Abstract
Spinal cord injury (SCI) causes abnormal liver function, the development of metabolic dysfunction-associated steatotic liver disease features and metabolic impairment in patients. Experimental models also demonstrate acute and chronic changes in the liver that may, in turn, affect SCI recovery. These changes have collectively been proposed to contribute to the development of a SCI-induced metabolic dysfunction-associated steatohepatitis (MASH). However, none of the existent studies have focused on hepatic stellate cells (HSCs), liver resident cells that are the primary drivers of collagen deposition and fibrosis following sustained liver damage. Here, we describe the transient activation of HSCs after a thoracic contusion in rats, considered a clinically relevant model of experimental SCI. We studied HSC during the time course of SCI, from 1 to 45 days post injury. We found a transient activation of HSCs after SCI, beginning with the acute downregulation of Glial Fibrillar Acidic Protein 1dpi. This is followed by a morphological and phenotypical transformation into alpha-smooth muscle actin (ACTA2/SMA) immunoreactive myofibroblast-like cells, peaking at 14 days post-injury and returning to control-like levels at later timepoints (45 days post-injury). These changes are not accompanied by fibrosis development but collagen deposition in peri-portal areas is observed at 45 days.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-87131-3.
Keywords: GFAP, Hepatic stellate cells, Fibrosis, Spinal cord, Liver, SMA
Subject terms: Neuroscience, Diseases of the nervous system, Glial biology, Regeneration and repair in the nervous system, Liver, Spinal cord diseases
Introduction
Spinal cord injury (SCI) is a condition that extends beyond neural damage. Depending on the lesion level, a number of organ imbalances develop that, in turn, influence the neurological status of the patient1–3. One such organ is the liver, whose malfunction may partly explain the various metabolic disturbances observed in SCI patients3, and the systemic inflammation profile in the early stages4–7. SCI triggers an acute-phase response (APR), increasing levels of cytokines and chemokines and neutrophil infiltration in the liver5,8–10 as early as 1–2 h post-injury, peaking at 6–12 h5,8,9,11. Additionally, during the initial days, liver macrophages/Kupfer cells get activated12, blood flow is impared10,13 and glucose metabolism is affected11. But liver activation goes beyond the early stages after SCI: there is chronic activation of macrophages/Kupfer cells12,14,15, accumulation of lipid droplets within hepatocytes, characteristic of hepatic steatosis7,12,14–16, modification of lipid metabolism from the first week7,12, and accumulation of iron and ferritin12,14. The evidence of chronic inflammation and lipid accumulation have led to propose a SCI-induced Metabolic dysfunction-associated Steatohepatitis (MASH)3,7,12.
In recent years, some roles of liver-resident cell types such as hepatocytes and Kupffer cells after SCI have been described4,6,10,12 but there has been little attention to another crucial population: the hepatic stellate cells (HSCs). HSCs are located in the Space of Disse, between liver sinusoidal endothelial cells and hepatocytes, and comprise about 10% of resident liver cells17,18. In healthy conditions, HSCs maintain a quiescent phenotype, expressing glial fibrillary acidic protein (GFAP) in their cytoskeleton17,19,20, storing vitamin A, regulating liver microcirculation and acting as immune regulators17,21. Following liver damage/stress, HSCs activate and differentiate into SMA+ myofibroblast-like cells and produce considerable amounts of extracellular matrix (ECM), becoming the main fibrogenic cell type in the liver, regardless of the etiology17,18,22.
Although mild hepatic fibrosis and inflammation may be part of the wound-healing response17,18, prolonged hepatic injury or stress can impair regeneration and result in excessive ECM accumulation and the formation of a fibrous scar17,18,23. This may escalate to serious liver conditions, from severe MASH to cirrhosis and, eventually, hepatocellular carcinoma17,18,23. Fibrosis, however, can be reversed after the cessation of the fibrogenic stimulus, by inducing HSC death, senescence or deactivation23–25. Indeed, HSC deactivation is becoming increasingly explored to resolve fibrosis and liver damage24,25. Here, we describe for the first time the course of HSCs activation in response to a clinically relevant model of SCI.
Results
Spinal cord injury induces a transient loss of GFAP immunoreactivity in liver hepatic stellate cells
GFAP is a marker of HSC in their resting state19,20. In controls, a high density of GFAP + star-shaped like cells (HSCs) were found evenly distributed in the tissue, also around the central veins, where proportional area was quantified (Fig. 1A,G). We found that 1d after injury GFAP-ir was substantially reduced using either polyclonal or monoclonal antibodies against the protein (Fig. 1B,H) but this reduction is transient. At 3 days, signal reduction is not statistically significant vs. control levels (Fig. 1F,L), being fully recovered from 14 days to 45 dpi (Fig. 1A–L).
Fig. 1.
Time course of GFAP immunoreactivity (GFAP-ir) in the liver of spinal cord injured rats. (A) Control animals show even distribution of GFAP+ cells around central vein with a stellate morphology consistent with resting HSC phenotype. (B) GFAP-ir markedly decreases 1-day post-injury, but rapidly returns to control levels from 3 to 45dpi (C–E). (F) Quantification of GFAP-ir proportional area shows that GFAP-ir decrease is statistically significant at 1dpi. (G–L) Similar results are obtained when using a monoclonal anti-GFAP antibody. (L) Quantification of GFAP-ir proportional area shows that GFAP-ir decrease is statistically significant at 1dpi. Statistics: one way ANOVA followed by Tukey’s multiple comparison test; *, p < 0.05 vs. CONTROL, 14 and 45dpi; Magnification bars, 100 μm. Lines represent Mean ± SEM.
