Abstract
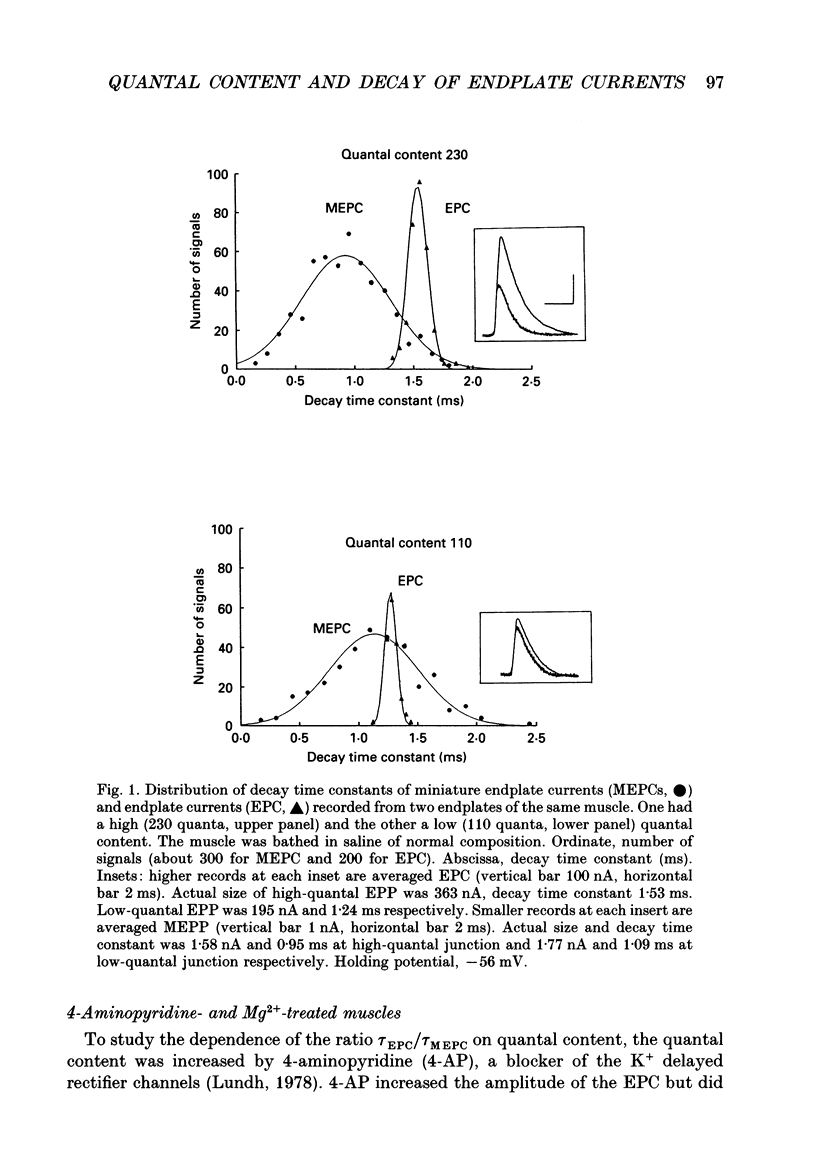
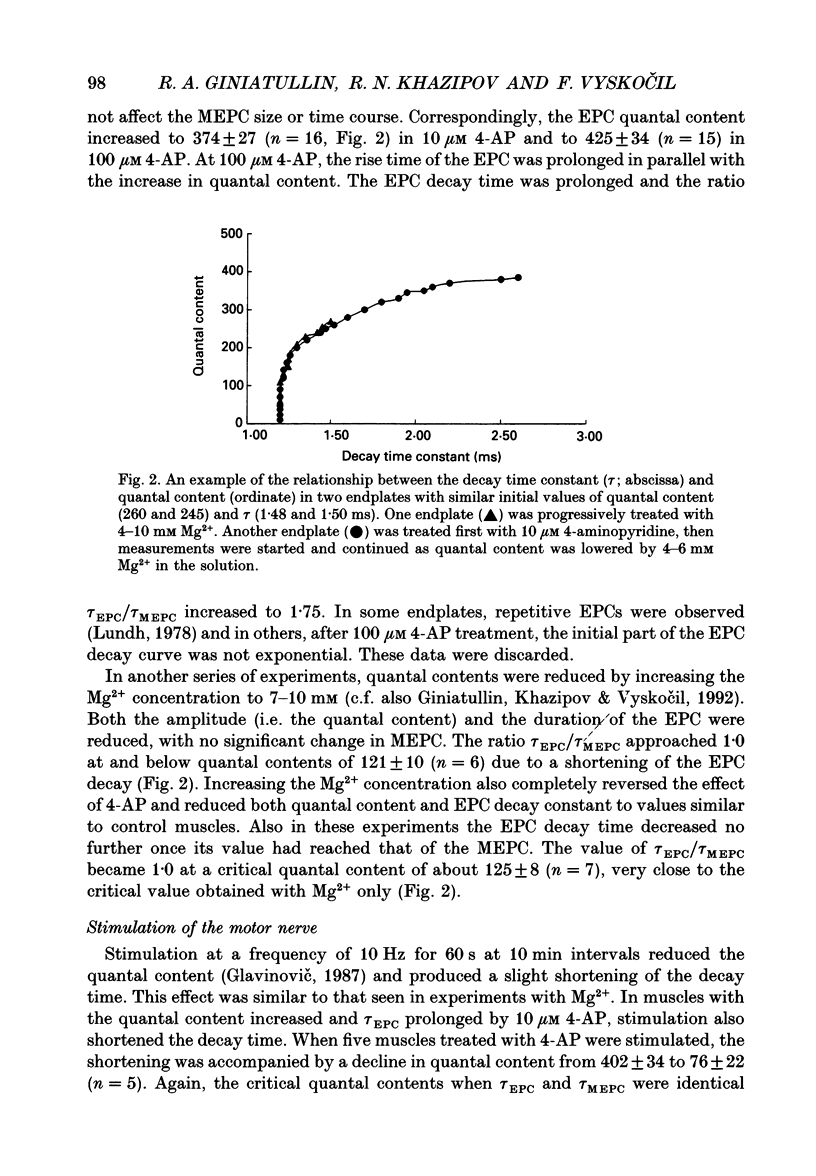
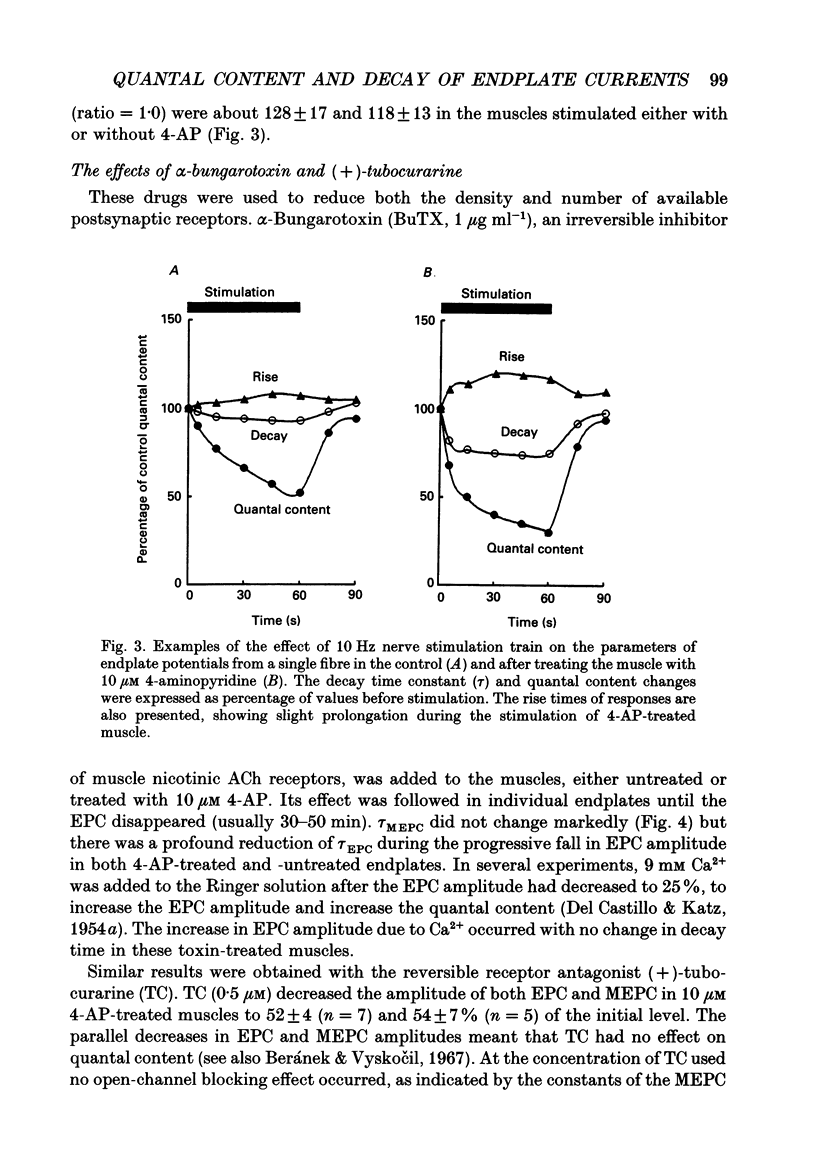
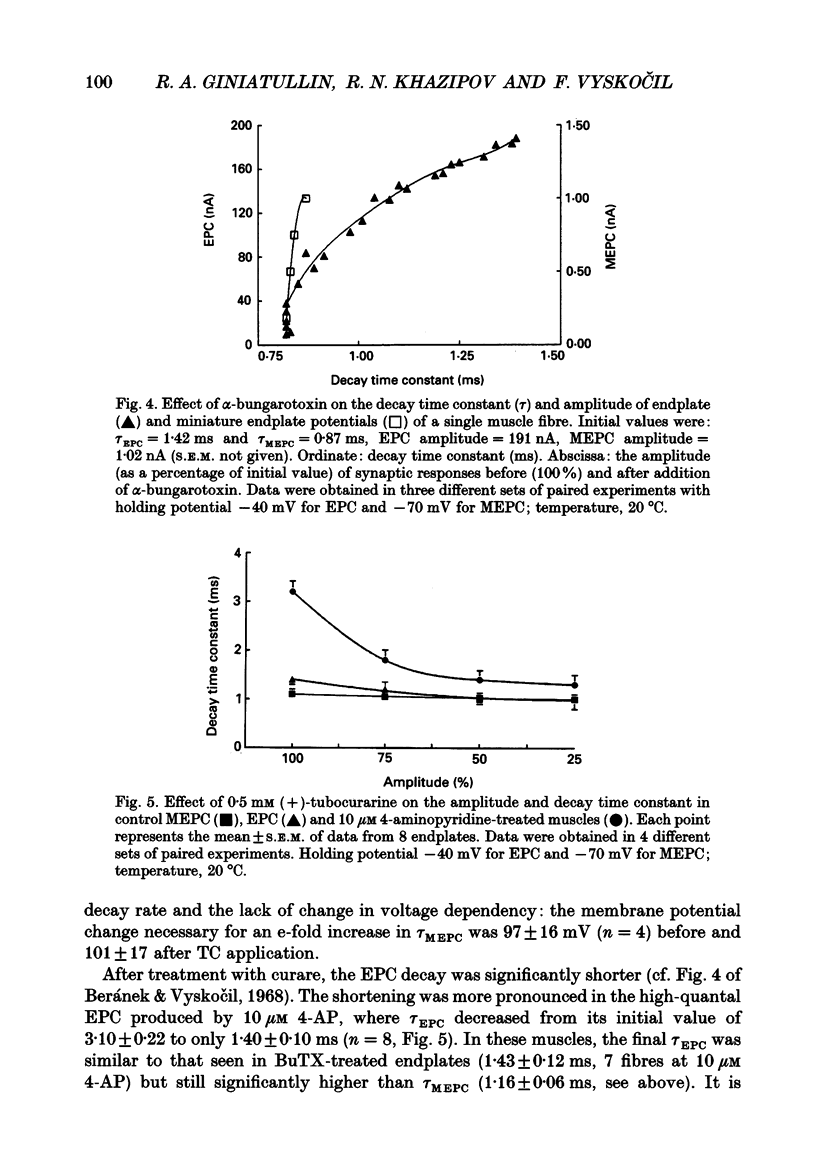
1. The relationship between quantal content and prolongation of endplate currents (EPC) was studied in the frog sartorius with intact synaptic acetylcholinesterase. 2. The prolongation of EPC was more pronounced in endplates with a higher quantal content both before and after potentiation of quantal release by 4-aminopyridine (4-AP). When the quantal content of EPC was lowered, either by high Mg2+ or repetitive stimulation, the EPC decay constant was reduced. 3. A certain critical value of about 120 quanta per nerve impulse was found, at which point the decay of EPC remained constant even through the quantal content was reduced further. 4. The reduction in both density and number of postsynaptic receptors, produced by alpha-bungarotoxin and (+)-tubocurarine led to a profound reduction in EPC decay during the progressive fall in EPC amplitude in both 4-AP-treated and -untreated endplates. Both drugs are known to produce a shortening of EPC in anti-cholinesterase (anti-ChE)-treated muscles, due to a decrease in receptor density and less frequent repetitive binding of ACh. 5. It is assumed that the prolongation of multiquantal EPC is caused by an increased ACh concentration near the receptors, which may provide the opportunity for repetitive binding even with full cholinesterase activity. The critical quantum content of about 120 might be the number of quanta at which the probability of multiple release at single active zones is increased above zero.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barstad J. A., Lilleheil G. Transversaly cut diaphragm preparation from rat. An adjuvant tool in the study of the physiology and pbarmacology of the myoneural junction. Arch Int Pharmacodyn Ther. 1968 Oct;175(2):373–390. [PubMed] [Google Scholar]
- Bartol T. M., Jr, Land B. R., Salpeter E. E., Salpeter M. M. Monte Carlo simulation of miniature endplate current generation in the vertebrate neuromuscular junction. Biophys J. 1991 Jun;59(6):1290–1307. doi: 10.1016/S0006-3495(91)82344-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beránek R., Vyskocil F. The action of tubocurarine and atropine on the normal and denervated rat diaphragm. J Physiol. 1967 Jan;188(1):53–66. doi: 10.1113/jphysiol.1967.sp008123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beránek R., Vyskocil F. The effect of atropine on the frog sartorius neuromuscular junction. J Physiol. 1968 Mar;195(2):493–503. doi: 10.1113/jphysiol.1968.sp008470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin R. A., Khazipov R. N., Vyskocil F. Critical quantum content for shortening of endplate currents in the frog skeletal muscle. Physiol Res. 1992;41(4):331–332. [PubMed] [Google Scholar]
- Glavinović M. I. Synaptic depression in frog neuromuscular junction. J Neurophysiol. 1987 Jul;58(1):230–246. doi: 10.1152/jn.1987.58.1.230. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Estimates of quantal content during 'chemical potentiation' of transmitter release. Proc R Soc Lond B Biol Sci. 1979 Aug 31;205(1160):369–378. doi: 10.1098/rspb.1979.0070. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. J., Durant N. N., Reynolds L. S., Volle R. L., Henderson E. G. Characterization of end-plate conductance in transected frog muscle: modification by drugs. J Pharmacol Exp Ther. 1981 Jan;216(1):62–69. [PubMed] [Google Scholar]
