Abstract
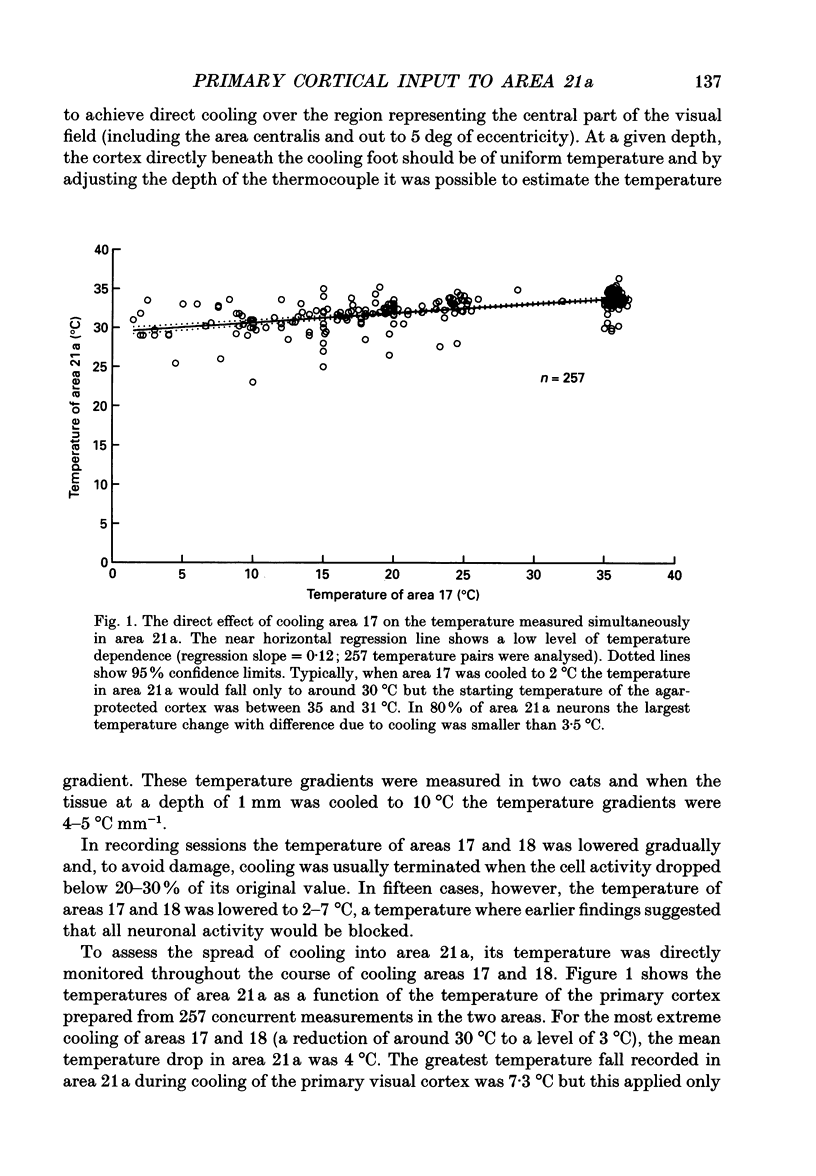
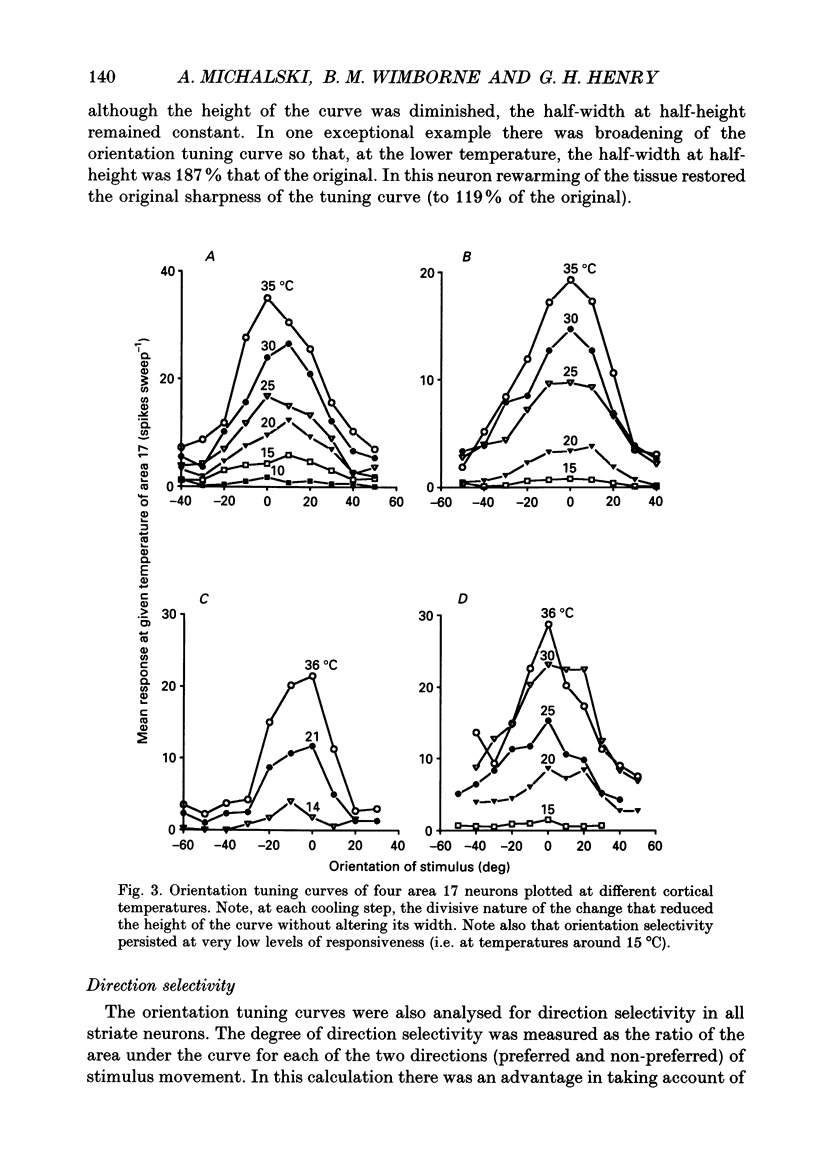
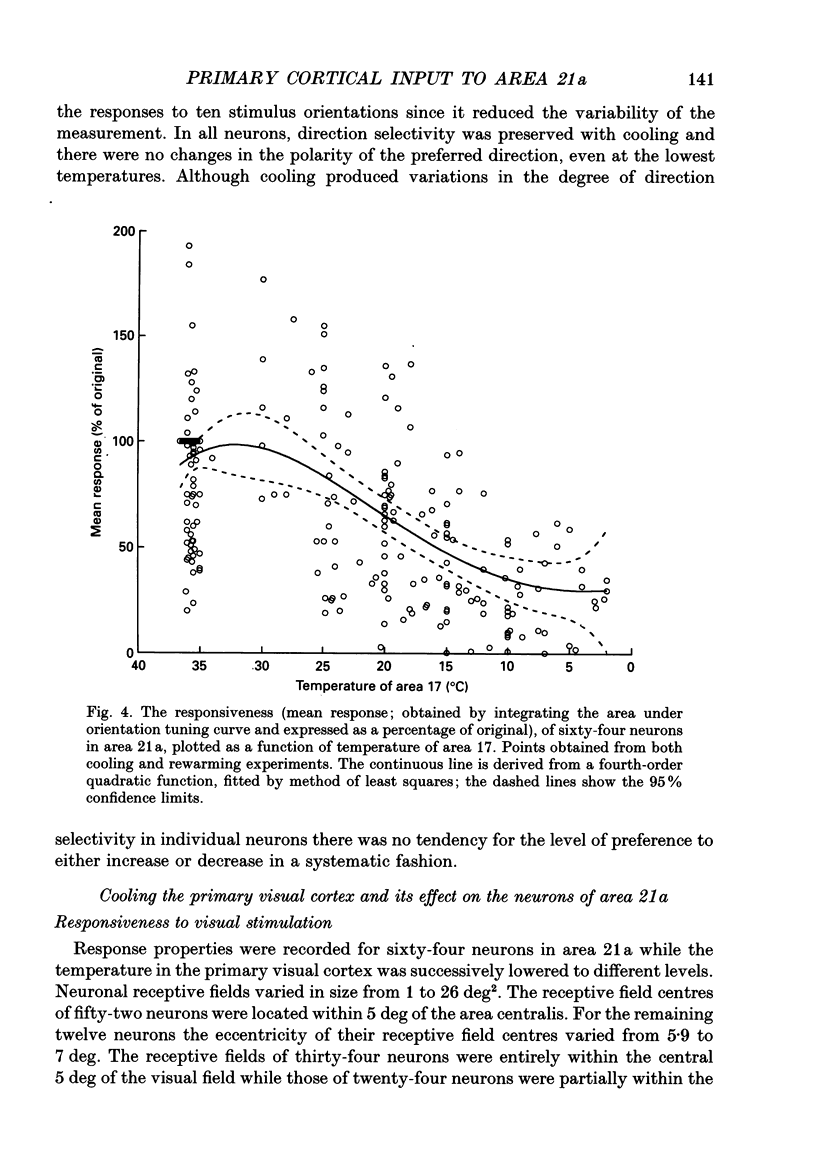
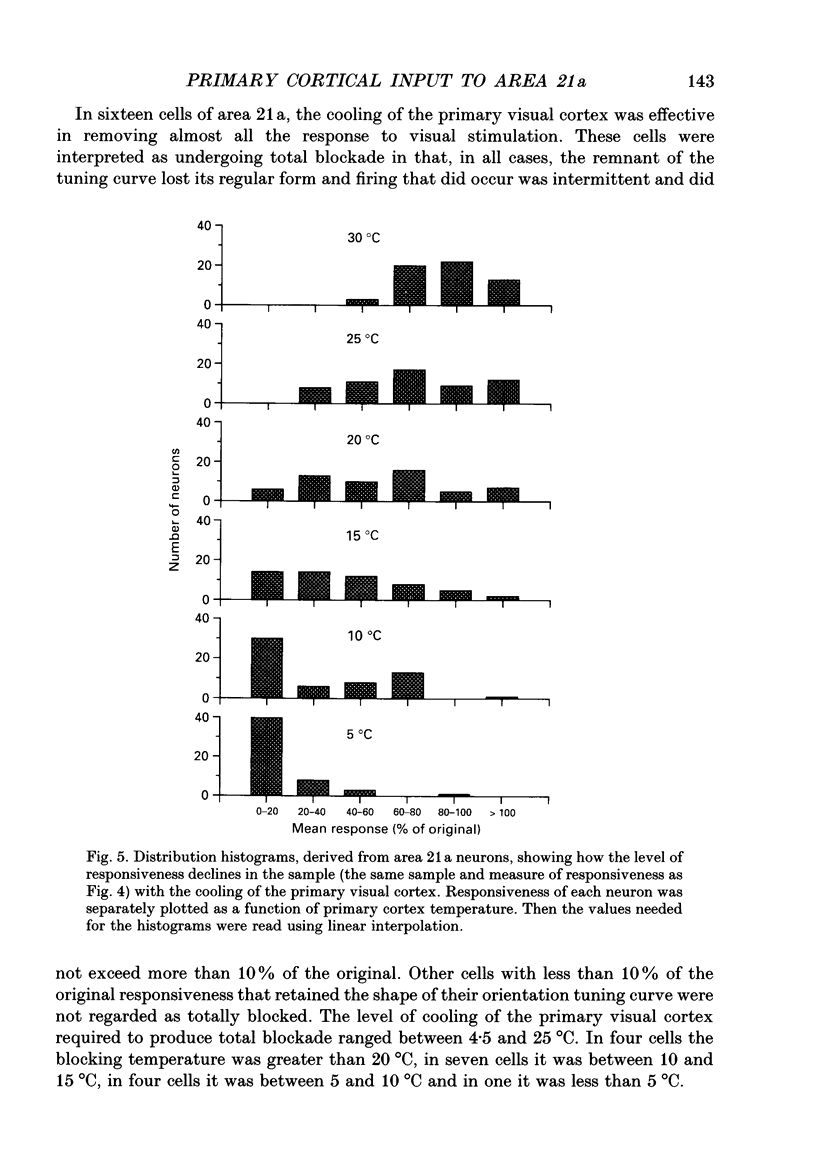
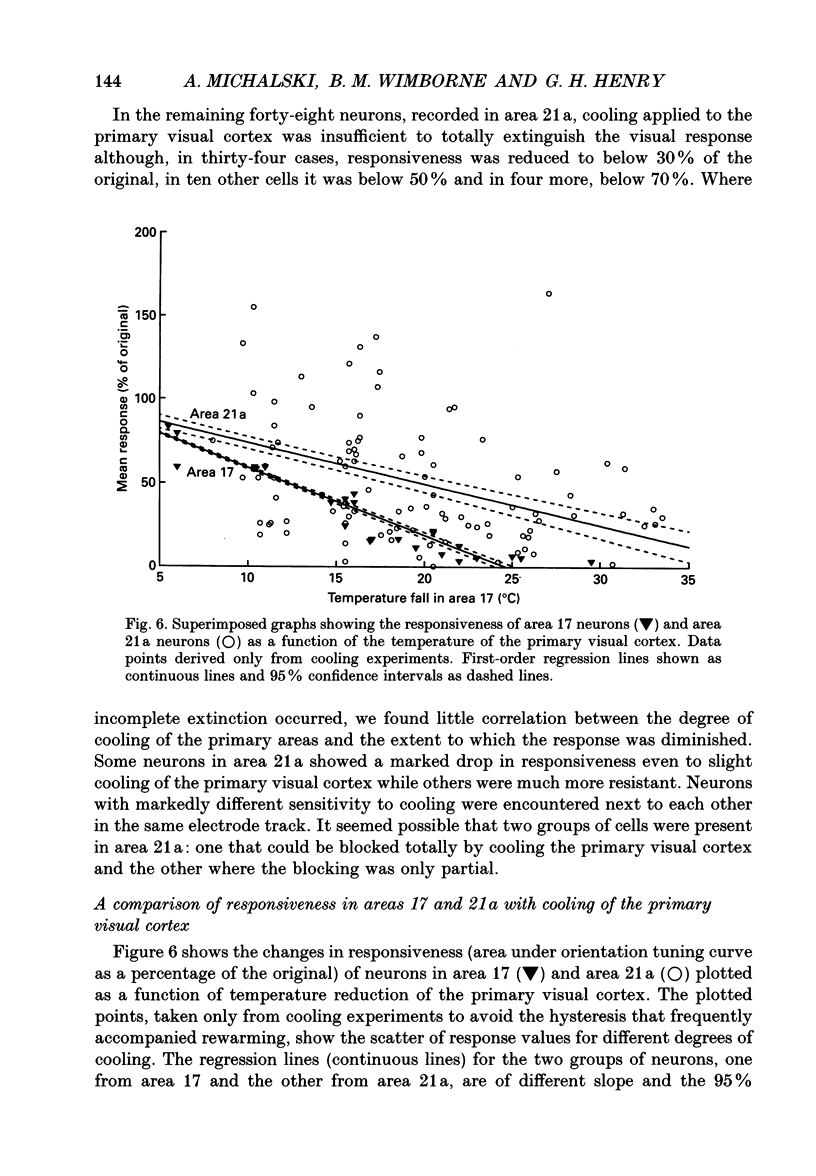
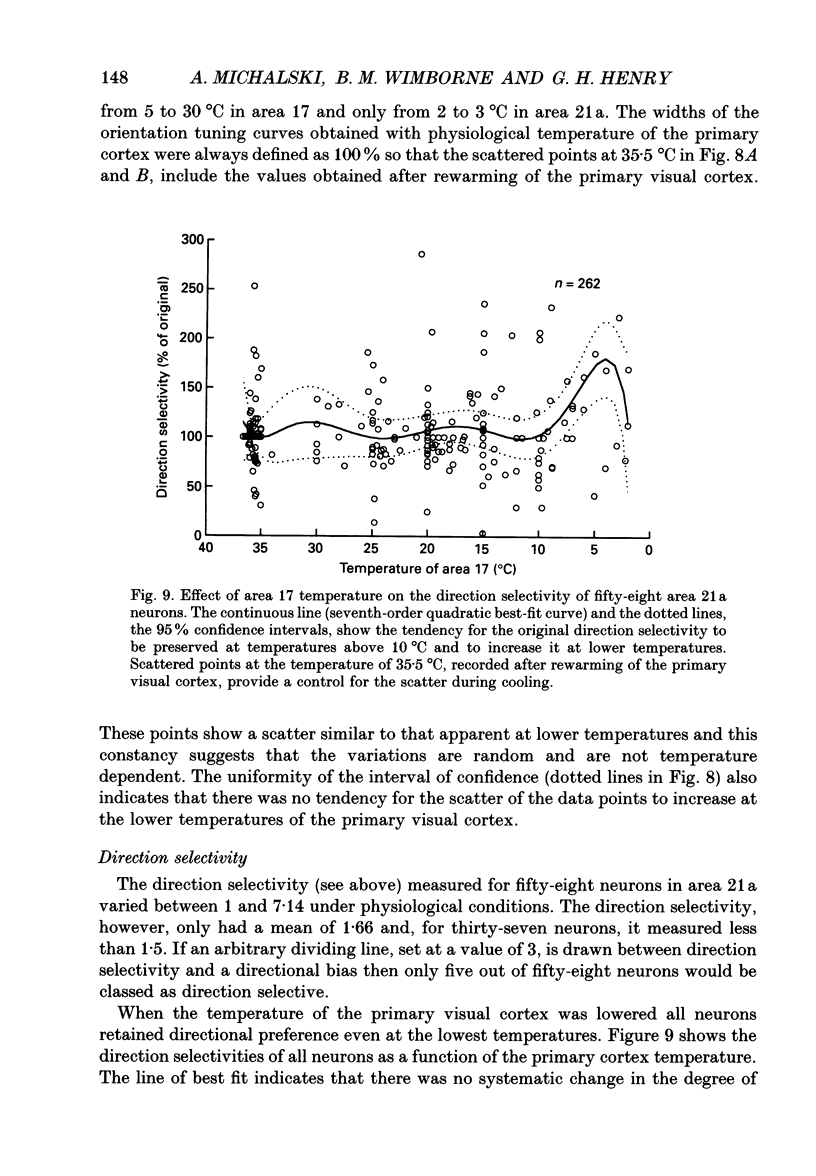
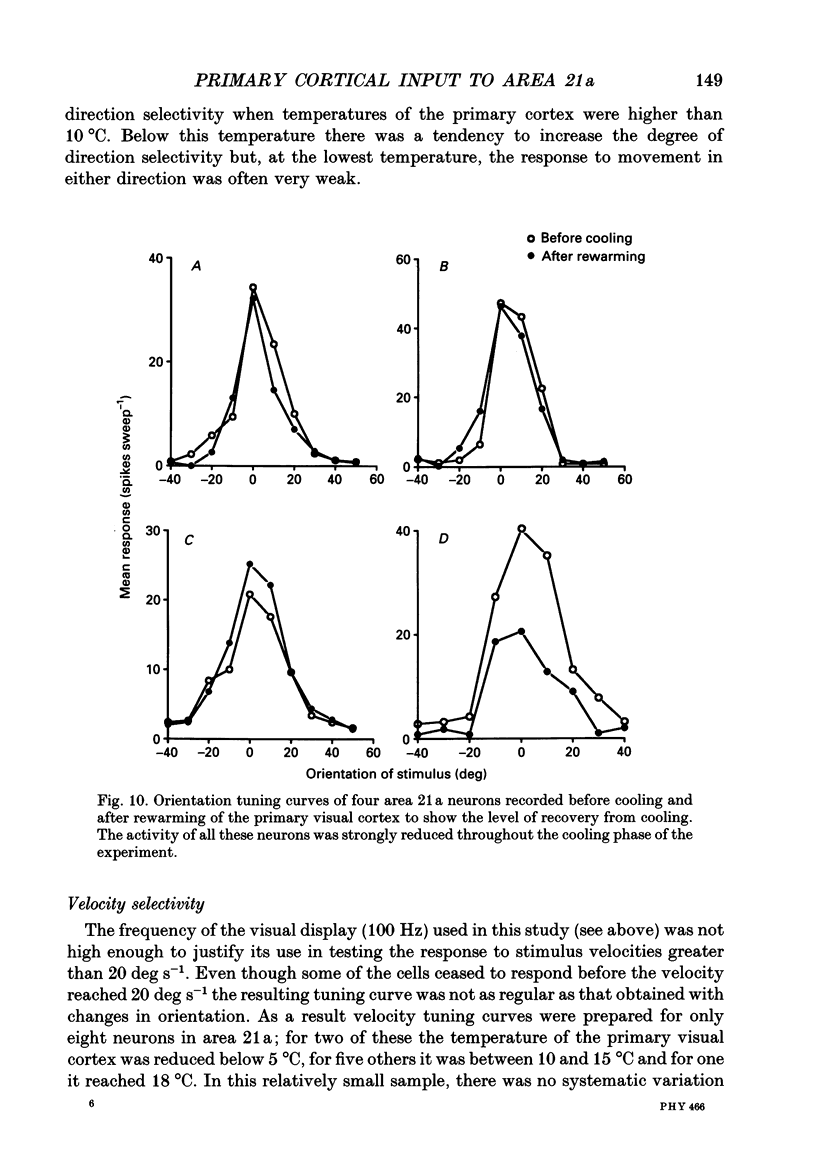
1. Responses of sixty-four neurons in cortical area 21a were studied with areas 17 and 18 reversibly deactivated by cooling. From anatomical studies, most of area 21a input in the cat originates from these primary areas with no input from the A laminae of the lateral geniculate nucleus. 2. Both responses and spontaneous activity of all sixty-four area 21a neurons were markedly reduced when primary areas were cooled. In sixteen cells the responses were totally blocked. Temperatures of primary cortex required to produce total blockade varied between 25 and 4.5 degrees C. 3. The effect of cooling the primary visual cortex on the shape of orientation tuning curves was analysed in thirteen neurons from area 17 and in fifty-eight neurons from area 21a. In both areas, the width of the curve, when measured at its