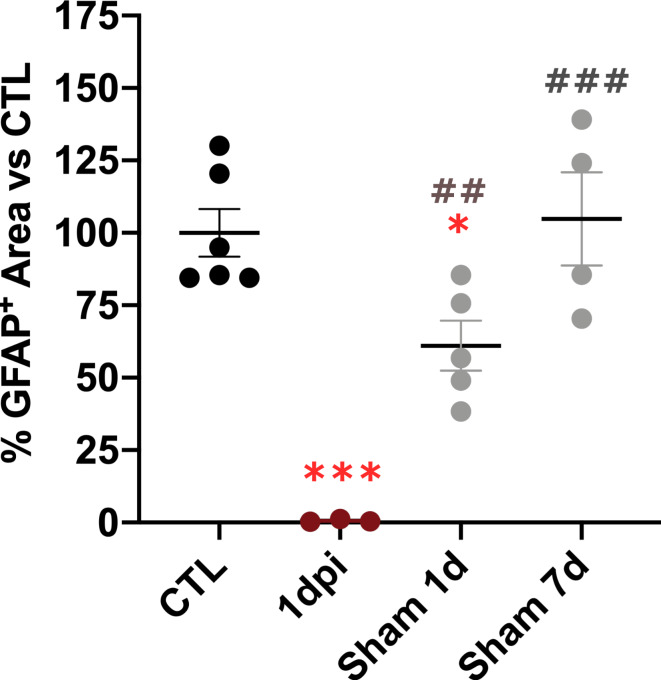
Since liver can be acutely affected by surgery and anesthetics, we measured GFAP-ir also in sham-operated animals, sacrificed 1 day after the lesion. We observed a moderate but significant reduction in GFAP-ir in sham animals compared to controls at 1 day post-surgery (Fig. 2). However, GFAP-ir in sham animals remained significantly higher than in SCI animals at the same time point. This suggest that, although acute surgical and peri-operative interventions may activate HSCs, the most pronounced acute reduction in GFAP-ir is primarily mediated by spinal cord injury.
Fig. 2.
GFAP proportional area in SCI vs. sham-operated animals at 1 and 7 days post injury. Proportional GFAP-immunreactivity (GFAP-ir) area in liver significantly decreases in SCI animals vs. Control and sham operated animals. Sham animals show a moderate, although significant GFAP-ir decrease vs. Control at 1 day, while this significance is lost at 7 days. Statistics: one way ANOVA followed by Tukey’s multiple comparison test; ***, p < 0.001 vs. CONTROL, *, p < 0.05 vs. CONTROL, ##, p < 0.01 vs. 1dpi, ###, p < 0.001 vs. 1dpi. Lines represent Mean ± SEM.
Hepatic stellate cells undergo a phenotypic transformation from day 1 to 14 after SCI, that includes morphological changes and overexpression of SMA protein
SCI induced the appearance of SMA+ cells early after damage. The abundance of these cells is maximal at 14 dpi, returning to normal levels at 45 dpi (Fig. 3A–F). SMA + cells show few processes and a more ameboid shape than the stellated GFAP + cells. In controls and sham-operated animals (1 and 7 days of survival), SMA-ir was limited to the walls of central veins or portal triads (Fig. 1A). Notably, when using more sensitive set ups (i.e. confocal microscopy), a small amount of SMA+ cells can be observed in SCI livers as soon as 1dpi in the proximities of central veins or portal triads (yellow arrowheads at Fig. 3H).
Fig. 3.
Time course of SMA immunoreactivity (SMA-ir) in the liver of spinal cord injured rats and relation with GFAP. (A) SMA expression in control animals is limited to vessels (in the image, central vein), and mostly absent 1 or 3 dpi (B, C). (D) From 7dpi, SMA+ cells with morphological resemblance of myofibroblast can be observed, peaking at 14dpi (E). (F) Quantification of SMA-ir proportional area shows that it peaks with statistical significance 14 dpi, returning to non-significant levels at 45dpi. (G) Confocal images of double GFAP (green) and SMA (magenta) labelling in control animals, show absence of double labelled cells. (H) Scarce double GFAP+/SMA+ cells can be observed 1dpi (yellow arrowheads) around the central vein, but are prominent 14dpi (I). Dashed line depicts square shown in higher magnification at (J–L). (J–L) Higher magnification images show SMA+ cells with remaining GFAP-ir in their processes (white arrows). Magnification bars = 100 μm. Lines represent Mean ± SEM.
Confocal microscopy studies using double labeling with GFAP and SMA confirmed our observations. We found stellated GFAP+ cells evenly distributed in the control tissue but virtually no SMA+ cell (Fig. 3G). However, some SMA+ cells could be found 1 dpi in the vicinity of central veins and portal triads (Fig. 3H, yellow arrowheads) that were abundant and extended to the rest of the tissue at 14 dpi (Fig. 3I–L). High magnification images showed some of these cells with SMA-ir in the perikaryon and GFAP+ processes (Fig. 3J–L, white arrows).
