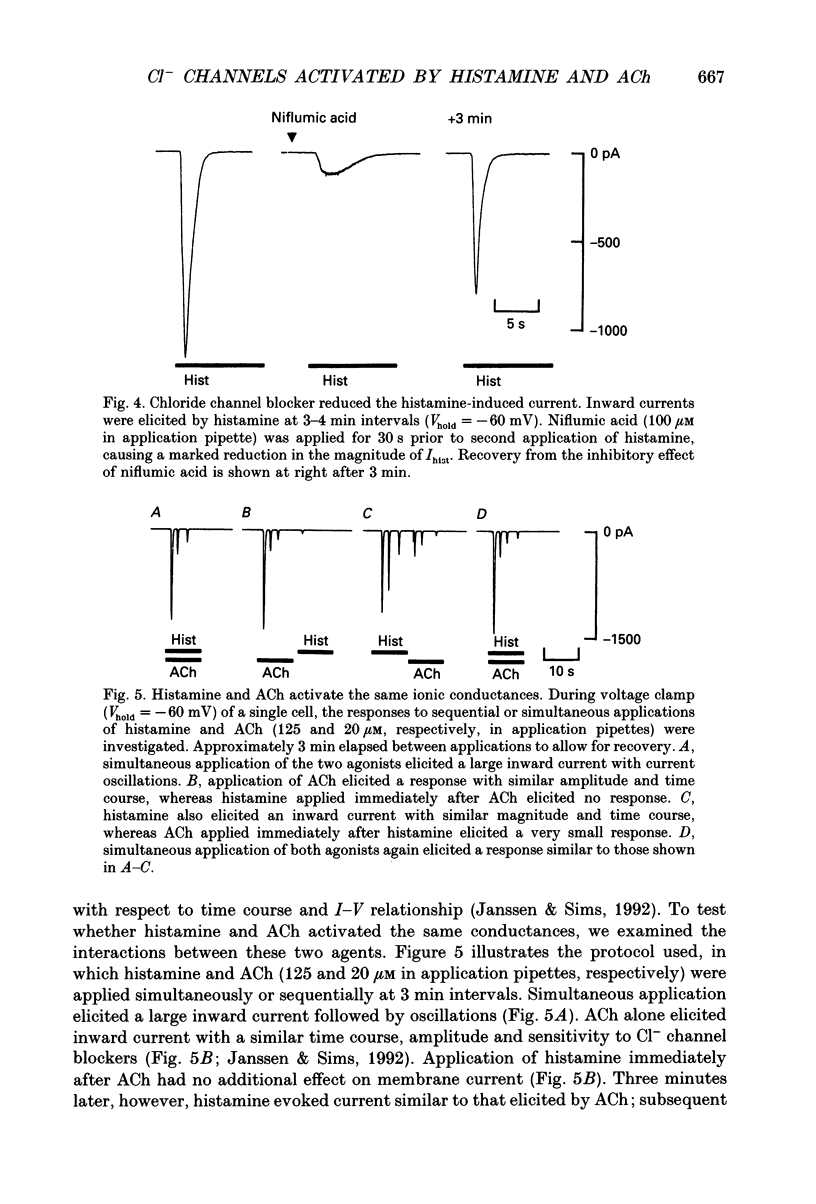







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Ahmed F., Foster R. W., Small R. C., Weston A. H. Some features of the spasmogenic actions of acetylcholine and histamine in guinea-pig isolated trachealis. Br J Pharmacol. 1984 Sep;83(1):227–233. doi: 10.1111/j.1476-5381.1984.tb10139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A., Peralta E. G., Winslow J. W., Ramachandran J., Capon D. J. Functionally distinct G proteins selectively couple different receptors to PI hydrolysis in the same cell. Cell. 1989 Feb 10;56(3):487–493. doi: 10.1016/0092-8674(89)90251-1. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Comparison of the excitatory actions of substance P, carbachol, histamine and prostaglandin F2 alpha on the smooth muscle of the taenia of the guinea-pig caecum. Br J Pharmacol. 1983 Nov;80(3):409–420. doi: 10.1111/j.1476-5381.1983.tb10710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Clark J. P., Kitamura K., Lang R. J. Evidence that histamine and carbachol may open the same ion channels in longitudinal smooth muscle of guinea-pig ileum. J Physiol. 1981 Nov;320:363–379. doi: 10.1113/jphysiol.1981.sp013955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilvers E. R., Barnes P. J., Nahorski S. R. Characterization of agonist-stimulated incorporation of myo-[3H]inositol into inositol phospholipids and [3H]inositol phosphate formation in tracheal smooth muscle. Biochem J. 1989 Sep 15;262(3):739–746. doi: 10.1042/bj2620739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp L. H., Vivaudou M. B., Walsh J. V., Jr, Singer J. J. Acetylcholine increases voltage-activated Ca2+ current in freshly dissociated smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2092–2096. doi: 10.1073/pnas.84.7.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Désilets M., Driska S. P., Baumgarten C. M. Current fluctuations and oscillations in smooth muscle cells from hog carotid artery. Role of the sarcoplasmic reticulum. Circ Res. 1989 Sep;65(3):708–722. doi: 10.1161/01.res.65.3.708. [DOI] [PubMed] [Google Scholar]
- Ehrlich B. E., Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988 Dec 8;336(6199):583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Finney M. J., Karlsson J. A., Persson C. G. Effects of bronchoconstrictors and bronchodilators on a novel human small airway preparation. Br J Pharmacol. 1985 May;85(1):29–36. doi: 10.1111/j.1476-5381.1985.tb08827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall I. P., Donaldson J., Hill S. J. Inhibition of histamine-stimulated inositol phospholipid hydrolysis by agents which increase cyclic AMP levels in bovine tracheal smooth muscle. Br J Pharmacol. 1989 Jun;97(2):603–613. doi: 10.1111/j.1476-5381.1989.tb11992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Ito Y. A role for inositol 1,4,5-trisphosphate in the initiation of agonist-induced contractions of dog tracheal smooth muscle. Br J Pharmacol. 1985 Sep;86(1):191–199. doi: 10.1111/j.1476-5381.1985.tb09449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. J. Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol Rev. 1990 Mar;42(1):45–83. [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J Physiol. 1992;453:197–218. doi: 10.1113/jphysiol.1992.sp019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Kitazawa T., Somlyo A. V., Somlyo A. P. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989 Oct 25;264(30):17997–18004. [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Role of G-proteins in muscarinic receptor inward and outward currents in rabbit jejunal smooth muscle. J Physiol. 