Abstract
The traditional “nine steaming and nine basking” method for processing Polygonati Rhizoma has been practiced in China for over two millennia. However, research on its impact on glycans, particularly low molecular weight fructans, is limited. Therefore, dynamic changes in glycans were analyzed based on the two common species, Polygonatum filipes and Polygonatum cyrtonema. Results revealed the significant degradation of low molecular weight fructans within the first three processing cycles, with complete degradation by the seventh cycle, suggesting that the traditional technique may be excessive. Molecular weight analysis indicated the aggregation, degradation, and reaggregation of polysaccharides, with a notable decrease in fructose and an increase in galactose. This suggested that fructans were the primary constituents before processing, while galactans prevailed afterward. No significant differences in carbohydrate changes were found between the two species. This study enhances our understanding of the traditional processing mechanisms and promotes the efficient utilization of Polygonati Rhizoma.
Keywords: Polygonatum filipes, Polygonatum cyrtonema, Low molecular weight fructans, Polysaccharides, HPAEC-PAD, Processed Polygonati Rhizoma
Graphical abstract
Highlights
-
•
Low molecular weight fructan degraded in the first three heat processing cycles.
-
•
The major type of polysaccharide changed from fructan to galactan after processing.
-
•
No significant glycan difference was observed between PF and PC during processing.
-
•
Low molecular weight fructan can be the indicator of Polygonati Rhizoma processing.
1. Introduction
Polygonatum Mill. is a perennial herb in the genus Polygonatum, family Asparagaceae, widely distributed in temperate regions of the Northern Hemisphere, including China, Japan, Korea, and North America (Li, Ma, et al., 2021). The rhizome of Polygonatum, known as “Polygonati Rhizoma”, has been used as both food and medicine in China for over two thousand. In traditional Chinese medicine, Polygonati Rhizoma is believed to have the functions of strengthening the spleen, moistening the lungs, invigorating qi and nourishing yin (Shi et al., 2023). Recent studies have shown that Polygonati Rhizoma contains active components such as polysaccharides, saponins and flavonoids (Liu, Tang, et al., 2022; Xu et al., 2023), which have been confirmed to have positive effects in treating of diabetes, Alzheimer's disease, and enhancing immunity (Luo et al., 2022; Yu et al., 2024).
Consumption of raw Polygonati Rhizoma can cause numbness and a stinging sensation in the upper respiratory tract, so it must be processed before consumption. Steaming is the most basic processing method, with a long history and numerous variations, including nine cycles of steaming and basking, steaming with wine, and steaming with black beans (Li et al., 2024). Among these, the “nine steaming and nine basking” method has been handed down to the present day. This method involves steaming the Polygonati Rhizoma for several hours, then drying it in the sun, and repeating this cycle nine times. After processing, its throat-irritating properties disappear and its pharmacological activity enhanced (He et al., 2021). With the development of this method, differences in processing techniques have emerged across regions, and a standardized processing method has not yet been established, leading to inconsistent product quality (Chen et al., 2022). Therefore, it is necessary to further investigate the underlying mechanisms of the “nine steaming and nine basking” method.
Studies have investigated the changes in the basic physicochemical properties, sensory quality, and flavor characteristics of Polygonati Rhizoma during the repeated steaming and basking process (Cheng et al., 2022). Some researchers have also examined changes in the functional compounds such as flavonoids, alkaloids, and saponins during this process (Nie et al., 2023; Zhu et al., 2022). Regarding the carbohydrates in Polygonati Rhizoma, most studies focus on the changes in polysaccharides during the processing (Wu et al., 2022). However, researches have shown that low molecular weight fructans were present in Polygonati Rhizoma. Zhang et al. (2021) isolated highly branched fructans with an average degree of polymerization of 23 and a molecular weight of 3.8 kDa from the raw rhizomes of Polygonatum cyrtonema. And our previous research indicated that low molecular weight fructans with a degree of polymerization around 10 –20, were the primary carbohydrates in Polygonati Rhizoma (Xia, Zhang, et al., 2023). These fructans have potential benefits in maintaining intestinal health, protecting against lung injury, and modulating immune function (He et al., 2021). However, changes in these low molecular weight fructans during the repeated steaming and basking process have rarely been reported. A comprehensive analysis of the dynamic alterations of these functional carbohydrates during the processing cycles remains to be investigated.
Therefore, this study focused on two common species, Polygonatum filipes (PF) and Polygonatum cyrtonema (PC), applying them to the nine cycles of steaming and basking process. The qualitative and quantitative analyses were conducted on the functional carbohydrates with different molecular weights, including low molecular weight fructans, polysaccharides, free monosaccharides, and disaccharides. The aim of this study was to provide a theoretical basis for understanding the mechanism of the traditional “nine steaming and nine basking” method on the dynamic changes of carbohydrates, thereby promoting the efficient utilization of Polygonati Rhizoma resources.
2. Materials and methods
2.1. Materials and chemicals
Fresh rhizomes of PF and PC, grown naturally for over five years, were collected from Ninghai County and Qingyuan County in Zhejiang Province, China, respectively.
Carbohydrate standards, including fucose (Fuc), arabinose (Ara), rhamnose (Rha), glucose (Glc), mannose (Man), galactose (Gal), fructose (Fru), galacturonic acid (GalA), sucrose (Suc) and dextran standards with the weight-average molecular weights (Mw) of 1000 Da and 5000 Da, were purchased from Sigma-Aldrich Co. (St. Louis, Mo, USA). Sucrase was obtained from Shanghai Yuanye (Shanghai, China). Chromatographic-grade reagents, such as 50 % NaOH solution and sodium acetate (NaOAc) were supplied by the Fisher Scientific (Atlanta, GA, USA). Trifluoroacetic acid (TFA) and sodium borohydride (NaBH4) were purchased from Shanghai Aladdin Biochemical Technology Co. Ltd. (Shanghai, China). All other reagents were of analytical grade and were obtained from commercial suppliers.
2.2. Samples preparation
After removing the fibrous root, the fresh rhizomes were cleaned, steamed for 3 h and basked until their surfaces were dry and wrinkled. This process was repeated eight times to produce nine different processed Polygonati Rhizoma samples. The raw PF and PC samples were labeled as P0 and S0, respectively, after the initial drying process. The nine steamed samples were named according to the number of processing cycles: P1 ∼ P9 for PF samples and S1 ∼ S9 for PC samples (Fig. 1). After freeze-drying, the samples were ground and passed through a 40-mesh sieve. The resulting powder was stored in a dryer to maintain moisture balance.
Fig. 1.
Samples from different steaming and basking cycles of PF (P0 ∼ P9) and PC (S0 ∼ S9).
