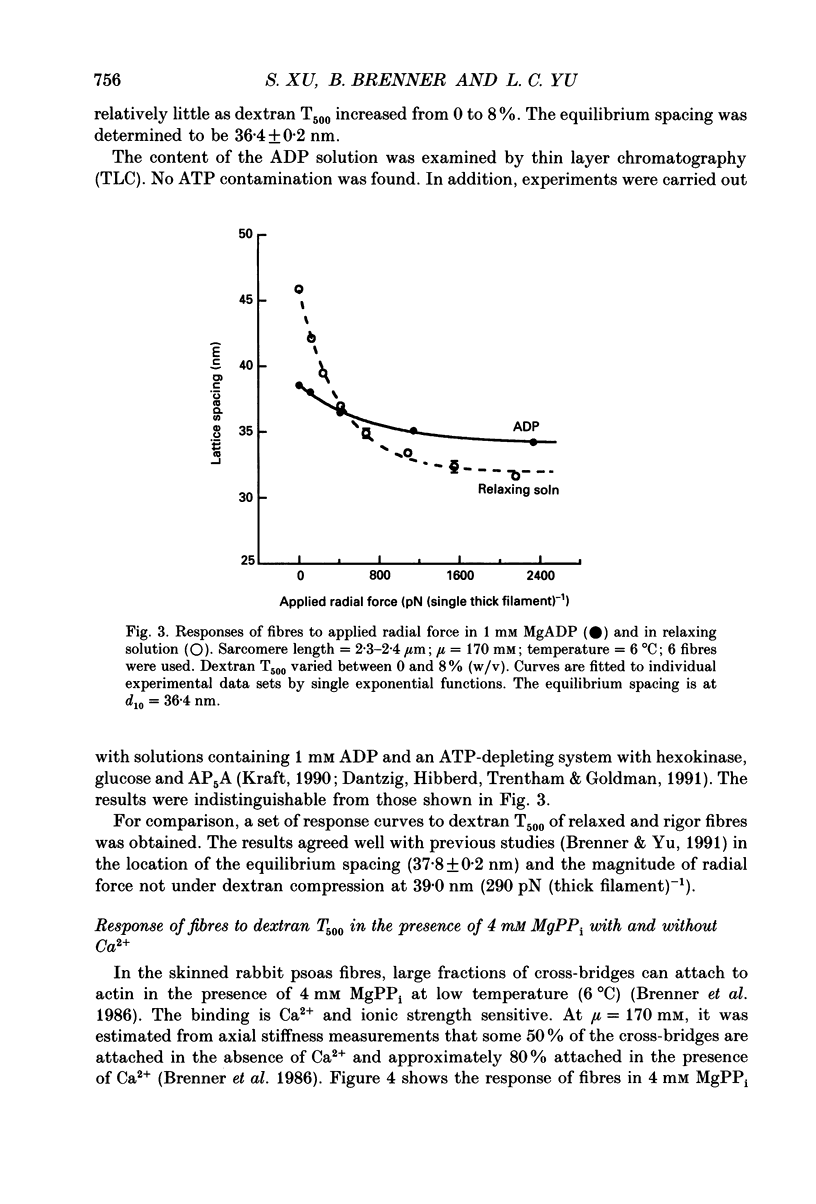
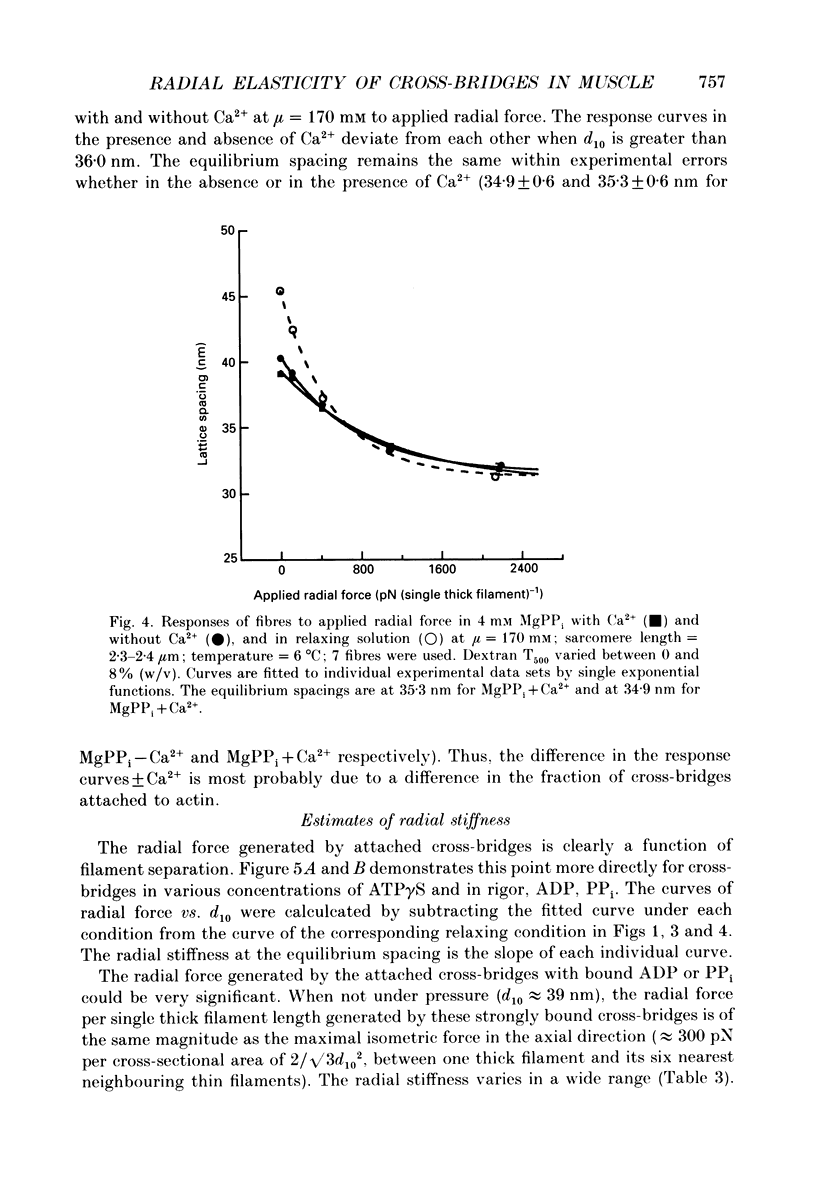
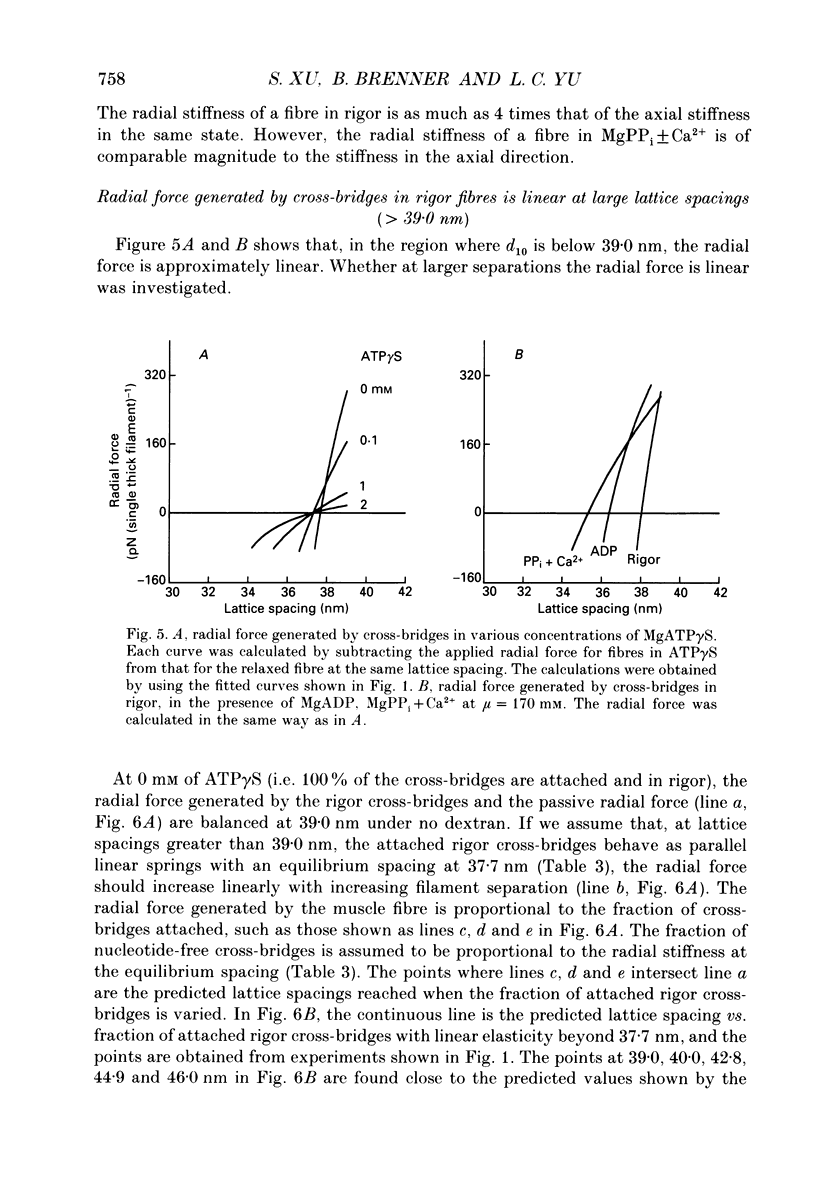
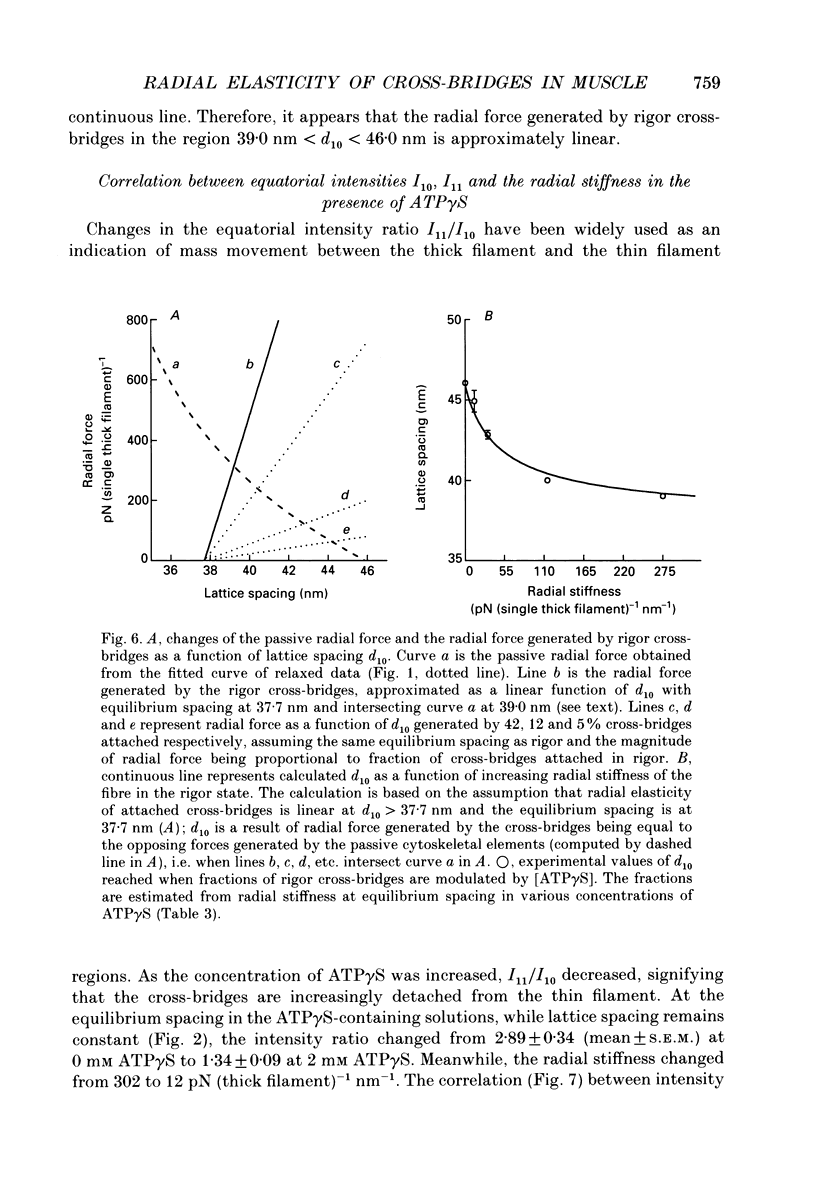
Abstract
1. In a single skinned fibre of rabbit psoas muscle, upon attachment of cross bridges to actin in the presence of ADP or pyrophosphate (PPi), the separation between the contractile filaments, as determined by equatorial X-ray diffraction, is found to decrease, suggesting that force is generated in the radial direction. 2. The single muscle fibres were subjected to compression by 0-8% of dextran T500. The changes in lattice spacings by dextran compression were compared with changes induced by cross-bridge attachment to actin. Based on this comparison, the magnitude and the direction of the radial force generated by the attached cross-bridges were estimated. The radial cross-bridge force varied with filament separation, and the magnitude of the radial cross-bridge force reached as high as the maximal axial force produced during isometric contraction. 