1990 Aug;427:395–419. doi: 10.1113/jphysiol.1990.sp018178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Kawai M., Takewaki T., Ohashi H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J Physiol. 1992 May;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlikoff M. I., Murray R. K., Reynolds E. E. Histamine-induced calcium release and phorbol antagonism in cultured airway smooth muscle cells. Am J Physiol. 1987 Oct;253(4 Pt 1):C561–C566. doi: 10.1152/ajpcell.1987.253.4.C561. [DOI] [PubMed] [Google Scholar]
- Kume H., Kotlikoff M. I. Muscarinic inhibition of single KCa channels in smooth muscle cells by a pertussis-sensitive G protein. Am J Physiol. 1991 Dec;261(6 Pt 1):C1204–C1209. doi: 10.1152/ajpcell.1991.261.6.C1204. [DOI] [PubMed] [Google Scholar]
- Madison J. M., Brown J. K. Differential inhibitory effects of forskolin, isoproterenol, and dibutyryl cyclic adenosine monophosphate on phosphoinositide hydrolysis in canine tracheal smooth muscle. J Clin Invest. 1988 Oct;82(4):1462–1465. doi: 10.1172/JCI113752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi J., Couture R., Caranikas S., Regoli D. Pharmacological effects of peptides on tracheal smooth muscle. Pharmacology. 1982;25(1):39–50. doi: 10.1159/000137722. [DOI] [PubMed] [Google Scholar]
- Murray R. K., Bennett C. F., Fluharty S. J., Kotlikoff M. I. Mechanism of phorbol ester inhibition of histamine-induced IP3 formation in cultured airway smooth muscle. Am J Physiol. 1989 Oct;257(4 Pt 1):L209–L216. doi: 10.1152/ajplung.1989.257.4.L209. [DOI] [PubMed] [Google Scholar]
- Murray R. K., Kotlikoff M. I. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol. 1991 Apr;435:123–144. doi: 10.1113/jphysiol.1991.sp018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neliat G., Masson F., Gargouil Y. M. Modulation of the spontaneous transient outward currents by histamine in single vascular smooth muscle cells. Pflugers Arch. 1989;414 (Suppl 1):S186–S187. doi: 10.1007/BF00582298. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Oike M., Kitamura K., Kuriyama H. Histamine H3-receptor activation augments voltage-dependent Ca2+ current via GTP hydrolysis in rabbit saphenous artery. J Physiol. 1992 Mar;448:133–152. doi: 10.1113/jphysiol.1992.sp019033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P., Bolton T. B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J Physiol. 1991 Sep;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Wakui M. Oscillating intracellular Ca2+ signals evoked by activation of receptors linked to inositol lipid hydrolysis: mechanism of generation. J Membr Biol. 1990 Nov;118(2):93–105. doi: 10.1007/BF01868467. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Neto F. A., Mattera R., Hildebrandt J. D., Codina J., Field J. B., Birnbaumer L., Sekura R. D. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol. 1985;109:566–572. doi: 10.1016/0076-6879(85)09115-7. [DOI] [PubMed] [Google Scholar]
- Sims S. M. Cholinergic activation of a non-selective cation current in canine gastric smooth muscle is associated with contraction. J Physiol. 1992 Apr;449:377–398. doi: 10.1113/jphysiol.1992.sp019091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims S. M., Walsh J. V., Jr, Singer J. J. Substance P and acetylcholine both suppress the same K+ current in dissociated smooth muscle cells. Am J Physiol. 1986 Oct;251(4 Pt 1):C580–C587. doi: 10.1152/ajpcell.1986.251.4.C580. [DOI] [PubMed] [Google Scholar]