2.3. Extraction and separation of different molecular weight carbohydrates
Different powder samples (1 g each) were dissolved in 20 mL of deionized water, and extraction was performed using magnetic stirring in a water bath at 90 °C for 1 h. The supernatant was immediately filtered using 500-mesh fabric, and the residue was washed three times with hot water to transfer soluble extracts. After centrifugation (12,000 ×g, 10 min), the combined supernatant was adjusted to 50 mL, mixed with 200 mL of absolute ethanol, and stored at 4 °C for 12 h. The resulting precipitate and supernatant were separated using 1800-mesh fabric. Ethanol was removed from the supernatant via rotary evaporation to obtain low molecular weight carbohydrates, while the precipitate was redissolved, dialyzed with a 10,000 Da membrane for 72 h, and lyophilized to isolate polysaccharides. This method enabled the separation of carbohydrates with different molecular weights from Polygonati Rhizoma at various processing stages (Xia, Zhang, et al., 2023).
2.4. Molecular weight distribution of low molecular weight carbohydrates
The molecular weight distribution of low molecular weight carbohydrates was analyzed using a Waters 1525 HPLC-RID with a Superdex Peptide 10/300 GL column (Li et al., 2019). The mobile phase was 0.3 mol/L NH4HCO3 at a flow rate of 0.4 mL/min, with an injection volume of 50 μL. Data were analyzed using Waters Breeze GPC software. Dextran standards (1 mg/mL, 1000 Da and 5000 Da), sucrose, and glucose were used as references. All samples were filtered through a 0.22 μm membrane before injection.
2.5. Contents of free monosaccharides and disaccharides
The contents of free monosaccharides and disaccharides were analyzed using a Dionex ICS 5000+ chromatographic system (Thermo Scientific, Waltham, MA, USA) equipped with a pulsed amperometric detector and a Dionex Carbopac PA-10 column (250 mm × 4 mm). The mobile phases were deionized water (A), 200 mmol/L NaOH (C), and 100 mmol/L NaOAc with 24 mmol/L NaOH (D). The gradient program was: 0–15 min, 87 % A and 24 % C; 15.1–18 min, 100 % C; 18.1–29 min, 100 % D; 29.1–40 min, 87 % A and 24 % C. The injection volume was 25 μL, with a flow rate of 1 mL/min, and the column temperature was maintained at 35 °C. Detection used an Ag/AgCl reference electrode with an Au working electrode (Xia, Mei, et al., 2023; Xia, Zhang, et al., 2023). Standard solutions of Glc, Fru, and Suc, along with sample solutions, were diluted and filtered through a 0.22 μm membrane prior to analysis.
2.6. Contents of low molecular weight fructans
The quantitative method of low molecular weight fructans in Polygonati Rhizoma was optimized based on the method proposed by Tran et al. (2022). The method operates on the following principles: sucrose is enzymatically hydrolyzed into monosaccharides by sucrase, which are subsequently reduced to sugar alcohols using NaBH4. Low molecular weight fructans are then hydrolyzed into monosaccharides through acid hydrolysis. The fructan content is indirectly quantified by analyzing the monosaccharides using HPAEC-PAD.
The experimental procedure was as follows: 0.1 mL of the extract was mixed with 0.2 mL of 20 U/mL sucrase solution in a 10 mL centrifuge tube and hydrolyzed at 40 °C for 30 min. Next, 0.2 mL of 0.5 mol/L NaBH4 was added, and the reaction continued at 40 °C for 1 h. Subsequently, 0.1 mL of 1 mol/L acetic acid was added, followed by 0.2 mL of 4 mol/L TFA after 10 min. Acidolysis was performed at 80 °C for 15 min. After the reaction, the solvent was evaporated under nitrogen at 40 °C, and the residue was dissolved in 5 mL of deionized water. Samples were filtered through a 0.22 μm membrane prior to HPAEC-PAD analysis, using the same conditions as for free monosaccharides. The content of low molecular weight fructans was calculated as follows:
where F is the content of low molecular weight fructans, C1 and C2 represent the concentrations of glucose and fructose, respectively, V is the volume of the low molecular weight fructans solution, λ is the dilution factor, and M is the dry weight of the sample powder.
2.7. Yields of polysaccharides
Following the method of Zheng et al. (2024), the yields of crude polysaccharides were obtained by calculating the difference in weight of polysaccharide before and after lyophilization. The results were expressed as dry weight.
2.8. Molecular weight of polysaccharides
The molecular weight and distribution of polysaccharides from differently processed Polygonati Rhizoma were analyzed using size exclusion chromatography combined with multi-angle laser light scattering (SEC-MALLS), following the method of Chen et al. (2021). The SEC-MALLS-RI system consisted of a Waters 2695e HPLC system (USA), a Shodex OHpak SB-G guard column, two size exclusion chromatography columns (SB-806 HQ and SB-804 HQ, 300 mm × 7.8 mm, Shodex, Japan), a DAWN HELEOS II multi-angle light scattering detector (λ0 = 660 nm, Wyatt Technology, USA), and a SHIMADZU RID-20A refractive index detector.
Freeze-dried polysaccharides were dissolved in deionized water, vortexed, and prepared into a 3 mg/mL solution. The solution was filtered through a 0.22 μm membrane prior to analysis. The mobile phase was 0.15 mol/L NaCl containing 0.02 % Procolin, with the column temperature maintained at 40 °C. Each injection volume was 50 μL, and isocratic elution was performed at a flow rate of 0.5 mL/min for 60 min. The refractive index increment (dn/dc) was set at 0.138 mL/g, and data were acquired and processed using ASTRA 7.3.2 software.
2.9. Monosaccharide composition analysis of polysaccharides
Seven monosaccharide standards (Fuc, Rha, Ara, Gal, Glc, Man, and GalA) were prepared as mixed standard solutions with gradient concentrations. Fructose standards were prepared at 500, 400, 200, 100, and 50 μmol/L. A 3 mg/mL polysaccharide solution was used for hydrolysis under two conditions: (1) 0.3 mL polysaccharide solution mixed with 0.1 mL of 4 mol/L TFA was hydrolyzed at 80 °C for 4 h (Sample 1); (2) 0.5 mL polysaccharide solution mixed with an equal volume of 4 mol/L TFA was hydrolyzed in a sealed ampoule at 110 °C for 7 h (Sample 2). After nitrogen drying, both samples were dissolved in 6 mL deionized water. Monosaccharide composition was analyzed by HPAEC-PAD (Xia, Mei, et al., 2023; Xia, Zhang, et al., 2023). Sample 1 conditions matched those in Section 2.6, while Sample 2 used gradient elution: 0–15 min, 87 % A and 13 % C; 15.1–35 min, 100 % D.