3. One key parameter of the radial elasticity, i.e. the equilibrium spacing where the radial force is zero, was found to depend on the ligand bound to the myosin head. In the presence of ADP, the equilibrium spacing was 36 nm. In the presence of MgPPi the equilibrium spacing shifted to 35 nm and Ca2+ had little effect on the equilibrium spacing. 4. The equilibrium spacing was independent of the fraction of cross-bridges attached to actin. The fraction of cross-bridges attached in rigor was modulated from 100% to close to 0% by adding up to 10 mM of ATP gamma S in the rigor solution. The lattice spacing remained at 38 nm, the equilibrium spacing for nucleotide-free cross-bridges at mu = 170 mM. 5. Radial force generated by cross-bridges in rigor at large lattice spacings (38 nm < or = d10 < or = 46 nm) appeared to vary linearly with lattice spacing. 6. The titration of ATP gamma S to fibres in rigor provided a correlation between the radial stiffness of the nucleotide-free cross-bridges and the equatorial intensities. The relation between the equatorial intensity ratio I11/I10 and radial stiffness appeared to be approximately linear. 7. The fibres under different conditions showed a wide range of radial stiffness, which was not proportional to the apparent axial stiffness of the fibre. If the apparent axial stiffness is a measure of the fraction of cross-bridges bound to actin, it follows that the radial elastic constant is state dependent; or vice versa.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



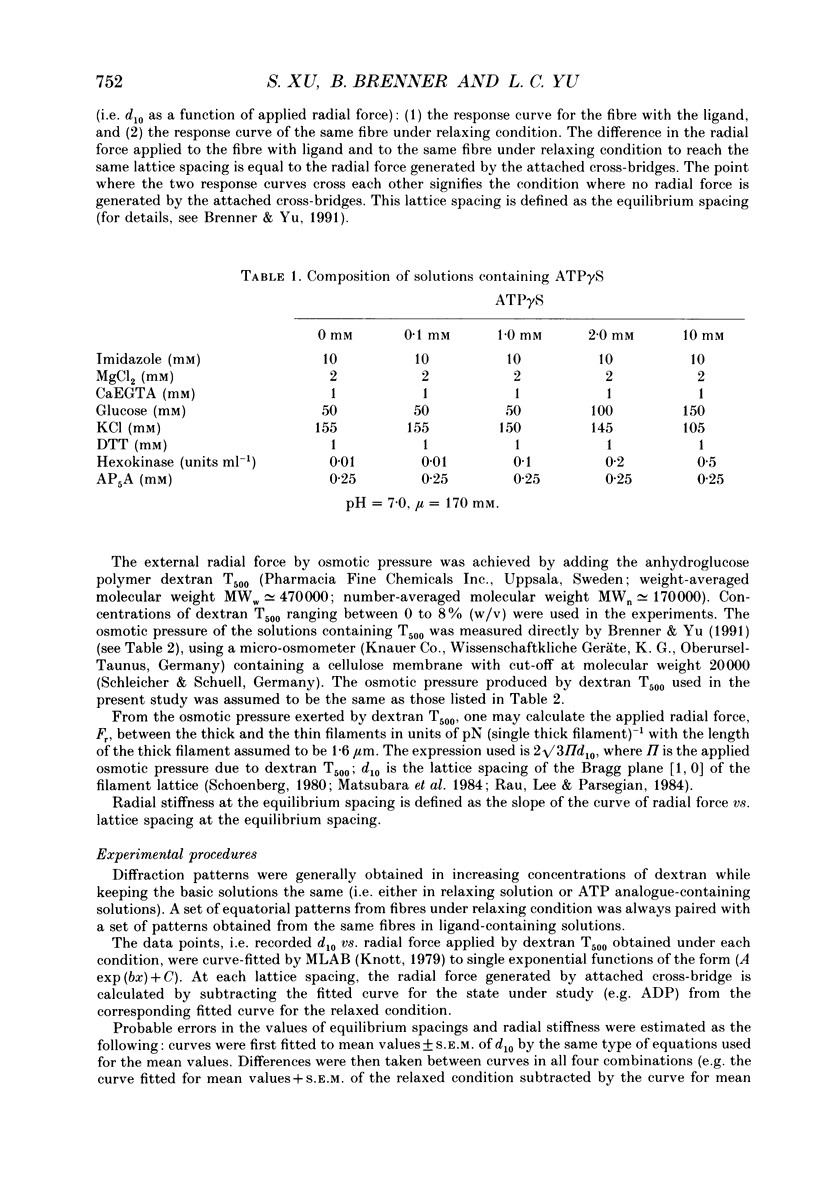
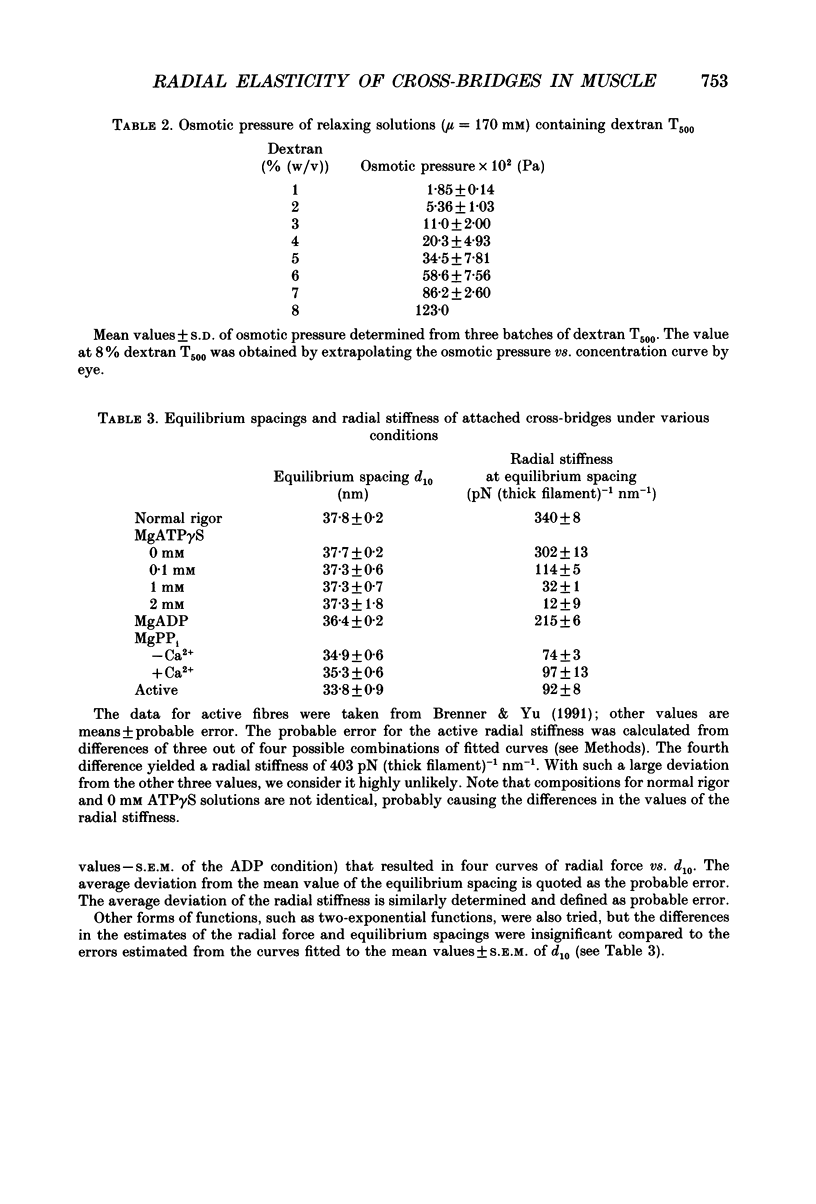
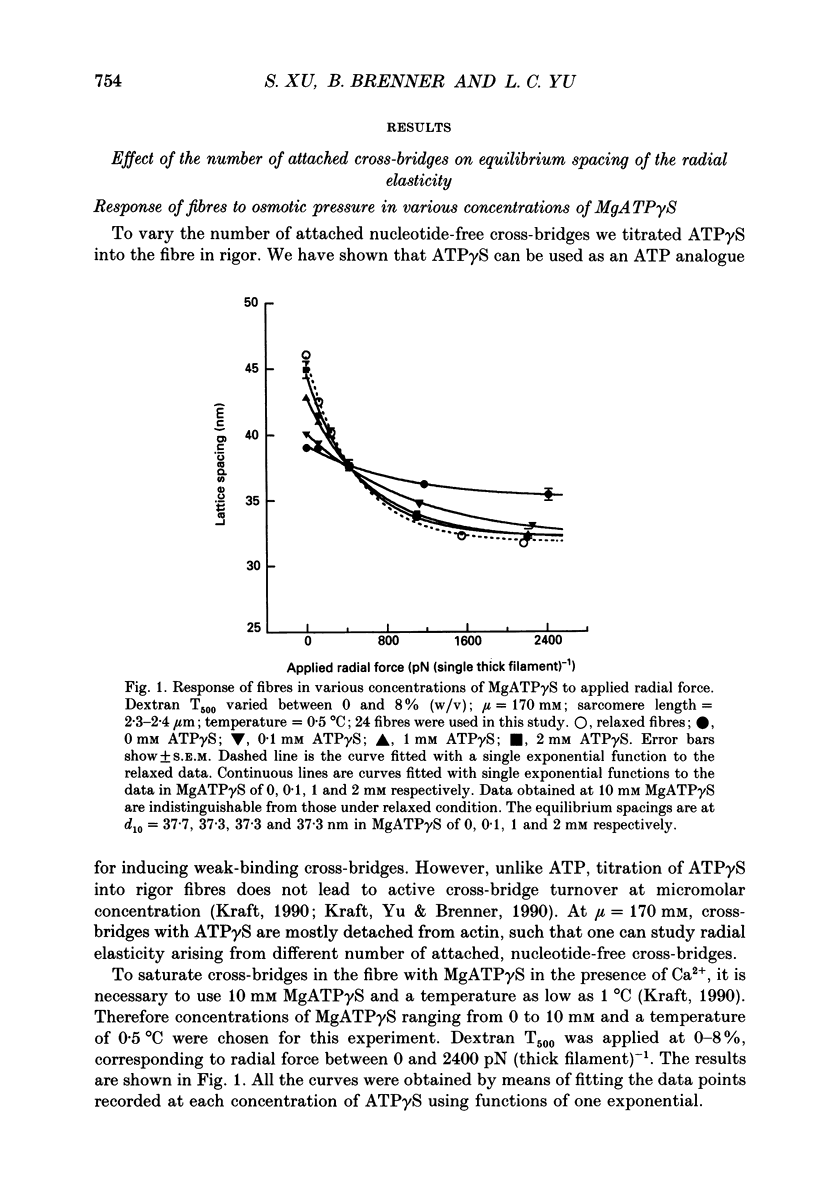
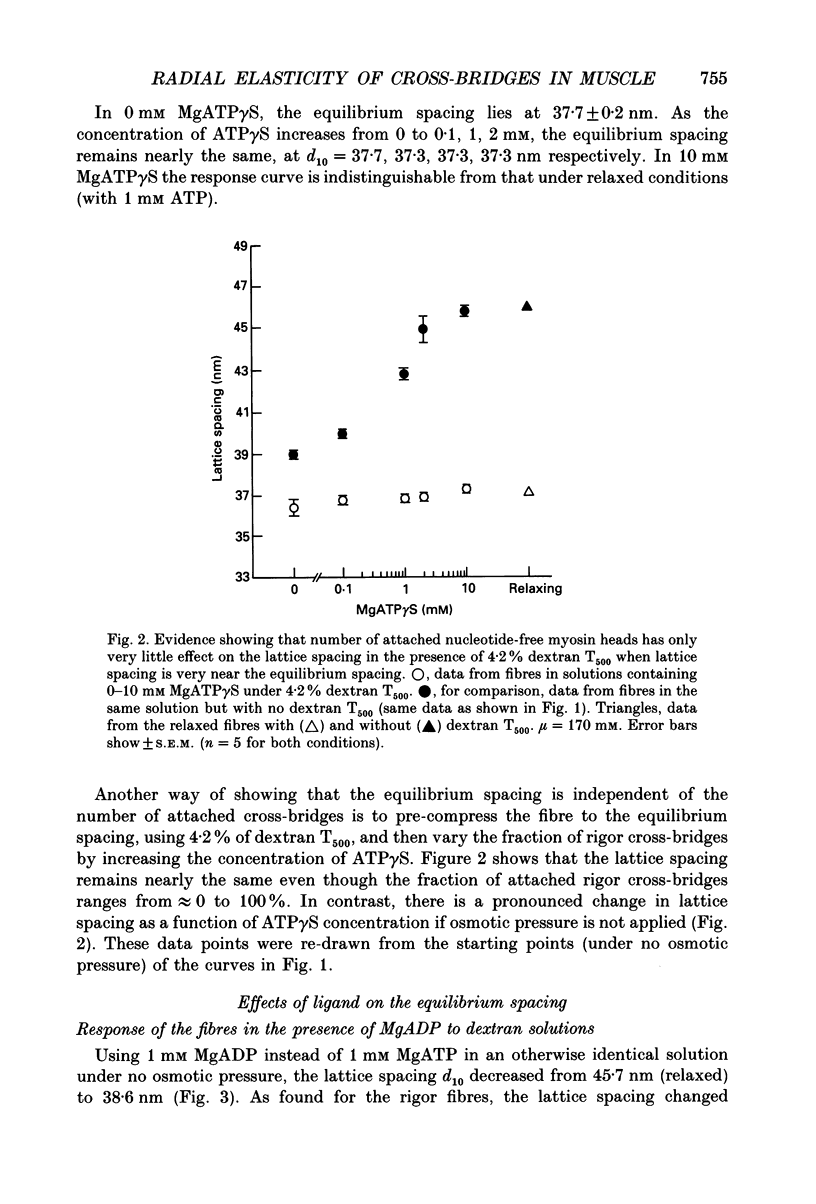





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner B. Rapid dissociation and reassociation of actomyosin cross-bridges during force generation: a newly observed facet of cross-bridge action in muscle. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10490–10494. doi: 10.1073/pnas.88.23.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys J. 1983 Jan;41(1):99–102. doi: 10.1016/S0006-3495(83)84411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C. Characterization of radial force and radial stiffness in Ca(2+)-activated skinned fibres of the rabbit psoas muscle. J Physiol. 1991 Sep;441:703–718. doi: 10.1113/jphysiol.1991.sp018774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C. Equatorial x-ray diffraction from single skinned rabbit psoas fibers at various degrees of activation. Changes in intensities and lattice spacing. Biophys J. 1985 Nov;48(5):829–834. doi: 10.1016/S0006-3495(85)83841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Greene L. E., Eisenberg E., Schoenberg M. Ca2+-sensitive cross-bridge dissociation in the presence of magnesium pyrophosphate in skinned rabbit psoas fibers. Biophys J. 1986 Dec;50(6):1101–1108. doi: 10.1016/S0006-3495(86)83554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Podolsky R. J. X-ray diffraction evidence for cross-bridge formation in relaxed muscle fibers at various ionic strengths. Biophys J. 1984 Sep;46(3):299–306. doi: 10.1016/S0006-3495(84)84026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Bagni M. A., Griffiths P. J., Ashley C. C., Maeda Y. Detection of radial crossbridge force by lattice spacing changes in intact single muscle fibers. Science. 1990 Dec 7;250(4986):1409–1411. doi: 10.1126/science.2255911. [DOI] [PubMed] [Google Scholar]
- Cooke R., Franks K. All myosin heads form bonds with actin in rigor rabbit skeletal muscle. Biochemistry. 1980 May 13;19(10):2265–2269. doi: 10.1021/bi00551a042. [DOI] [PubMed] [Google Scholar]
- Dantzig J. A., Hibberd M. G., Trentham D. R., Goldman Y. E. Cross-bridge kinetics in the presence of MgADP investigated by photolysis of caged ATP in rabbit psoas muscle fibres. J Physiol. 