HSC transient activation does not involve significant collagen deposition nor the development of fibrosis
We aimed to study if HSC activation also involved a functional switch that may lead to pathological accumulation of collagen and fibrosis using picrosirius red staining (PSR+). We observed that relative PSR+ area in the liver did not significantly change at any time of the SCI time course vs. control (Fig. 4A–G). We also used clinically validated scales to blindly score the fibrotic stage of the liver at any time after lesion (Fig. 4H–J), observing that most animals showed very low or non-fibrotic features, scoring 0 in Ishak, METAVIR and Batts-Ludwig scales during the acute/subacute phase (up to 14 days after injury). However, in chronic phase, at 45 dpi, 66% of animals showed Ishak score 1, METAVIR F1 (mild to moderate fibrosis) and Batts-Ludwig stage 1 (mild fibrosis), a difference statistically significant when compared to controls.
Fig. 4.
Evaluation of collagen deposition in the liver using Picrosirius Red Staining. (A) Control animals show (B–E) relative PSR + area in the liver did not significantly change at any time of the SCI time course vs. control, except in some animals at 45dpi, not reaching statistical significance (G). But blind scoring using clinically validated scales for estimating fibrotic stage of the liver (H–J), showed statistically significant differences between control vs. 45dpi, when 66% of animals showed Ishak score 1, METAVIR F1 (mild to moderate fibrosis) and Batts-Ludwig stage 1 (mild fibrosis). Statistics: (A-F) one way ANOVA followed by Tukey’s multiple comparison test; (H–J) Fisher exact test *, p < 0.05 45dpi vs. CONTROL, Magnification bars, 100 μm.
Discussion
Spinal cord injury (SCI) causes abnormal liver function and the development of Metabolic dysfunction-Associated Steatotic Liver Disease (MASLD) in patients26–29. Experimental models also demonstrate acute and chronic liver changes that may affect SCI recovery3,5,7–12,14–16,30–33. Here, we describe a transient activation of hepatic stellate cells (HSCs) in SCI rats, beginning with acute GFAP downregulation, followed by morphological and phenotypical transformation into SMA+ myofibroblast-like cells. This transformation peaks at 14 days post-injury (dpi) and returns to control-like levels by 45 dpi. These changes do not result in fibrosis during the times studied, but mild collagen deposition occurs in peri-portal areas at 45 days.
HSCs play multiple roles in normal liver function, but remain non-proliferative in a quiescent state17,21. They activate following liver damage of various etiologies and become primarily responsible for extracellular matrix deposition, which can lead to fibrosis if the harmful stimulus persists17,18,22,34. Most literature on chronic liver damage describes HSC activation involving a phenotypic change from GFAP + ramified cells, rich in retinoic acid, to SMA+ collagen-secreting fibroblast-like cells with fewer processes17,22,34. We observed a similar transition after SCI, beginning with a rapid, but transient downregulation of GFAP. This is unlikely to be a technical artifact, since it was observed with poly and monoclonal antibodies (Fig. 1). Early changes in the liver induced by SCI (macrophage activation, neutrophil infiltration, increased pro-inflammatory cytokines, decreased liver blood flow, elevated catecholamines, oxygen free radical accumulation and lipid peroxidation)5,7,9,10,12,13,30–33 are known activators of HSCs17, and may underlie the transient decrease in GFAP-ir. However, the exact mechanism behind this remains unclear: It might involve rapid downregulation of gene and protein expression (42–44); transient protein modifications or proteolysis affecting antigen recognition 35–37 or competition of our antibody with high levels of natural circulating anti-GFAP auto-antibodies, known to increase after SCI in patients38,39. Further experiments are needed to clarify this.
We do not interpret GFAP-ir decrease as HSC death or disappearance for different reasons: (i) GFAP-ir cells can be detected at 1dpi when using more sensitive techniques or less restrictive IHC settings (i.e. when using monoclonal antibody settings, Fig. 1, or immunofluorescence and confocal imaging, Fig. 3); (ii) after 1dpi we see no features of extensive cell death in HE staining (data not shown) neither increases in proliferation (Supplementary Figure S1), required to replenish HSC population in case of death or disappearance.
Regardless the underlying mechanism, we observed that GFAP-ir loss precedes the appearance of SMA+ myofibroblast-like cells, likely derive from transformed HSCs. Accordingly, cells expressing both GFAP in their processes and SMA in their cell bodies are observed from 1 to 14dpi (Fig. 3). Transient HSC activation, like the one we observe, is part of physiological liver repair mechanisms, such as after chemically induced damage, where fibrosis resolves and liver regenerates. In these cases, HSCs get deactivated into a near-baseline phenotype, although they may remain primed for subsequent fibrogenic stimuli23,40.