2.10. FT-IR spectrum and methylation degree analysis of polysaccharides
The functional groups of polysaccharides were analyzed using a Nicolet iS10 FTIR spectrometer (Thermo Nicolet, USA). Freeze-dried polysaccharide samples from different processed Polygonati Rhizoma were mixed with KBr, ground into pellets, and scanned in the 4000–400 cm−1 wavenumber range with a resolution of 4 cm−1 over 32 scans (Yu et al., 2022). Data were processed using Thermo Scientific OMNIC software. The degree of methylesterification (DM%) of the polysaccharides was calculated using the following formula:
2.11. Statistical analysis
All results were calculated and expressed on a dry-weight basis. SPSS software (IBM) was used for significance analysis of differences between means. Origin 2021 software was applied for the data processing. The clustering analysis was performed using the online platform available at https://www.chiplot.online/.
3. Results and discussion
3.1. Molecular weight distribution of low molecular weight carbohydrates
Gel permeation chromatography (GPC) was used to analyze the molecular weight distribution of low molecular weight carbohydrates. Fig. 2. shows the GPC chromatogram of low molecular weight carbohydrates from different processing cycles of PF and PC. The 5000 Da dextran (5 K Dextran) exhibited a broad peak between 20–35 min, while the 1000 Da dextran (1 K Dextran) showed a peak between 30–40 min, with some overlap due to heterogeneity. Disaccharides and monosaccharides were eluted at approximately 41 min and 43 min, respectively.
Fig. 2.
GPC chromatograms of low molecular weight carbohydrates from PF (a) and PC (b).
By comparing the retention time with the standards, the molecular weight distribution of the carbohydrates in the samples can be inferred. As shown in Fig. 2., P0 and S0 exhibited broad peaks between 20–44 min, similar to those of the dextran standards, indicating that the raw rhizomes of PF and PC contained substantial amounts of low molecular weight polysaccharides with average molecular weights between 1000 Da and 5000 Da. Previous research (Xia, Zhang, et al., 2023) demonstrated that raw Polygonatum sibiricum (PS) rhizomes contain abundant low molecular weight fructans. Given that PF, PC, and PS belong to the Polygonatum genus, it is likely that the low molecular weight polysaccharides in raw PF and PC are also fructans. Under identical extraction and analysis conditions, the peak area of low molecular weight fructans in S0 was significantly larger than in P0, indicating a higher concentration of these fructans in raw PC rhizomes.
In addition, small amounts of free monosaccharides and disaccharides were detected in both P0 and S0. After the first processing cycle, the peak signal of these sugars increased significantly, while the retention time of low molecular weight fructans was delayed by approximately 2 min. With additional processing cycles, the retention time of low molecular weight fructans in both PF and PC continued to shift backward, accompanied by a steady decrease in peak signal intensity. These results indicated that low molecular weight fructans in PF and PC were significantly degraded into free disaccharides and monosaccharides during the steaming process. By the fifth processing cycle, most of the low molecular weight fructans had been degraded. Further investigation is needed to clarify the dynamic relationship among low molecular weight fructans, disaccharides, and monosaccharides throughout the nine steaming and basking cycles.
3.2. Qualitative and quantitative analysis of low molecular weight carbohydrates
Ion chromatography, known for its high column efficiency, rapid analysis, and excellent sensitivity, is widely used for carbohydrate quantification and has been successfully applied to identify fructan content using HPAEC-PAD (Mechelke et al., 2017). For Polygonati Rhizoma, which lacks starch, some research has employed strong acid hydrolysis to convert fructans into monosaccharides, using the difference in monosaccharide contents before and after hydrolysis used for quantification (Xia, Mei, et al., 2023; Xia, Zhang, et al., 2023). However, this method has limitations: it cannot distinguish between hydrolysis-derived monosaccharides and free monosaccharides originally present in the sample, leading to overestimation of fructan content, particularly when free fructose or glucose levels are high (Tran et al., 2022). Moreover, under strong acidic conditions, fructose and glucose can degrade into by-products such as 5-HMF, formic acid, acetic acid, and levulinic acid, further compromising measurement accuracy (Lee et al., 2019). Therefore, this study utilized sucrase hydrolysis and sodium borohydride reduction to eliminate the interference of free monosaccharides and disaccharides. Mild acid hydrolysis was then applied to selectively degrade the low molecular weight fructans, followed by ion chromatography for the quantitative analysis of low molecular weight fructans during various processing cycles of Polygonati Rhizoma.
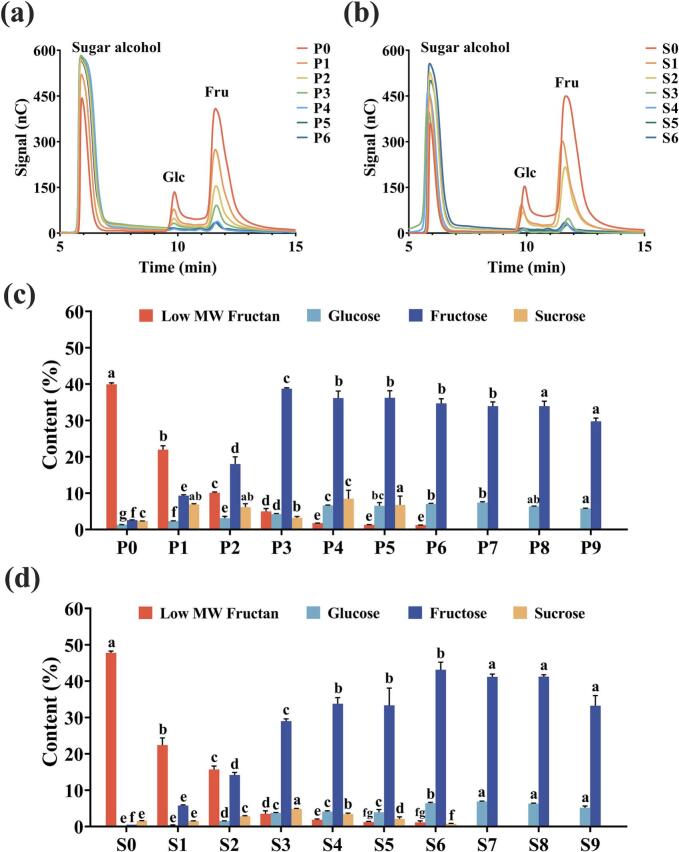
Fig. 3. (a) and (b) display the HPAEC-PAD chromatograms of low molecular weight fructans hydrolyzed from PF and PC at various processing stages. Peaks for glucose and fructose were observed around 10 min and 11 min, respectively, while the peak at approximately 6 min corresponded to sugar alcohols formed from free monosaccharides after sodium borohydride reduction. These results indicated that low molecular weight fructans were degraded into glucose and fructose under mild acidolysis. With increasing processing cycles, the signal intensities for glucose and fructose gradually decreased. The low molecular weight fructans in PF and PC was calculated based on the chromatographic peak areas. As shown in Fig. 3. (c) and (d), the initial content of low molecular weight fructans in raw PF and PC was 39.93 % and 47.78 %, respectively. Zong et al. (2024) extracted an inulin-type fructan with a molecular weight of 3.6 kDa from Polygonatum sibiricum, with a yield of approximately 21.8 %, which is structurally similar to the low molecular weight fructans identified in this study. These findings suggest that there are variations in the content of low molecular weight fructans among different species of Polygonati Rhizoma, and beyond the widely studied inulin, Polygonati Rhizoma may serve as a promising natural source of fructans.