half-height, was preserved even when spike activity was reduced to below 10% of the original level. All neurons also retained their original directional preferences during cooling of the primary visual cortex. 4. The responsiveness of forty-seven neurons from area 21a was tested after rewarming of primary cortex. All but one neuron recovered their initial responsiveness within half an hour of restoring physiological temperature of primary visual cortex. 5. The results of the present study give an indication of the extent to which area 21a is sequentially related to the primary visual cortex in the processing of information.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berson D. M., Graybiel A. M. Subsystems within the visual association cortex as delineated by their thalamic and transcortical affiliations. Prog Brain Res. 1983;58:229–238. doi: 10.1016/S0079-6123(08)60024-2. [DOI] [PubMed] [Google Scholar]
- Bullier J., Mustari M. J., Henry G. H. Receptive-field transformations between LGN neurons and S-cells of cat-striate cortex. J Neurophysiol. 1982 Mar;47(3):417–438. doi: 10.1152/jn.1982.47.3.417. [DOI] [PubMed] [Google Scholar]
- Bénita M., Condé H. Effects of local cooling upon conduction and synaptic transmission. Brain Res. 1972 Jan 14;36(1):133–151. doi: 10.1016/0006-8993(72)90771-8. [DOI] [PubMed] [Google Scholar]
- Douglas R. J., Martin K. A. A functional microcircuit for cat visual cortex. J Physiol. 1991;440:735–769. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B., Michalski A., Cleland B. G., Burke W. Effects of selective pressure block of Y-type optic nerve fibers on the receptive-field properties of neurons in area 18 of the visual cortex of the cat. Vis Neurosci. 1992 Jul;9(1):65–78. doi: 10.1017/s0952523800006374. [DOI] [PubMed] [Google Scholar]
- Ferster D. Orientation selectivity of synaptic potentials in neurons of cat primary visual cortex. J Neurosci. 1986 May;6(5):1284–1301. doi: 10.1523/JNEUROSCI.06-05-01284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahwiler B. H., Mamoon A. M., Schlapfer W. T., Tobias C. A. Effects of temperature on spontaneous bioelectric activity of cultured nerve cells. Brain Res. 1972 May 26;40(2):527–533. doi: 10.1016/0006-8993(72)90157-6. [DOI] [PubMed] [Google Scholar]