MASLD represents a histopathological spectrum from mild steatosis (more than 5% lipid content) to Metabolic dysfunction-Associated SteatoHepatitis (MASH), which can progress to cirrhosis and hepatocellular carcinoma41, fibrosis being present only in high grade MASH. After SCI, SCI-induced MASH has been proposed3 according to the changes observed (iron accumulation, increase in pro-inflammatory cytokines, Kupffer cell activation and lipid droplet accumulation)7,12,15. However, the histological features described (moderate lobular inflammation, limited hepatocyte lipid infiltration with no hepatocyte ballooning) suggest that it is likely limited to a mild MASH form (Grade 1)7,12,15,42. This is also supported by the moderate and transient elevation of liver enzymes, limited to sub-acute phase both in rats12,14 and humans28. To get a high-grade MASH in SCI rats requires a stronger inflammatory challenge, such as bile duct ligation combined with SCI15 or premorbid obesity16. Our data also suggest that SCI-induced MASLD during the acute and subacute phases in rats is limited. We found no significant changes in circulating liver enzymes beyond those induced by surgery (AST/ALT data in supplementary figure S2 and Supp Methods) neither in collagen deposition during SCI-induced HSC activation, consistent with the lack of fibrosis reported by McTigue’s lab up to 23 dpi12,15. Although other study reports increased collagen area in the liver from 3 dpi7, this may possibly due to different selection of the area of study and image analysis. While we used wide fields including central veins, portal triads, zones 1 to 3 and excluded blood vessels from the total area, Sun et al.7 seem to have focused on zone 3.
On the other hand, we detect mild fibrosis in 2 thirds of SCI animals at the chronic phase, 45 dpi, by blindly scoring liver images using three clinical pathological scores (Ishak, METAVIR, and Batts-Ludwig), which focus on collagen deposition around portal triads. This score is far from the high scores found in severe liver pathologies (scores 4–6 meaning severe fibrosis and cirrhosis). This warrants further studies in longer-surviving animals and SCI patients.
Finally, a possible limitation of the study is the use of only thoracic lesion (T8), what may arise the question about the activation of HSC after lesions in other spinal levels. Liver receives, predominantly, sympathetic innervation by the splanchnic nerves originated from neurons in the celiac and superior mesenteric ganglia, these being innervated by pre-ganglionic neurons of the thoracic spinal cord (T7-T12)43–45. Therefore, our lesion directly impacted the levels most involved in liver sympathetic innervation but previous works have shown that liver also responds after cervical and lumbar lesions12. Also, MASLD is also observed in both paraplegic and tetraplegic patients46. The exact contribution of liver innervation to these responses is not fully clear: (i) both cervical and thoracic lesions produce a similar liver response in rats, despite cervical lesions do not directly affect preganglionic neurons involved in liver control. Even lumbar lesions, located below the liver innervation level, show liver inflammation12 ; (ii) on the other hand, MASLD prevalence is higher in paraplegic vs. tetraplegic patients; (iii) a complete denervation of the liver does not critically affect liver function, as can be observed in organ transplantation, where full denervation does not prevent the new liver from assisting the transplanted patient47, even though some changes may be observed45,47; and (iv) lesions in upper thoracic and cervical levels cause an overload of sympathetic signaling in some organs, like the spleen48, in which an excessive secretion of norepinephrine is deleterious, but this has not been studied in the liver so far. Future studies are required to determine the precise contribution of liver neural control in cellular responses to SCI.
In conclusion, we present new data on HSC activation following SCI using a clinically relevant rat model. The involvement of HSCs and their interaction with other cell types and immune system in the liver may play a crucial role in modulating regenerative responses and preventing liver damage chronification that may lead to severe MASH and significant metabolic imbalances. Additionally, HSC may be target by anti-GFAP auto-antibodies which increase acute and subacutely in the serum of SCI patients38,39,49.
Methods
Animals
Experiments were performed on male Wistar rats (rattus norvegicus, 300 g) from our inbred colony and maintained in our animal facility on a 12/12-hour light/dark cycle, with ad libitum access to food and water. Animals were housed in pairs before surgery. After surgery, animals were housed individually in all groups, including sham animals, to avoid misinterpretations due to differences cause by this factor. Animals were handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals, the Guidelines for the Use of Animals in Neuroscience Research published by the Society for Neuroscience, and European Union guidelines (Council Directive 86/609/EEC). Experimental procedures were approved by the Ethical Committee for Animal Welfare at the National Paraplegics Hospital (CEEA) and by the Regional Authorized Body for Animal Welfare (OH-HNPT, approval ref #51-OH/2021). Special care was taken to use the minimum number of animals required for statistical accuracy. The current study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Experimental spinal cord injury (SCI)
All rats were lesioned between 09:30 and 15:30 in a fed state. Animals were anesthetized with an intraperitoneal injection of sodium pentobarbital (45 mg/kg: Normon Veterinary Division, Madrid, Spain) and Xilagesic (2% Xylacine, 10 mg/kg: Calier, Barcelona, Spain). Once the absence of reflexes was confirmed, rats were injected with a low dose of atropine (50 µg/kg body weight: Brown Medical, Barcelona, Spain) to reduce salivary and bronchial secretions, and to avoid the induction of bradycardia and possible cardiac arrest by xylazine or the surgical procedure. Artificial tears were applied to the eyes to prevent corneal abrasion and infection. Laminectomy of the 8th thoracic vertebra (T8) was performed as described previously39 and the animals were subjected to a moderate contusion using the Infinite Horizon (IH) spinal cord injury device (Precision Systems & Instrumentation [PSI], Lexington, KY). This device applied a defined controlled impact of 200 kdyn with no additional dwell time to the exposed spinal cord using computer software. Lesions were included in the study provided that (i) the infinite horizon device feedback was correct (i.e. adequate actual force, velocity and a smooth movement profile of the impounder), (ii) submeningeal congestion in the dorsal spinal cord was observed within minutes following contusion, and (iii) rats showed severe motor impairment during the first days (no presence of weight support or stepping 1–3 days after injury), according to the regular pattern of recovery we and others have observed for this type of lesion50–53. Animals were then hydrated and placed on heated blankets for 1 h. Postoperative care included subcutaneous injections of Buprex (buprenorphine, 0.05 mg/kg: Schering Plough, Madrid, Spain) during the first 3 days after lesion, and prophylactic subcutaneous injection of antibiotics (Marbocyl, marbofloxacin 1%, 0.5 mg/Kg, Madrid, Spain) on alternate days during the first week. Animals were fed with wet extruded rodent food and their bladders were manually expressed until self-control was recovered. Animals (n = 4–7) were sacrificed at 1, 3, 7, 14 or 45 days after injury by intracardial perfusion as described below.