Fig. 3.
HPAEC-PAD chromatograms of acid-hydrolyzed low molecular weight fructans from PF (a) and PC (b); quantitative analysis of low molecular weight carbohydrates in different processed samples from PF (c) and PC (d).
After one processing cycle, the content of low molecular weight fructans sharply decreased to 21.95 % in PF and 22.41 % in PC, continuing to decline with further processing. By cycles four to six, the content stabilized around 1 %, showing no significant differences. After seven cycles, low molecular weight fructans were nearly undetectable, indicating their almost complete degradation at this stage. In addition, small amounts of free disaccharides and monosaccharides were detected in the raw samples. In P0, the contents of sucrose, glucose, and fructose were 2.31 %, 1.31 %, and 2.57 %, respectively, while in S0, these contents were 1.56 %, 0.19 %, and 0.50 %. With increasing processing cycles, sucrose content initially rose before declining, degrading almost completely after the sixth steaming and basking cycle. Glucose and fructose also exhibited a similar trend. Notably, fructose content showed variations between PF and PC. In PF, fructose peaked at 38.71 % after three processing cycles, whereas in PC, it reached a maximum of 43.12 % after six processing cycles, with both declining gradually in subsequent cycles.
Based on the above results, it can be concluded that during the repeated processing, low molecular weight fructans were gradually degraded, leading to increased fructose and glucose content, which enhanced the sweetness of the Polygonati Rhizoma. Concurrently, the Maillard reaction occurred, producing dark-colored substances such as melanoidins, contributing to sample browning (Cui et al., 2021). Initially, the production of glucose and fructose from fructan degradation exceeded their consumption in the Maillard reaction. However, with additional processing cycles, low molecular weight fructans and sucrose were nearly depleted, while the Maillard reaction continued. This shift caused the depletion rate of glucose and fructose to surpass their production, resulting in reduced sweetness, increased bitterness and acidity, and further browning of the sample (Nie et al., 2023).
3.3. Yields and molecular weight distribution of polysaccharides
The yields of polysaccharides extracted from PF and PC at different processing stages are presented in Table 1. Compared to raw Polygonati Rhizoma, the yields of polysaccharides slightly increased after initial processing. As the number of processing cycles increased, the yields stabilized with no significant changes. This suggests that appropriate steaming and basking treatments effectively disrupted the cell wall structure of raw Polygonati Rhizoma, facilitating polysaccharide release (Jing et al., 2023). However, the enhancement effect appeared to be limited, as the yields of crude polysaccharides had no significant increase with further processing cycles.
Table 1.
Yields and molecular weight distribution of polysaccharides from different processed PF (P0 ∼ P9) and PC (S0 ∼ S9).
| Samples | Yields (%) | Molecular weight distribution |
|||
|---|---|---|---|---|---|
| Mw (g/mol)a | Mn (g/mol)b | PD (Mw/Mn)c | Rz (nm)d | ||
| P0 | 4.98 ± 0.43e | 1.67 × 105 | 1.51 × 104 | 11.11 | 45.6 |
| P1 | 5.42 ± 0.44e | 4.38 × 105 | 2.43 × 104 | 18.03 | 63.6 |
| P2 | 7.76 ± 0.53bc | 2.49 × 105 | 1.75 × 104 | 14.24 | 67.2 |
| P3 | 5.57 ± 0.17e | 1.41 × 105 | 2.52 × 104 | 6.01 | 51.8 |
| P4 | 7.13 ± 0.10cd | 1.07 × 105 | 2.65 × 104 | 4.04 | 31.9 |
| P5 | 8.77 ± 0.18a | 9.00 × 104 | 3.30 × 104 | 2.73 | 31.9 |
| P6 | 8.26 ± 0.37ab | 7.11 × 104 | 2.64 × 104 | 2.69 | 32.0 |
| P7 | 8.09 ± 0.18ab | 1.00 × 105 | 2.54 × 104 | 3.94 | 38.5 |
| P8 | 8.22 ± 0.57ab | 1.56 × 105 | 3.66 × 104 | 4.26 | 40.8 |
| P9 | 6.72 ± 0.58d | 2.67 × 105 | 5.12 × 104 | 5.22 | 59.8 |
| S0 | 6.48 ± 0.77c | 1.59 × 105 | 1.60 × 104 | 9.91 | 51.5 |
| S1 | 4.61 ± 0.61d | 2.61 × 106 | 4.21 × 104 | 61.93 | 46.2 |
| S2 | 6.41 ± 0.27c | 2.98 × 105 | 2.99 × 104 | 9.96 | 50.8 |
| S3 | 7.95 ± 0.57ab | 2.90 × 105 | 2.79 × 104 | 10.41 | 59.4 |
| S4 | 6.96 ± 0.81bc | 2.63 × 105 | 5.11 × 104 | 5.15 | 41.0 |
| S5 | 7.29 ± 0.62bc | 2.00 × 105 | 4.85 × 104 | 4.13 | 39.9 |
| S6 | 7.78 ± 0.27ab | 1.75 × 105 | 4.38 × 104 | 4.00 | 44.9 |
| S7 | 8.08 ± 0.57ab | 6.17 × 104 | 4.18 × 104 | 1.48 | 40.9 |
| S8 | 7.98 ± 0.21ab | 1.04 × 105 | 5.19 × 104 | 2.00 | 30.8 |
| S9 | 8.60 ± 0.90a | 1.10 × 105 | 5.50 × 104 | 2.01 | 34.7 |
Note: a: weight-average of molar mass; b: number-average of molar mass; c: polydispersity index; d: z-average of root mean square radius of gyration.
The size exclusion chromatograms of Polygonati Rhizoma polysaccharides at various processing stages (Fig. S1) revealed multiple distinct, symmetric peaks. As summarized in Table 1, the polysaccharides from different processing cycles exhibited considerable polydispersity and heterogeneity under consistent extraction conditions. Interestingly, the molecular weight increased significantly after the first cycle, potentially due to the rise in galacturonic acid content. The strong electronegativity of the oxygen atoms in the carboxyl or hydroxyl groups of uronic acids could promote hydrogen bonding with hydroxyl groups on other carbohydrate chains, leading to larger molecular aggregates and an increase in molecular weight (Wang et al., 2019).