- Lundh H. Effects of 4-aminopyridine on neuromuscular transmission. Brain Res. 1978 Sep 22;153(2):307–318. doi: 10.1016/0006-8993(78)90409-2. [DOI] [PubMed] [Google Scholar]
- Magazanik L. G., Fedorov V. V., Snetkov V. A. Faktory, opredeliaiushchie dlitel'nost' odinochnogo postsinapticheskogo otveta v nervno-myshechnykh soedineniiakh. Neirofiziologiia. 1984;16(5):590–602. [PubMed] [Google Scholar]
- Magazanik L. G., Nikolsky E. E., Giniatullin R. A. End-plate currents evoked by paired stimuli in frog muscle fibres. Pflugers Arch. 1984 Jun;401(2):185–192. doi: 10.1007/BF00583880. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- Thomsen R. H., Wilson D. F. Effects of 4-aminopyridine and 3,4-diaminopyridine on transmitter release at the neuromuscular junction. J Pharmacol Exp Ther. 1983 Oct;227(1):260–265. [PubMed] [Google Scholar]
- Volkova I. N., Nikol'skii E. E., Poletaev G. I. Blokirovanie potentsialov deistviia i sokrashchenii skeletnoi myshtsy liagushki poperechnym rassecheniem myshechnykh volokon. Fiziol Zh SSSR Im I M Sechenova. 1975 Sep;61(9):1433–1436. [PubMed] [Google Scholar]