Animals in “Control” group were age-matched with no interventions. Sham animals were included also for comparison. These received the same surgery protocol and post-operative care than SCI animals except for the contusion. A group of sham animals (n = 5) was perfused 1 day after surgery while other (n = 5) was perfused 7 days after surgery.
A sample size of 4–7 rats per group was planned based on our preliminary studies showing controlled variability in the quantification strategies used here. 51 rats were bred for the experiments, of which 47 survived the experimental manipulations (lesioned rats not fulfilling criteria of appropriate lesion explained above were eliminated from the study). Rats were randomly assigned to different SCI survival times and sham groups by a blind observer.
Tissue processing
Rats were deeply anesthetized with sodium pentobarbital (200 mg/kg) and perfused through the left cardiac ventricle with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer pH 7.4. Liver right median lobe was extracted and postfixed for 2 h at 4ºC in the same fixative. 40 μm sections (for immunohistochemistry) or 30 μm sections (for Picrosirius Red staining) were then obtained with a vibrating microtome (Leica VT 1000 M) and used for histology. Uncutted tissue block was stored at − 18 ºC in Olmos solution and new sections were obtained when needed.
Immunohistochemistry
Free floating sections were rinsed in 0.1 M Phosphate-buffered saline (pH 7.4) (PB). Endogenous peroxidase activity was inhibited by incubating the sections in 0.1 M PB containing 2.4% H2O2 and 50% Methanol. Then, sections were extensively rinsed with 0.3% Bovine Serum Albumin (BSA), 0,1% Triton X-100 in 0.1 M PB (Rinse Solution, RS). Sections were incubated overnight with either donkey anti- Glial fibrillar acidic protein (GFAP, #Z-0334, Dako, Glostrup, Denmark; 1:1000) or mouse anti- Smooth muscle actin (SMA, clone 1A4, #A2547, SIGMA, Saint Louis, USA; 1:1000) in 0.1 M PB with 5% BSA and 0.3% Triton X-100. After careful rinsing, the sections were incubated for 2 h at room temperature (RT) with a horseradish peroxidase (HRP)-conjugated Donkey anti rabbit or Donkey anti mouse secondary antibodies (Jackson Immunoresearch, West Grove, Pensilvania, USA; 1:300). The peroxidase reaction product was visualized by incubating the sections in a solution of 0.03% diaminobenzidine and 0.01% hydrogen peroxide in 0.1 M PB. Finally, the sections were mounted on gelatin-coated slides, coverslipped and analyzed with a Leica DMR microscope.
For fluorescent multiple staining, sections were rinsed in RS and incubated with both rabbit anti-GFAP and mouse anti-SMA overnight. After three rinses in RS, sections were incubated with Alexa-488 conjugated Donkey anti rabbit and Alexa-555 conjugated Donkey anti mouse (1:1000; Jackson Immunoresearch, West Grove, Pensilvania, USA). To reduce tissue autofluorescence, sections were mounted on gelatin covered slides, dried overnight and incubated 5 min with a 0.5% solution of Sudan Black B (#199664, Sigma Aldrich) in 70% ethanol as described elsewhere54. After quick rinses with 70% ethanol and PB, nuclei were counterstained with Bisbenzimide (Hoechst 33258, 1:5000, ThermoFisher), rinsed in PB, dried overnight and covered with Fluoromount (F4680, Sigma-Aldrich). Samples were analyzed with a LEICA SP5 confocal microscope in our Microscopy Facility (Hospital Nacional de Paraplejicos, Toledo, SESCAM).
Handling and analysis of histological materials was performed by team members unaware of the experimental group of each animal. Histological analyses were performed in several batches, with samples assigned to batches randomly. All batches included control animals for an appropriate calibration of eventual different outcomes between batches. Specificity of antibodies used for histology was confirmed in our and others previous works. Negative controls were included by omitting the primary antibodies, observing absence of specific staining. For immunofluorescence, confocal settings were set so that no signal was observed in negative control samples. These settings were maintained for all the acquisitions.