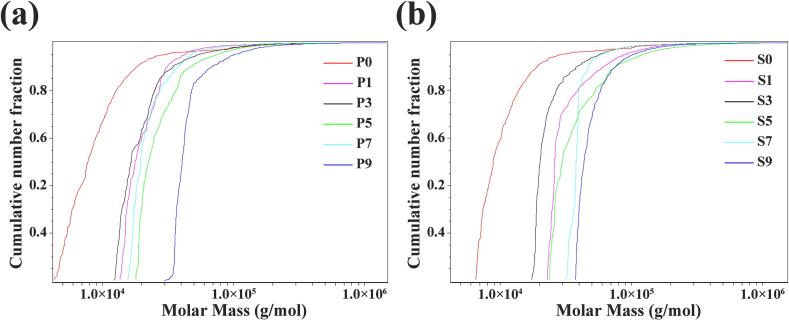
As the processing cycles increased, the molecular weight gradually declined, reaching its lowest point after the sixth cycle for PF and the seventh for PC, before a slow recovery. This trend is consistent with findings by (Wu et al., 2022), who observed an initial molecular weight increase in Polygonatum cyrtonema polysaccharides during the first four hours of steaming, followed by a gradual decline with extended processing. The cumulative molar mass distribution of polysaccharides (Fig. 4), showed a shift toward higher molecular mass after nine processing cycles, indicating an overall trend of increasing molecular weight. These results suggest that Polygonati Rhizoma polysaccharides undergo dynamic changes during repeated processing, including phases of aggregation, degradation, and reaggregation.
Fig. 4.
Cumulative molar mass distribution of polysaccharides from different processed PF (P0 ∼ P9) and PC (S0 ∼ S9).
The molecular weight distribution of polysaccharides is closely associated with the polydispersity index (Mw/Mn). A higher polydispersity index indicates a broader molecular weight distribution, while a value closer to 1 suggests a more homogeneous polysaccharides composition (Du et al., 2022). As shown in Table 1, the polydispersity index of Polygonati Rhizoma polysaccharides increased after the first processing cycle, suggesting a broader molecular weight distribution. However, with subsequent processing cycles, the polydispersity index decreased, indicating a gradual shift toward a more homogeneous composition. Rz, which reflects molecular size, revealed dynamic changes across the processing stages. Larger Rz values correspond to larger molecular sizes (Nie et al., 2020). The molecular size initially increased, then decreased, and subsequently increased again during the repeated processing cycles. This trend suggests that the molecular structure of Polygonati Rhizoma polysaccharides became more compact as processing continued.
In summary, appropriate processing could improve the yields of polysaccharides from Polygonati Rhizoma. During repeated cycles of steaming and basking, the polysaccharide molecules experienced aggregation, degradation, and reaggregation, resulting in an initial increase in molecular weight followed by a subsequent decrease.
3.4. Monosaccharide composition analysis of polysaccharides
Strong acid hydrolysis is commonly employed for the structural analysis of plant polysaccharides, as it ensures complete hydrolysis, facilitating the chromatographic determination of monosaccharide composition. Polygonati Rhizoma polysaccharides have been found to be particularly rich in fructose (Gao et al., 2024). However, harsh acidolysis conditions can damage the structure of fructose, resulting in the loss of critical structural information within the polysaccharides (Zong et al., 2024). To provide a more comprehensive assessment of monosaccharide composition across different processing stages, this study combined both strong and mild acidolysis during polysaccharide pretreatment.
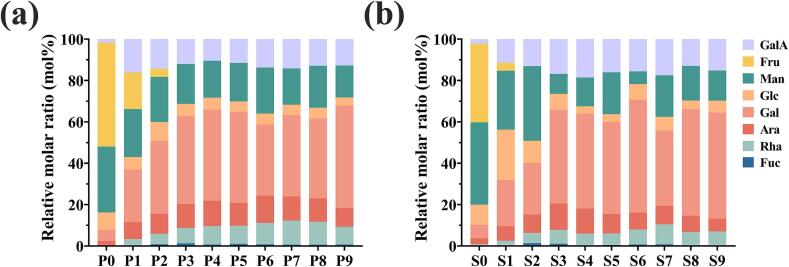
The monosaccharide composition of polysaccharides from different processing stages is illustrated in Fig. 5. Polysaccharides from raw Polygonati Rhizoma were primarily composed of Fru, Man, Glc, and Gal, with smaller amounts of Ara, GalA, Rha, and Fuc. In the P0 sample, fructose accounted for the highest relative content at 50.23 mol%, which significantly decreased to 17.69 mol% after the first processing cycle and was nearly completely degraded after the third cycle. A similar pattern was observed in PC samples, highlighting fructose as the most abundant and variable monosaccharide in Polygonati Rhizoma polysaccharides. However, this fluctuation in fructose content has not been extensively reported in previous studies on Polygonati Rhizoma polysaccharides. This discrepancy could be attributed to variations in experimental conditions, such as the hydrolysis process, which may lead to the degradation of fructose (Li, Chen, et al., 2021; Li, Ma, et al., 2021). Additionally, studies using 1-phenyl-3-methyl-5-pyrazolone (PMP) derivatization for monosaccharide identification may overlook fructose due to its lack of an aldehyde group, which prevents it from reacting with the PMP reagent (Wang et al., 2018). This highlights a limitation in current analytical methods that could result in incomplete characterization of polysaccharide composition. In comparison, other works on polysaccharide analysis have addressed similar challenges by using alternative derivatization methods (Liu et al., 2018; Zhang et al., 2024), which may provide a more accurate representation of the polysaccharide structures in Polygonati Rhizoma.
Fig. 5.
Monosaccharide composition analysis of polysaccharides from different processed PF (a) and PC (b) samples.
In addition to the significant changes in fructose, the relative content of Man in PF polysaccharides decreased significantly after the first processing cycle, while the proportions of Gal, GalA, Ara and Rha, increased noticeably. After the third processing cycle, Gal emerged as the dominant monosaccharide, replacing Fru. With continued processing, the relative content of Man and Glc progressively declined, Gal exhibited a fluctuating increase, and the relative content of GalA initially increased before decreasing. Similar trends were observed in the PC polysaccharide samples. Zhao et al. (2023) found that polysaccharides from the Polygonatum cyrtonema rhizomes processed for four cycles exhibited better antioxidant activity, which may be associated with the increase in Gal content (Duan & Yu, 2019). These findings suggest that the compositional changes in Polygonati Rhizoma polysaccharides during processing may influence their functional properties. Further studies are warranted to elucidate the mechanisms driving the improved probiotic effects of these polysaccharides after processing.
Based on the results, conclusion can be inferred that fructans were the dominant polysaccharides. The repeated processing cycles significantly altered the composition and structure of Polygonati Rhizoma polysaccharides. After three processing cycles, fructose was nearly completely degraded, resulting in polysaccharides that were predominantly heteropolysaccharides, including galactoglucans, galactomannans, and galactoarabinans. Additionally, the gradual increase in the relative content of GalA, Ara, and Rha throughout processing suggests that the processed Polygonati Rhizoma polysaccharides may include homogalacturonan (HG) and rhamnogalacturonan-I (RG-I) pectic domains (Zhao et al., 2020).
