Abstract
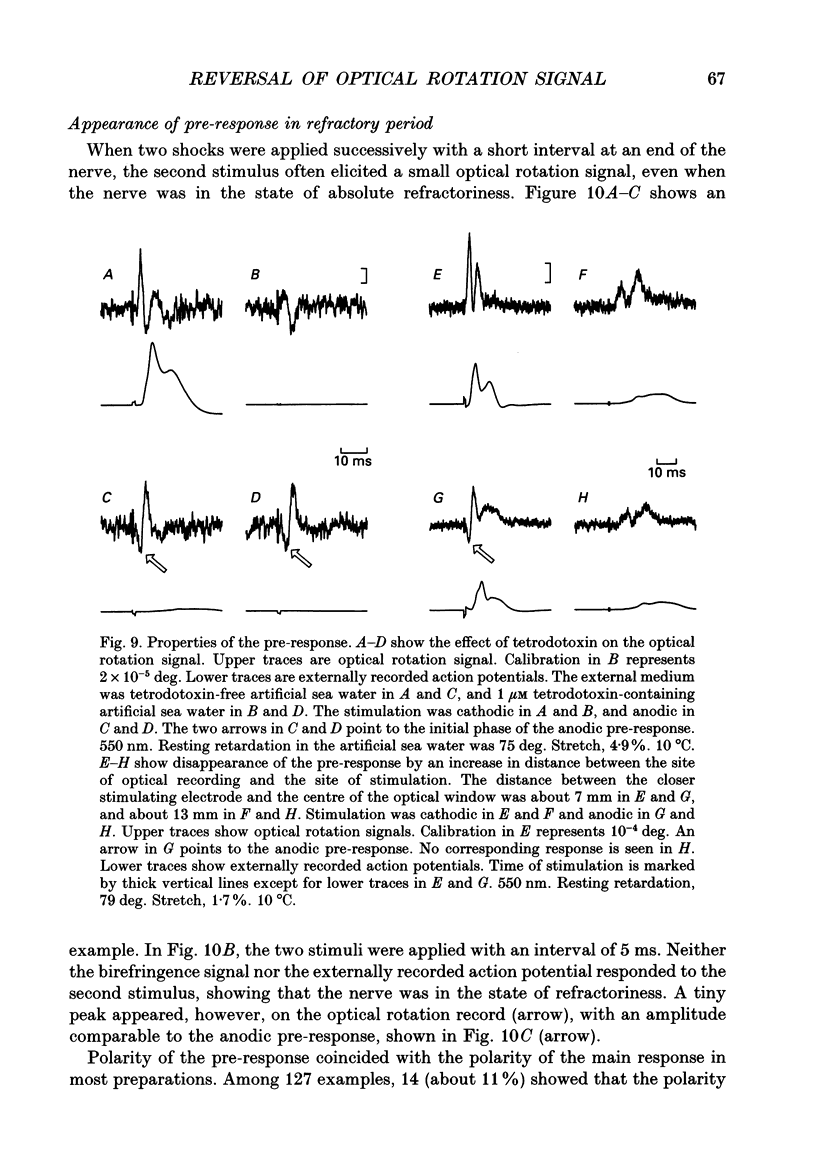
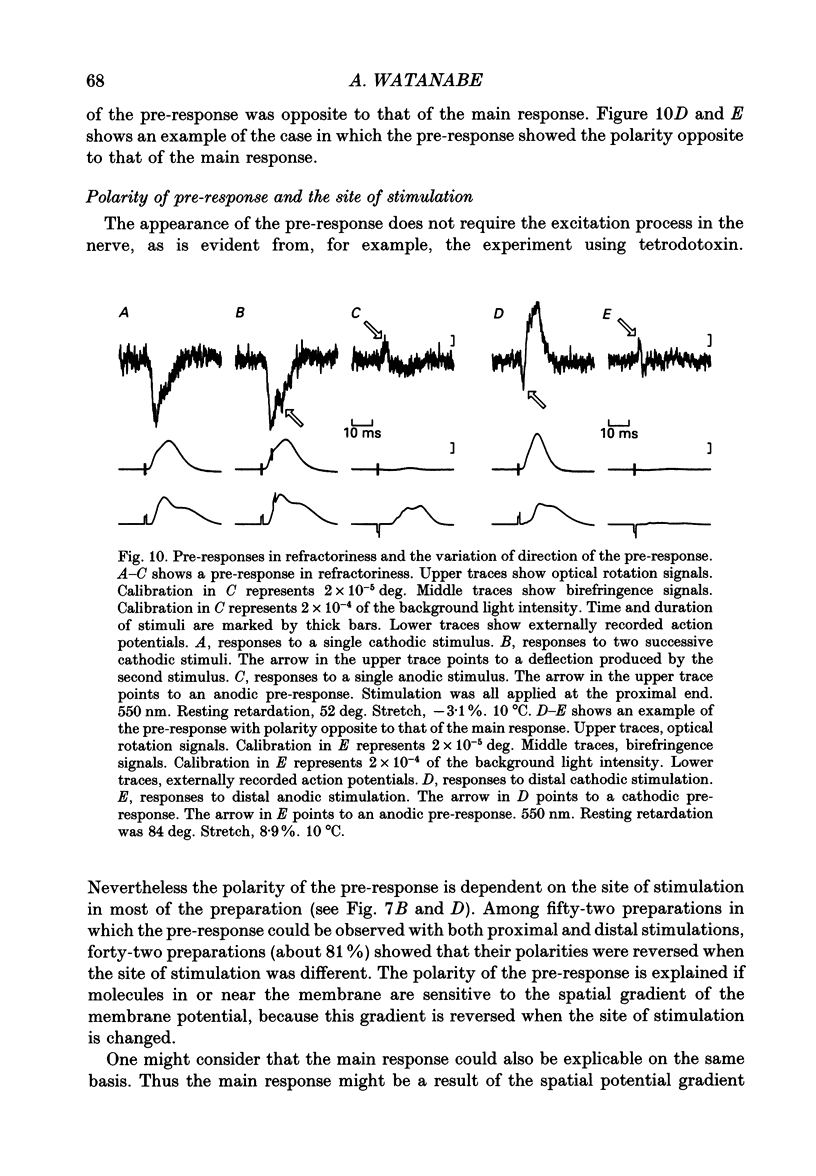
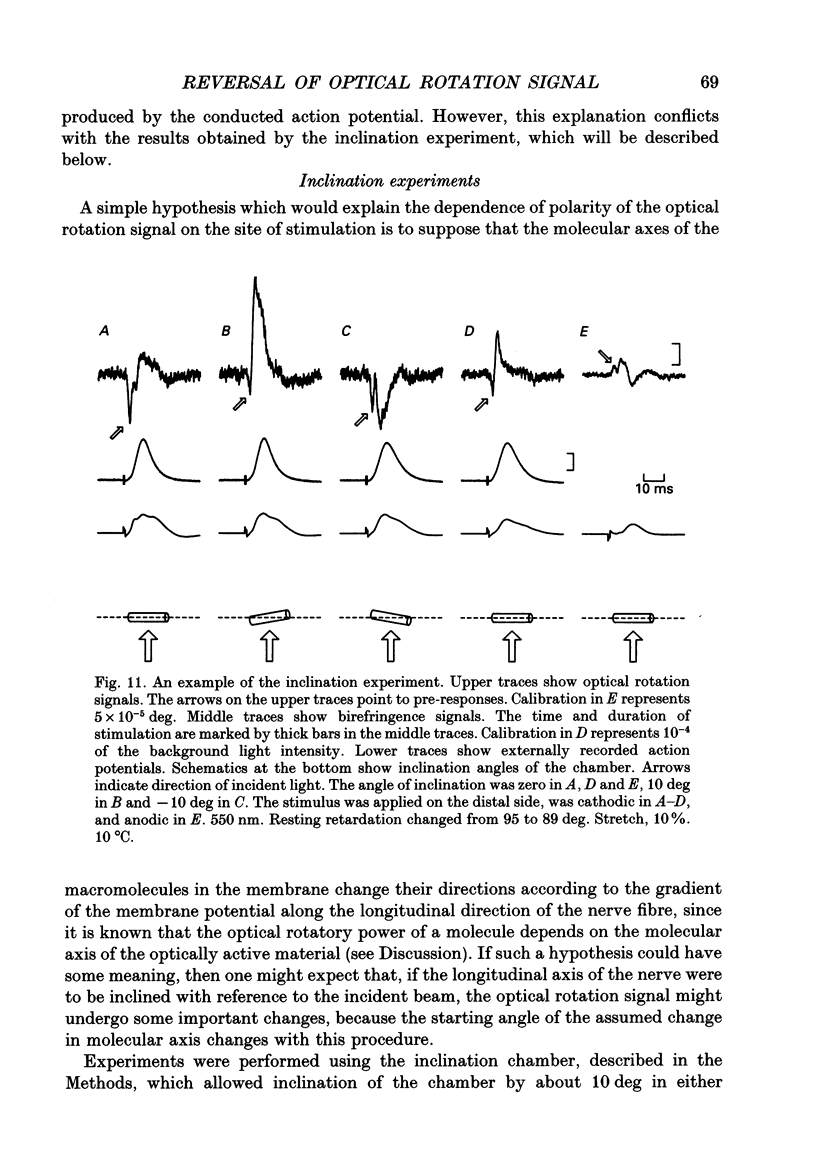
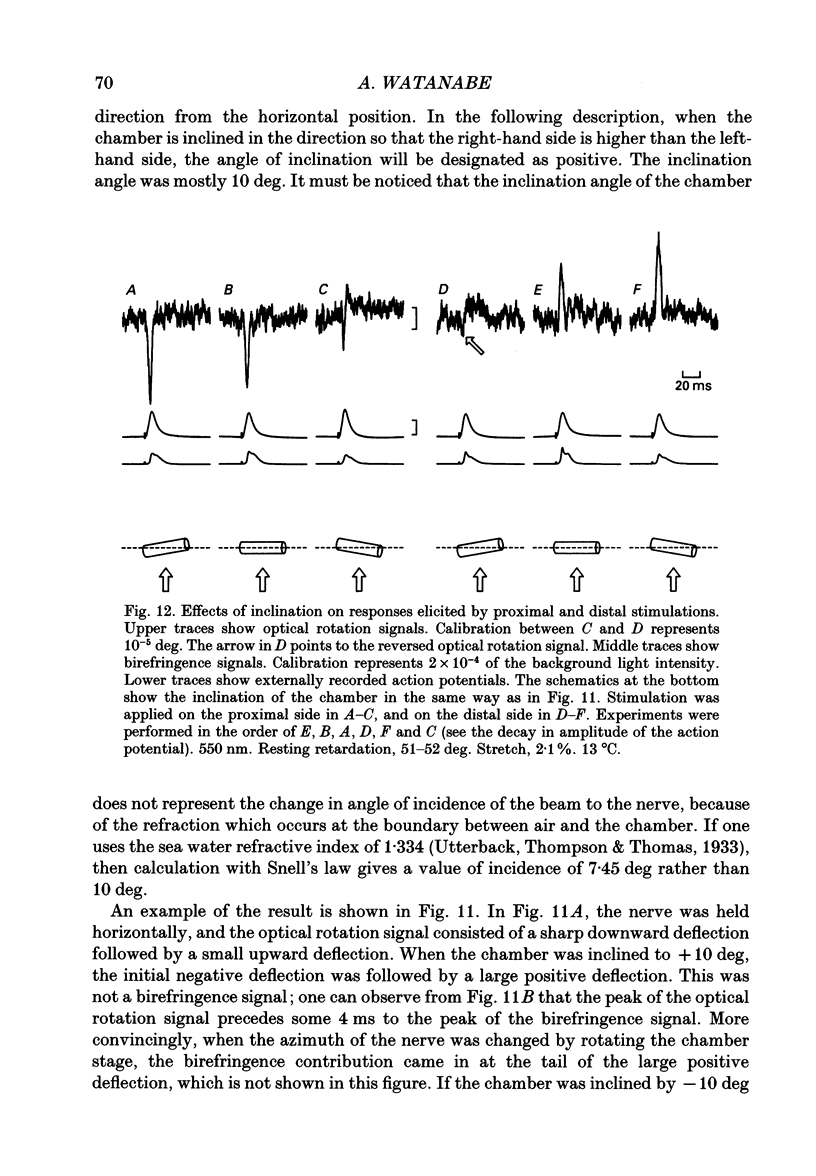
1. The optical rotation signal of nerve associated with excitation was recorded from peripheral nerve taken from a walking leg of a spiny lobster and its properties were analysed. 2. The polarity of the optical rotation signal was reversed when the site of stimulation was changed with reference to the site of optical recording, so that the direction of impulse conduction was reversed, in most of the preparations. 3. Apart from the main response, which is associated with the conducted impulse, a pre-response was found to exist, which manifested itself on anodic stimulation, in a tetrodotoxin-treated nerve, or during the refractory period of the nerve, when the site of stimulation was close to the site of optical recording. The polarity of the pre-response was also reversed when the site of stimulation was changed with reference to the site of optical recording. 