Biotin free rat-on-rat immunohistochemistry against GFAP
To strengthen our results on liver GFAP expression, we repeated the immunohistochemistry using a second anti-GFAP antibody (rat monoclonal anti-GFAP, #13–0300, ThermoScientific; 1:300). Since primary antibody was produced in rat, we used a rat on rat immunohistochemistry protocol adapted from55. In organs with high endogenous biotin content like liver, biotin-based systems are not recommended for signal amplification. We, therefore, used a biotin-free protocol, in which signal is amplified using HRP-conjugated polymers. Briefly, after initial rinses with PB and endogenous peroxidase blockade (as described above), tissue Fc receptors were blocked by incubating sections at RT with Fc Receptor Blocker (#NB309, Innovex biosciences, 1:2 dilution). In parallel, we formed primary/secondary antibody complexes by incubating primary antibody with Fab fragments of Goat anti-rat IgG (#112-007-003, Jackson IR) at 1:2 ratio in RS. After 45 min of incubation, primary/secondary mix was saturated with normal rat serum (#Jackson, 1:1000) for 10 min. Sections were then incubated with this solution overnight at 4ºC and 2 additional hours at RT. After several PB rinses, sections were incubated with Goat-on-Rodent HRP-Polymer (Biocare #902-GHP516-080717) according to manufacturer instructions, but diluting Goat probe and Goat-on-rodent HRP-polymer 1:4 in PB. Final rinses were made in RS and PB and the peroxidase reaction visualized as mentioned above.
GFAP and SMA proportional area measurement
We quantified the area occupied by GFAP or SMA immunoreactivity on regions around central veins (708 μm x 533 μm) captured with an Olympus DP71 camara attached to an Olympus BX61 microscope, using 20x objective. Images were analyzed with Fiji56 as follows: images were converted to 8 bit and background subtracted (rolling ball radius 10). Then, an automatic threshold mask was applied by using the Triangle method, to estimate immunoreactive area. This area was referred to the total tissue area in the picture, measured as that after excluding central vein light, to avoid potential changes in the space of reference and relative immunoreactive area due to variations in central vein.
Picrosirius red staining
Liver sections (30 μm thick) were mounted on Superfrost Plus Slides (Menzel, Thermo Scientific, Braunschweig, Germany) and dried overnight. Sections were then stained for picrosirius red according to published protocols57. Briefly, slides were immersed in a 0.1% solution of Sirius red F3B (Direct Red 80, #365548-5G, SIGMA ALDRICH) in 1.2% saturated aqueous picric acid solution (#A2520, PanReac Applichem). After 1 h staining at RT, slides were rinsed twice in acidified water (0.5% acetic acid), dried again and dehydrated in three changes of 100% ethanol, two changes of xylene and covered with DePeX mounting medium.
Picrosirius red proportional area measurement
Random photomicrographs of SR stained liver sections were obtained with an Olympus DP71 camara attached to an Olympus BX61 microscope, using 10x objective (N.A. 0.25), avoiding tissue borders or irregular non-specific staining. We evaluated an average of 8 images per animal (at least 4) using Fiji56 estimating SR + area using the automatic color thresholding (color space YUV) with triangle method. Total area was measured avoiding the light of the big vessels (cv or portal triad) and SR + area was related to this measurement.
Liver fibrosis clinical scoring based on Ishak, MetaVIR and Batts-Ludwig scales
Micropictures of picrosirius red staining obtained as described above were blindly evaluated by an expert pathologist according to Ishak, MetaVIR and Batts-Ludwig liver fibrosis scales58,59. These scales score collagen deposition from no histological liver alteration (score 0) to cirrhosis (6 for Ishak; F4/Stage 4 for MetaVIR and Batts-Ludwig), being severe fibrosis indicated by 4–5 points in Ishak or 3 in MetaVIR and Batts-Ludwig. Statistical frequency comparisons vs. control group were performed using Fisher´s exact test.
Statistics
Statistical analyses were carried out with the GraphPad Prisma software (v8.4). Student’s t test, one-way ANOVA followed by Tukey’s multiple comparison test were performed when appropriate (detailed in figure legends), i.e., when normality and homoscedasticity requirements were fulfilled. Normality was assessed with Shapiro’s test and homoscedasticity with Levene’s test. In the cases where these requirements were not fulfilled, the non-parametric factorial analysis was used.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Prof. Carmen Guaza (Cajal Institute, CSIC, Madrid) for generous gift of the rat anti-GFAP antibody. This work was funded by the Ministry of Science and Innovation of Spain (MCIN/ AEI /10.13039/501100011033), grant ID PID2020-120652RB-I00 (to DGO and AAM). Funding agency had no role in study design, collection, analysis or interpretation of data, writing the report or decision to submit the article for publication.
Author contributions
Study concept and design: AA-M, DG-O; acquisition of data, analysis and interpretation of data: IF-C, AB, JJ-G, MW, BP-T, CB-J, AA-M, DG-O; drafting of the manuscript: DG-O; critical revision of the manuscript for important intellectual content: AA-M; statistical analysis: DG-O, obtained funding: AA-M, DG-O; administrative, technical or material support: MDC; study supervision: AA-M, DG-O.
Data availability
All data generated or analysed during this study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Angel Arevalo-Martin, Email: aarevalom@sescam.jccm.es.
Daniel Garcia-Ovejero, Email: dgarciao@sescam.jccm.es.