3.5. FT-IR spectrum analysis
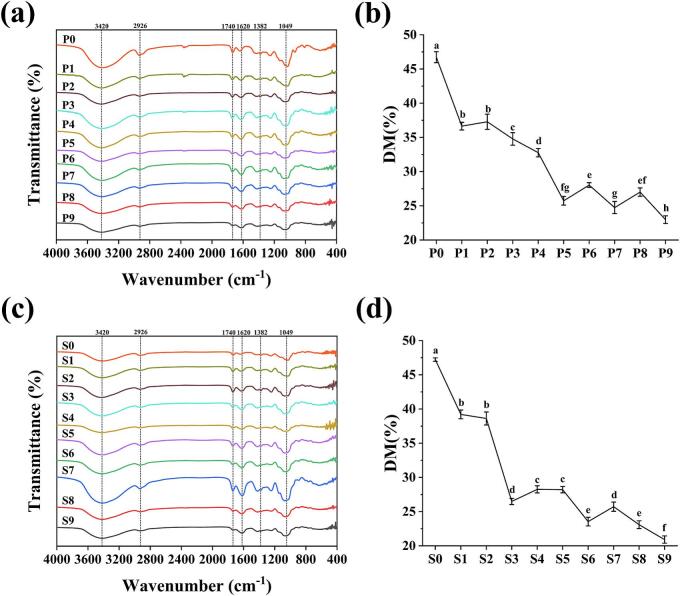
FT-IR is an essential method for the structural identification of polysaccharides. The FT-IR spectra of Polygonati Rhizoma polysaccharides at different processing stages are shown in Fig. 6. (a) and (c). All samples exhibited a broad absorption peak around 3420 cm−1, attributed to the stretching vibration of the —OH groups in the carbohydrate chains (Yu et al., 2022). A signal peak near 2926 cm−1 corresponded to —CH2 groups, while signals around 1736 cm−1 and 1620 cm−1 were associated with the stretching vibrations of methyl esterified carboxyl groups (—COOCH3) and non-methylated carboxyl groups (—COO—), respectively (Liu, Tang, et al., 2022). These results indicated the presence of acetylated uronic acid in all PF and PC samples, aligning with the previously observed monosaccharide composition results.
Fig. 6.
FT-IR spectra of polysaccharides from different processed PF (a) and PC (c); degree of methylesterification quantification of polysaccharides from different processed PF (b) and PC (d) samples.
The degree of methylesterification (DM%) of Polygonati Rhizoma polysaccharides was estimated using the peak areas at 1736 cm−1 and 1620 cm−1, with the results presented in Fig. 6. (b) and (d). The polysaccharides in raw Polygonati Rhizoma exhibited a higher DM%, which decreased significantly during processing, reaching its lowest value after nine cycles. This trend indicates a gradual reduction in methylesterification with repeated processing and aligns with the monosaccharide composition results, where the relative content of GalA increased progressively.
3.6. Relative contents and clustering analysis of carbohydrates
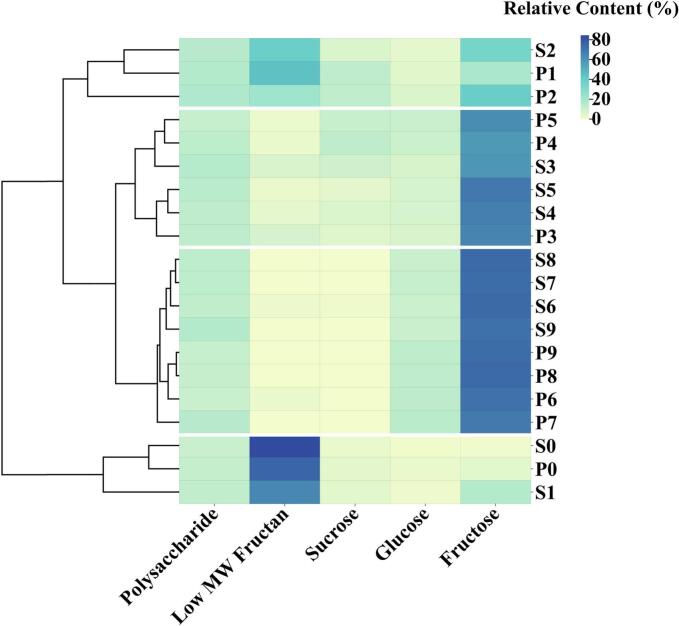
To further investigate the changes in carbohydrates of different molecular weights in Polygonati Rhizoma during steaming and basking, the relative contents of carbohydrates from all samples were analyzed (Fig. S2). The primary carbohydrates in raw Polygonati Rhizoma were low molecular weight fructans, comprising 75.57 % of the total carbohydrates in PF and 84.55 % in PC. Significant reductions were observed during the first three processing cycles, with fructan content decreasing by 67.20 % in P3 and 77.42 % in S3 compared to P0 and S0. Concurrently, the relative contents of Fru, Glc, and Suc increased sharply, with free monosaccharides and disaccharides exceeding 75 % of total carbohydrates after three cycles. However, the relative content of polysaccharides exhibited minimal fluctuation during the repeated processing. By the seventh cycle, the carbohydrates in PF and PC were primarily composed of free monosaccharides and structurally stable polysaccharides.
Clustering analysis of PF and PC samples at different processing stages (Fig. 7), classified the twenty samples into four distinct groups based on the relative contents of various carbohydrate components. The first group included P0, S0, and S1; the second group consisted of P1 and P2; the third group encompassed samples processed for three to five cycles, while the fourth group comprised those processed for six to nine cycles. Low molecular weight fructans emerged as a key factor in this classification, highlighting their potential as critical indicators for Polygonati Rhizoma processing. Moreover, no significant differences were observed in the carbohydrate composition between samples processed six and nine times, suggesting that additional steaming and basking beyond six cycles may be redundant.
Fig. 7.
Clustering analysis of carbohydrates contents in different processed PF and PC samples.
The traditional Chinese medicine practice of “nine steaming and nine basking” is believed to enhance the efficacy of Polygonati Rhizoma by reducing irritants, transforming key compounds, and improving absorption, thereby boosting its tonic effects (Su et al., 2023). However, our findings indicate significant degradation of beneficial low molecular weight fructans during repeated processing, suggesting that the current method may lead to over-processing and unnecessary depletion of valuable compounds. Therefore, developing a refined processing technique that effectively removes irritants while preserving low molecular weight fructans could open up a promising new research direction.
4. Conclusion
In summary, low molecular weight fructans were identified as the predominant carbohydrate component in both Polygonatum filipes (PF) and Polygonatum cyrtonema (PC). In addition to the prominent fructan source inulin, Polygonatum Mill. could serve as another natural source of fructans. However, these fructans degraded significantly within the first three processing cycles and were completely degraded by the seventh cycle, suggesting that the traditional “nine steaming and nine basking” may result in over-processing. The content of low molecular weight fructans could thus serve as a key indicator for optimizing the processing of Polygonati Rhizoma. As these fructans degraded, the levels of fructose, glucose, and sucrose initially increased but declined afterward due to the Maillard reaction. In contrast, polysaccharide yields remained relatively stable, though their composition became more homogeneous and condensed. Fructans were the primary carbohydrates in raw samples, while galactans predominated after processing. These patterns were consistent in both PF and PC, with no significant differences observed throughout the processing stages. This study could provide insights into the mechanism of the traditional “nine steaming and nine basking” method for Polygonati Rhizoma, contributing to the efficiency of industrial practices.