4. When the nerve was inclined from the horizontal level, so that the angle of incidence of light to the nerve was changed, the main response changed its amplitude and sometimes its polarity, whereas the pre-response remained practically unchanged. Thus the dependence on the angle of incidence was different between the pre-response and the main response. 5. It is suggested that the dependence of amplitude and polarity of the main response on the angle of incidence of light cannot be explained by the change in molecular axes of the membrane macromolecules, but can only be explained by their conformational change; and therefore the main response can be used as a monitor for the molecular conformation.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EYZAGUIRRE C., KUFFLER S. W. Processes of excitation in the dendrites and in the soma of single isolated sensory nerve cells of the lobster and crayfish. J Gen Physiol. 1955 Sep 20;39(1):87–119. doi: 10.1085/jgp.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Iwasa K. Rapid pressure changes and surface displacements in the squid giant axon associated with production of action potentials. Jpn J Physiol. 1982;32(1):69–81. doi: 10.2170/jjphysiol.32.69. [DOI] [PubMed] [Google Scholar]
- Terakawa S. Potential-dependent variations of the intracellular pressure in the intracellularly perfused squid giant axon. J Physiol. 1985 Dec;369:229–248. doi: 10.1113/jphysiol.1985.sp015898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A. Change in optical activity of a lobster nerve associated with excitation. J Physiol. 1987 Aug;389:223–253. doi: 10.1113/jphysiol.1987.sp016655. [DOI] [PMC free article] [PubMed] [Google Scholar]