References
- 1.Holmes, G. M. & Blanke, E. N. Gastrointestinal dysfunction after spinal cord injury. Exp. Neurol.320, 113009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guest, J., Datta, N., Jimsheleishvili, G., Gater, D. R. & Pathophysiology classification and comorbidities after traumatic spinal cord injury. J. Person. Med.1210.3390/jpm12071126 (2022). [DOI] [PMC free article] [PubMed]
- 3.Goodus, M. T. & McTigue, D. M. Hepatic dysfunction after spinal cord injury: a vicious cycle of central and peripheral pathology? Exp. Neurol.325, 113160 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Campbell, S. J. et al. Liver kupffer cells control the magnitude of the inflammatory response in the injured brain and spinal cord. Neuropharmacology55, 780–787 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Campbell, S. J. et al. Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with Leukocyte mobilization and recruitment to both the central nervous system and the liver. Am. J. Pathol.166, 1487–1497 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao, F., Brown, A., Dekaban, G. A., Omana, V. & Weaver, L. C. CD11d integrin blockade reduces the systemic inflammatory response syndrome after spinal cord injury. Exp. Neurol.231, 272–283 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun, X. et al. Liver-derived ketogenesis via overexpressing HMGCS2 promotes the recovery of spinal cord Injury. Adv. Biol.8, (2024). [DOI] [PubMed]
- 8.Campbell, S. J. et al. CINC-1 is identified as an acute-phase protein induced by focal brain injury causing leukocyte mobilization and liver injury. FASEB J.17, 1168–1170 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Hundt, H. et al. Assessment of hepatic inflammation after spinal cord injury using intravital microscopy. Injury42, 691–696 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Fleming, J. C. et al. Remote inflammatory response in liver is dependent on the segmental level of spinal cord injury. J. Trauma Acute Care Surg.72, (2012). [DOI] [PubMed]
- 11.Gaudet, A. D. et al. Spinal cord Injury in rats dysregulates diurnal rhythms of fecal output and liver metabolic indicators. J. Neurotrauma36, 1923–1934 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauerbeck, A. D. et al. Spinal cord injury causes chronic liver pathology in rats. J. Neurotrauma32, 159–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García-López, P., Martínez-Cruz, A., Guízar-Sahagún, G. & Castañeda-Hernández, G. Acute spinal cord injury changes the disposition of some, but not all drugs given intravenously. Spinal Cord45, 603–608 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Goodus, M. T., Sauerbeck, A. D., Popovich, P. G., Bruno, R. S. & McTigue, D. M. Dietary green tea extract prior to spinal cord injury prevents hepatic Iron overload but does not improve chronic hepatic and spinal cord pathology in rats. J. Neurotrauma35, 2872–2882 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodus, M. T. et al. Liver inflammation at the time of spinal cord injury enhances intraspinal pathology, liver injury, metabolic syndrome and locomotor deficits. Exp. Neurol.342, 113725 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodus, M. T. et al. Spinal cord injury-induced metabolic impairment and steatohepatitis develops in non-obese rats and is exacerbated by premorbid obesity. Exp. Neurol.11484710.1016/j.expneurol.2024.114847 (2024). [DOI] [PMC free article] [PubMed]
- 17.Tsuchida, T. & Friedman, S. L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol.14, 397–411 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Bataller, R. & Brenner, D. A. Liver fibrosis. J. Clin. Invest.115, 209–218 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niki, T. et al. Comparison of glial fibrillary acidic protein and desmin staining in normal and CCl4-induced fibrotic rat livers. Hepatology23, 1538–1545 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Cassiman, D., Libbrecht, L., Desmet, V., Denef, C. & Roskams, T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J. Hepatol.36, 200–209 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Kamm, D. R. & McCommis, K. S. Hepatic stellate cells in physiology and pathology. J. Physiol.600, 1825–1837 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mederacke, I. et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun.4, 2823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kisseleva, T. & Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol.18, 151–166 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Liu, X. et al. Identification of lineage-specific transcription factors that prevent activation of hepatic stellate cells and promote fibrosis resolution. Gastroenterology158, 1728–1744e14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arroyo, N. et al. GATA4 induces liver fibrosis regression by deactivating hepatic stellate cells. JCI Insight6, (2021). [DOI] [PMC free article] [PubMed]
- 26.Eisenberg, D. et al. Interaction between increasing body mass index and spinal cord injury to the probability of developing a diagnosis of nonalcoholic fatty liver disease. Obes. Sci. Pract.9, 253–260 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.COOPER, I. S., MacCARTY, C. S. & RYNEARSON, E. H. & Impairment of liver function subsequent to injury to the spinal cord. J. Lab. Clin. Med.38, 689–692 (1951). [PubMed] [Google Scholar]
- 28.Bloom, K. K. & Freed, M. M. Liver enzyme abnormalities in spinal cord injury. J. Am. Paraplegia Soc.12, 11–13 (1989). [DOI] [PubMed] [Google Scholar]
- 29.Sipski, M. L., Estores, I. M., Alexander, C. J., Guo, X. & Chandralapaty, S. K. Lack of justification for routine abdominal ultrasonography in patients with chronic spinal cord injury. J. Rehabil. Res. Dev.41, 101 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Campbell, S. J. et al. Hepatic nuclear factor κB regulates neutrophil recruitment to the injured brain. J. Neuropathol. Exp. Neurol.67, 223–230 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Bao, F., Omana, V., Brown, A. & Weaver, L. C. The systemic inflammatory response after spinal cord Injury in the rat is decreased by α4β1 integrin blockade. J. Neurotrauma29, 1626–1637 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guízar-Sahagún, G. et al. Systemic microcirculation after complete high and low thoracic spinal cord section in rats. J. Neurotrauma21, 1614–1623 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Dimova-Apostolova, G., Angelova, A. & Vaptzarova, K. Catecholamine concentration in rat liver after high level transection of the spinal cord. Life Sci.64, 2375–2381 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Carter, J. K. & Friedman, S. L. Hepatic stellate cell-immune interactions in NASH. Front. Endocrinol. (Lausanne)13, (2022). [DOI] [PMC free article] [PubMed]
- 35.Fert-Bober, J. et al. Chapter 6 - Traumatic brain injury: glial fibrillary acidic protein posttranslational modification. in Biomarkers for Traumatic Brain Injury (eds. Wu, A. H. B. & Peacock, W. F.) 77–91. 10.1016/B978-0-12-816346-7.00006-3 (Academic Press, 2020).