CRediT authorship contribution statement
Xingyu Mei: Writing – original draft, Visualization, Methodology, Investigation, Data curation. Jiabei Xia: Software, Methodology. Wenqing Li: Visualization, Investigation. Yufen Wu: Supervision, Investigation. Huan Cheng: Validation, Supervision. Shiguo Chen: Writing – review & editing, Supervision. Xingqian Ye: Writing – review & editing, Validation, Supervision. Jianle Chen: Writing – review & editing, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was financially supported by the Ningbo Municipal Bureau of Science and Technology (2022Z174), Key Research and Development Plan of Ningbo (2023Z119), and Science and Technology Research Project from Longquan (2024CYNY-003), China.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.102131.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Chen J., Cheng H., Zhi Z., Zhang H., Linhardt R.J., Zhang F.…Ye X. Extraction temperature is a decisive factor for the properties of pectin. Food Hydrocolloids. 2021;112 doi: 10.1016/j.foodhyd.2020.106160. [DOI] [Google Scholar]
- Chen Z., Zhu B., Chen Z., Cao W., Wang J., Li S., Zhao J. Effects of steam on polysaccharides from Polygonatum cyrtonema based on saccharide mapping analysis and pharmacological activity assays. Chinese Medicine. 2022;17(1):97. doi: 10.1186/s13020-022-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Ji H., Cheng X., Wang D., Li T., Ren K.…Liu X. Characterization, classification, and authentication of Polygonatum sibiricum samples by volatile profiles and flavor properties. Molecules. 2022;27(1) doi: 10.3390/molecules27010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H., Yu J., Zhai Y., Feng L., Chen P., Hayat K., Xu Y., Zhang X., Ho C.-T. Formation and fate of Amadori rearrangement products in Maillard reaction. Trends in Food Science & Technology. 2021;115:391–408. doi: 10.1016/j.tifs.2021.06.055. [DOI] [Google Scholar]
- Du B., Nie S., Peng F., Yang Y., Xu B. A narrative review on conformational structure characterization of natural polysaccharides. Food Frontiers. 2022;3(4):631–640. doi: 10.1002/fft2.150. [DOI] [Google Scholar]
- Duan G.-L., Yu X. Isolation, purification, characterization, and antioxidant activity of low-molecular-weight polysaccharides from Sparassis latifolia. International Journal of Biological Macromolecules. 2019;137:1112–1120. doi: 10.1016/j.ijbiomac.2019.06.177. [DOI] [PubMed] [Google Scholar]
- Gao Y., Wang J., Xiao Y., Yu L., Tang Q., Wang Y., Zhou J. Structure characterization of an agavin-type fructan isolated from Polygonatum cyrtonema and its effect on the modulation of the gut microbiota in vitro. Carbohydrate Polymers. 2024;330 doi: 10.1016/j.carbpol.2024.121829. [DOI] [PubMed] [Google Scholar]
- He L., Yan B., Yao C., Chen X., Li L., Wu Y., Song Z., Song S., Zhang Z., Luo P. Oligosaccharides from Polygonatum Cyrtonema Hua: Structural characterization and treatment of LPS-induced peritonitis in mice. Carbohydrate Polymers. 2021;255 doi: 10.1016/j.carbpol.2020.117392. [DOI] [PubMed] [Google Scholar]
- Jing Y., Yan M., Zhang H., Liu D., Qiu X., Hu B.…Wu L. Effects of extraction methods on the physicochemical properties and biological activities of polysaccharides from Polygonatum sibiricum. Foods. 2023;12(10):2088. doi: 10.3390/foods12102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-H., Chen K.-T., Lin J.-A., Chen Y.-T., Chen Y.-A., Wu J.-T., Hsieh C.-W. Recent advances in processing technology to reduce 5-hydroxymethylfurfural in foods. Trends in Food Science & Technology. 2019;93:271–280. doi: 10.1016/j.tifs.2019.09.021. [DOI] [Google Scholar]
- Li J., Li S., Liu S., Wei C., Yan L., Ding T.…Chen S. Pectic oligosaccharides hydrolyzed from citrus canning processing water by Fenton reaction and their antiproliferation potentials. International Journal of Biological Macromolecules. 2019;124:1025–1032. doi: 10.1016/j.ijbiomac.2018.11.166. [DOI] [PubMed] [Google Scholar]
- Li X., Chen Q., Liu G., Xu H., Zhang X. Chemical elucidation of an arabinogalactan from rhizome of Polygonatum sibiricum with antioxidant activities. International Journal of Biological Macromolecules. 2021;190:730–738. doi: 10.1016/j.ijbiomac.2021.09.038. [DOI] [PubMed] [Google Scholar]
- Li X.-L., Ma R.-H., Zhang F., Ni Z.-J., Thakur K., Wang S., Zhang J.-G., Wei Z.-J. Evolutionary research trend of Polygonatum species: A comprehensive account of their transformation from traditional medicines to functional foods. Critical Reviews in Food Science and Nutrition. 2021:1–18. doi: 10.1080/10408398.2021.1993783. [DOI] [PubMed] [Google Scholar]
- Li Y., Chen Z., Zhang C. Historical evolution and processing mechanism of ‘nine steaming and nine drying’ of traditional Chinese medicine preparation. Pharmaceutical Biology. 2024;62(1):436–446. doi: 10.1080/13880209.2024.2354345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Tang W., Han C., Nie S. Advances in Polygonatum sibiricum polysaccharides: Extraction, purification, structure, biosynthesis, and bioactivity. Frontiers in Nutrition. 2022;9 doi: 10.3389/fnut.2022.1074671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Dong Z., Zhu X., Xu H., Zhao Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. International Journal of Biological Macromolecules. 2018;107:796–802. doi: 10.1016/j.ijbiomac.2017.09.051. [DOI] [PubMed] [Google Scholar]
- Luo S., Zhang X., Huang S., Feng X., Zhang X., Xiang D. A monomeric polysaccharide from Polygonatum sibiricum improves cognitive functions in a model of Alzheimer’s disease by reshaping the gut microbiota. International Journal of Biological Macromolecules. 2022;213:404–415. doi: 10.1016/j.ijbiomac.2022.05.185. [DOI] [PubMed] [Google Scholar]
- Mechelke M., Herlet J., Benz J.P., Schwarz W.H., Zverlov V.V., Liebl W., Kornberger P. HPAEC-PAD for oligosaccharide analysis—Novel insights into analyte sensitivity and response stability. Analytical and Bioanalytical Chemistry. 2017;409(30):7169–7181. doi: 10.1007/s00216-017-0678-y. [DOI] [PubMed] [Google Scholar]