- Geisert E. E., Jr Cortical projections of the lateral geniculate nucleus in the cat. J Comp Neurol. 1980 Apr 15;190(4):793–812. doi: 10.1002/cne.901900410. [DOI] [PubMed] [Google Scholar]
- Girard P., Bullier J. Visual activity in area V2 during reversible inactivation of area 17 in the macaque monkey. J Neurophysiol. 1989 Dec;62(6):1287–1302. doi: 10.1152/jn.1989.62.6.1287. [DOI] [PubMed] [Google Scholar]
- Girard P., Salin P. A., Bullier J. Visual activity in areas V3a and V3 during reversible inactivation of area V1 in the macaque monkey. J Neurophysiol. 1991 Nov;66(5):1493–1503. doi: 10.1152/jn.1991.66.5.1493. [DOI] [PubMed] [Google Scholar]
- Girard P., Salin P. A., Bullier J. Visual activity in macaque area V4 depends on area 17 input. Neuroreport. 1991 Feb;2(2):81–84. doi: 10.1097/00001756-199102000-00004. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Dreher B., Bishop P. O. Orientation specificity of cells in cat striate cortex. J Neurophysiol. 1974 Nov;37(6):1394–1409. doi: 10.1152/jn.1974.37.6.1394. [DOI] [PubMed] [Google Scholar]