- 36.Chen, M. H., Hagemann, T. L., Quinlan, R. A., Messing, A. & Perng, M. D. Caspase cleavage of GFAP produces an assembly-compromised proteolytic fragment that promotes filament aggregation. ASN Neuro5, AN20130032 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, Y. B. et al. Rapid increase in immunoreactivity to GFAP in astrocytes in vitro induced by acidic pH is mediated by calcium influx and calpain I. Brain Res.864, 220–229 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Hergenroeder, G. W., Moore, A. N., Schmitt, K. M., Redell, J. B. & Dash, P. K. Identification of autoantibodies to glial fibrillary acidic protein in spinal cord injury patients. Neuroreport27, 90–93 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arevalo-Martin, A. et al. Elevated autoantibodies in subacute human spinal cord injury are naturally occurring antibodies. Front. Immunol.10.3389/fimmu.2018.02365 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Troeger, J. S. et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology143, 1073–1083e22 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi, Y. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol.20, 15539 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology41, 1313–1321 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Yi, C. X., la Fleur, S. E., Fliers, E. & Kalsbeek, A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim. Biophys. Acta (BBA) Mol. Basis Dis.1802, 416–431 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Jensen, K. J., Alpini, G. & Glaser, S. Hepatic nervous system and neurobiology of the liver. Compr. Physiol. 655–665. 10.1002/cphy.c120018 (2013). [DOI] [PMC free article] [PubMed]
- 45.Kandilis, A. N., Papadopoulou, I. P., Koskinas, J., Sotiropoulos, G. & Tiniakos, D. G. Liver innervation and hepatic function: new insights. J. Surg. Res.194, 511–519 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Rankin, K. C., O’Brien, L. C., Segal, L., Khan, M. R. & Gorgey, A. S. Liver adiposity and metabolic profile in individuals with chronic spinal cord injury. Biomed. Res. Int. 1364818 (2017). [DOI] [PMC free article] [PubMed]
- 47.Colle, I., Van Vlierberghe, H., Troisi, R. & De Hemptinne, B. Transplanted liver: consequences of denervation for liver functions. Anat. Rec. Discov. Mol. Cell Evol. Biol.280A, 924–931 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Noble, B. T., Brennan, F. H. & Popovich, P. G. The spleen as a neuroimmune interface after spinal cord injury. J. Neuroimmunol.321, 1–11 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Hergenroeder, G. W. et al. Increased levels of circulating glial fibrillary acidic protein and collapsin response mediator protein-2 autoantibodies in the acute stage of spinal cord Injury predict the subsequent development of neuropathic pain. J. Neurotrauma35, 2530–2539 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arevalo-Martin, A., Garcia-Ovejero, D. & Molina-Holgado, E. The endocannabinoid 2-arachidonoylglycerol reduces lesion expansion and white matter damage after spinal cord injury. Neurobiol. Dis.38, (2010). [DOI] [PubMed]
- 51.Arevalo-Martin, A. et al. Early endogenous activation of CB1 and CB2 receptors after spinal cord Injury is a protective response involved in spontaneous recovery. PLoS ONE7, e49057 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Ovejero, D. et al. The endocannabinoid system is modulated in response to spinal cord injury in rats. Neurobiol. Dis.33, (2009). [DOI] [PubMed]
- 53.Garcia-Ovejero, D. et al. Progesterone reduces secondary damage, preserves white matter, and improves locomotor outcome after spinal cord contusion. J. Neurotrauma31, (2014). [DOI] [PMC free article] [PubMed]
- 54.Meyronet, D. et al. The workflow from post-mortem human brain sampling to cell microdissection: a brain net Europe study. J. Neural Transm.122, 975–991 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Goodpaster, T. & Randolph-Habecker, J. A. Flexible mouse-on-mouse immunohistochemical staining technique adaptable to biotin-free reagents, immunofluorescence, and multiple antibody staining. J. Histochem. Cytochem.62, 197–204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dapson, R. W., Fagan, C., Kiernan, J. A. & Wickersham, T. W. Certification procedures for Sirius red F3B (CI 35780, direct red 80). Biotech. Histochem.86, 133–139 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Chowdhury, A. B. & Mehta, K. J. Liver biopsy for assessment of chronic liver diseases: a synopsis. Clin. Exp. Med.23, 273–285 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Standish, R. A. An appraisal of the histopathological assessment of liver fibrosis. Gut55, 569–578 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are available from the corresponding author on reasonable request.