1991 Jan;432:639–680. doi: 10.1113/jphysiol.1991.sp018405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Brunsvold N. J., Thomas D. D. Effects of AMPPNP on the orientation and rotational dynamics of spin-labeled muscle cross-bridges. Biophys J. 1988 Apr;53(4):513–524. doi: 10.1016/S0006-3495(88)83131-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Knott G. D. Mlab--a mathematical modeling tool. Comput Programs Biomed. 1979 Dec;10(3):271–280. doi: 10.1016/0010-468x(79)90075-8. [DOI] [PubMed] [Google Scholar]
- Lovell S. J., Harrington W. F. Measurement of the fraction of myosin heads bound to actin in rabbit skeletal myofibrils in rigor. J Mol Biol. 1981 Jul 15;149(4):659–674. doi: 10.1016/0022-2836(81)90352-1. [DOI] [PubMed] [Google Scholar]
- Lymn R. W. Myosin subfragment-1 attachment to actin. Expected effect on equatorial reflections. Biophys J. 1978 Jan;21(1):93–98. doi: 10.1016/S0006-3495(78)85510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Goldman Y. E., Simmons R. M. Changes in the lateral filament spacing of skinned muscle fibres when cross-bridges attach. J Mol Biol. 1984 Feb 15;173(1):15–33. doi: 10.1016/0022-2836(84)90401-7. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Umazume Y., Yagi N. Lateral filamentary spacing in chemically skinned murine muscles during contraction. J Physiol. 1985 Mar;360:135–148. doi: 10.1113/jphysiol.1985.sp015608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan D. W., Godt R. E. Radial forces within muscle fibers in rigor. J Gen Physiol. 1981 Jan;77(1):49–64. doi: 10.1085/jgp.77.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau D. C., Lee B., Parsegian V. A. Measurement of the repulsive force between polyelectrolyte molecules in ionic solution: hydration forces between parallel DNA double helices. Proc Natl Acad Sci U S A. 1984 May;81(9):2621–2625. doi: 10.1073/pnas.81.9.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M., Eisenberg E. ADP binding to myosin cross-bridges and its effect on the cross-bridge detachment rate constants. J Gen Physiol. 1987 Jun;89(6):905–920. doi: 10.1085/jgp.89.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umazume Y., Kasuga N. Radial stiffness of frog skinned muscle fibers in relaxed and rigor conditions. Biophys J. 1984 Apr;45(4):783–788. doi: 10.1016/S0006-3495(84)84222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. C. Analysis of equatorial x-ray diffraction patterns from skeletal muscle. Biophys J. 1989 Mar;55(3):433–440. doi: 10.1016/S0006-3495(89)82837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. C., Brenner B. Structures of actomyosin crossbridges in relaxed and rigor muscle fibers. Biophys J. 1989 Mar;55(3):441–453. doi: 10.1016/S0006-3495(89)82838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. P., Hartt J. E., Podolsky R. J. Equatorial x-ray intensities and isometric force levels in frog sartorius muscle. J Mol Biol. 1979 Jul 25;132(1):53–67. doi: 10.1016/0022-2836(79)90495-9. [DOI] [PubMed] [Google Scholar]