- Jasper H. H., Shacter D. G., Montplaisir J. The effect of local cooling upon spontaneous and evoked electrical activity of cerebral cortex. Can J Physiol Pharmacol. 1970 Sep;48(9):640–652. doi: 10.1139/y70-094. [DOI] [PubMed] [Google Scholar]
- Kalil R. E., Chase R. Corticofugal influence on activity of lateral geniculate neurons in the cat. J Neurophysiol. 1970 May;33(3):459–474. doi: 10.1152/jn.1970.33.3.459. [DOI] [PubMed] [Google Scholar]
- Mizobe K., Itoi M., Kaihara T., Toyama K. Neuronal responsiveness in area 21a of the cat. Brain Res. 1988 Jan 12;438(1-2):307–310. doi: 10.1016/0006-8993(88)91353-4. [DOI] [PubMed] [Google Scholar]
- Moseley J. I., Ojemann G. A., Ward A. A., Jr Unit activity during focal cortical hypothermia in the normal cortex. Exp Neurol. 1972 Oct;37(1):152–163. doi: 10.1016/0014-4886(72)90232-4. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Malpeli J. G. The effect of striate cortex cooling on area 18 cells in the monkey. Brain Res. 1977 May 6;126(2):366–369. doi: 10.1016/0006-8993(77)90734-x. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Stryker M., Cynader M., Berman N. Response characteristics of single cells in the monkey superior colliculus following ablation or cooling of visual cortex. J Neurophysiol. 1974 Jan;37(1):181–194. doi: 10.1152/jn.1974.37.1.181. [DOI] [PubMed] [Google Scholar]
- Schmielau F., Singer W. The role of visual cortex for binocular interactions in the cat lateral geniculate nucleus. Brain Res. 1977 Jan 21;120(2):354–361. doi: 10.1016/0006-8993(77)90914-3. [DOI] [PubMed] [Google Scholar]
- Sherk H. Area 18 cell responses in cat during reversible inactivation of area 17. J Neurophysiol. 1978 Jan;41(1):204–215. doi: 10.1152/jn.1978.41.1.204. [DOI] [PubMed] [Google Scholar]
- Sherk H. Location and connections of visual cortical areas in the cat's suprasylvian sulcus. J Comp Neurol. 1986 May 1;247(1):1–31. doi: 10.1002/cne.902470102. [DOI] [PubMed] [Google Scholar]
- Symonds L. L., Rosenquist A. C. Corticocortical connections among visual areas in the cat. J Comp Neurol. 1984 Oct 10;229(1):1–38. doi: 10.1002/cne.902290103. [DOI] [PubMed] [Google Scholar]