- Nie X., Wang L., Wang S., Yu N., Lu Y., Lyu W., Meng X. In vitro hypoglycemic and antioxidant activities of steamed Polygonatum cyrtonema Hua with various steaming degrees: Relationship with homoisoflavonoids. Food Bioscience. 2023;53 doi: 10.1016/j.fbio.2023.102518. [DOI] [Google Scholar]
- Nie X.-R., Fu Y., Wu D.-T., Huang T.-T., Jiang Q., Zhao L.…Qin W. Ultrasonic-assisted extraction, structural characterization, chain conformation, and biological activities of a pectic-polysaccharide from okra (Abelmoschus esculentus) Molecules. 2020;25(5) doi: 10.3390/molecules25051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Si D., Chen D., Zhang X., Han Z., Yu Q., Liu J., Si J. Bioactive compounds from Polygonatum genus as anti-diabetic agents with future perspectives. Food Chemistry. 2023;408 doi: 10.1016/j.foodchem.2022.135183. [DOI] [PubMed] [Google Scholar]
- Su L., Li X., Guo Z., Xiao X., Chen P., Zhang J.…Lu T. Effects of different steaming times on the composition, structure and immune activity of Polygonatum polysaccharide. Journal of Ethnopharmacology. 2023;310 doi: 10.1016/j.jep.2023.116351. [DOI] [PubMed] [Google Scholar]
- Tran S.H., Mac T.T.H., Vu T.T.A., Cao C.K., Le T.H.H., Dao V.D.…Povydysh M.N. Quantitative analysis of fructans in functional foods by high-performance anion-exchange chromatography with pulsed amperometric detection. Bioactive Carbohydrates and Dietary Fibre. 2022;28 doi: 10.1016/j.bcdf.2022.100320. [DOI] [Google Scholar]
- Wang S., Zhao L., Li Q., Liu C., Han J., Zhu L., Zhu D., He Y., Liu H. Rheological properties and chain conformation of soy hull water-soluble polysaccharide fractions obtained by gradient alcohol precipitation. Food Hydrocolloids. 2019;91:34–39. doi: 10.1016/j.foodhyd.2018.12.054. [DOI] [Google Scholar]
- Wang W., Chen F., Wang Y., Wang L., Fu H., Zheng F., Beecher L. Optimization of reactions between reducing sugars and 1-phenyl-3-methyl-5-pyrazolone (PMP) by response surface methodology. Food Chemistry. 2018;254:158–164. doi: 10.1016/j.foodchem.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Wu W., Huang N., Huang J., Wang L., Wu L., Wang Q., Zhao H. Effects of the steaming process on the structural properties and immunological activities of polysaccharides from Polygonatum cyrtonema. Journal of Functional Foods. 2022;88 doi: 10.1016/j.jff.2021.104866. [DOI] [Google Scholar]
- Xia J., Mei X., Cheng H., Chen S., Ye X., Chen J. The functional components of by-product resources from the aerial parts of Polygonatum cyrtonema Hua. Agriculture. 2023;13(9) doi: 10.3390/agriculture13091820. [DOI] [Google Scholar]
- Xia J., Zhang C., Zhu K., Mei X., Cheng H., Chen S., Ye X., Chen J. Identification of carbohydrate in Polygonatum sibiricum: Fructo-oligosaccharide was a major component. Food Quality and Safety. 2023;7 doi: 10.1093/fqsafe/fyad029. [DOI] [Google Scholar]
- Xu C., Xia B., Zhang Z., Lin Y., Li C., Lin L. Research progress in steroidal saponins from the genus Polygonatum: Chemical components, biosynthetic pathways and pharmacological effects. Phytochemistry. 2023;213 doi: 10.1016/j.phytochem.2023.113731. [DOI] [PubMed] [Google Scholar]
- Yu J., Wang X., Li D., Wang L., Wang Y. Development of soy protein isolate emulsion gels as extrusion-based 3D food printing inks: Effect of polysaccharides incorporation. Food Hydrocolloids. 2022;131 doi: 10.1016/j.foodhyd.2022.107824. [DOI] [Google Scholar]
- Yu J., Zhao L., Wang Z., Yue T., Wang X., Liu W. Correlations between the structure and anti-diabetic activity of novel polysaccharides from raw and “nine steaming nine sun-drying” Polygonti rhizome. International Journal of Biological Macromolecules. 2024;260 doi: 10.1016/j.ijbiomac.2023.129171. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chen H., Luo L., Zhou Z., Wang Y., Gao T., Yang L., Peng T., Wu M. Structures of fructan and galactan from Polygonatum cyrtonema and their utilization by probiotic bacteria. Carbohydrate Polymers. 2021;267 doi: 10.1016/j.carbpol.2021.118219. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Lin X., Su W. Study on the components changes of polysaccharides and saponins during nine steaming and drying of Polygonatum sibiricum. Journal of the Science of Food and Agriculture. 2024;104(11):6862–6874. doi: 10.1002/jsfa.13516. [DOI] [PubMed] [Google Scholar]
- Zhao P., Li X., Wang Y., Yan L., Guo L., Huang L., Gao W. Characterisation and saccharide mapping of polysaccharides from four common Polygonatum spp. Carbohydrate Polymers. 2020;233 doi: 10.1016/j.carbpol.2020.115836. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang Z., Fu R., Xie R., Wang B., Li Q. Structural characterization and antioxidant activity of processed polysaccharides PCP-F1 from Polygonatum cyrtonema Hua. Frontiers in Nutrition. 2023;10 doi: 10.3389/fnut.2023.1272977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Lan S., Zhang W., Nie B., Zhu K., Ye X., Hou Z., Chen S. Polysaccharide structure evaluation of Ganoderma lucidum from different regions in China based on an innovative extraction strategy. Carbohydrate Polymers. 2024;335 doi: 10.1016/j.carbpol.2024.122079. [DOI] [PubMed] [Google Scholar]
- Zhu S., Liu P., Wu W., Li D., Shang E., Guo S., Qian D., Yan H., Wang W., Duan J. Multi-constituents variation in medicinal crops processing: Investigation of nine cycles of steam-sun drying as the processing method for the rhizome of Polygonatum cyrtonema. Journal of Pharmaceutical and Biomedical Analysis. 2022;209 doi: 10.1016/j.jpba.2021.114497. [DOI] [PubMed] [Google Scholar]
- Zong X., Wang Z., Chen S., Li S., Xie M., Nie S., Yin J. Optimized acid hydrolysis conditions for better characterization the structure of inulin-type fructan from Polygonatum sibiricum. International Journal of Biological Macromolecules. 2024;256 doi: 10.1016/j.ijbiomac.2023.128030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.