- Symonds L. L., Rosenquist A. C. Laminar origins of visual corticocortical connections in the cat. J Comp Neurol. 1984 Oct 10;229(1):39–47. doi: 10.1002/cne.902290104. [DOI] [PubMed] [Google Scholar]
- Tusa R. J., Palmer L. A., Rosenquist A. C. The retinotopic organization of area 17 (striate cortex) in the cat. J Comp Neurol. 1978 Jan 15;177(2):213–235. doi: 10.1002/cne.901770204. [DOI] [PubMed] [Google Scholar]
- Tusa R. J., Rosenquist A. C., Palmer L. A. Retinotopic organization of areas 18 and 19 in the cat. J Comp Neurol. 1979 Jun 15;185(4):657–678. doi: 10.1002/cne.901850405. [DOI] [PubMed] [Google Scholar]
- Vidyasagar T. R. A model of striate response properties based on geniculate anisotropies. Biol Cybern. 1987;57(1-2):11–23. doi: 10.1007/BF00318712. [DOI] [PubMed] [Google Scholar]
- Wieniawa-Narkiewicz E., Wimborne B. M., Michalski A., Henry G. H. Area 21a in the cat and the detection of binocular orientation disparity. Ophthalmic Physiol Opt. 1992 Apr;12(2):269–272. doi: 10.1111/j.1475-1313.1992.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Wimborne B. M., Henry G. H. Response characteristics of the cells of cortical area 21a of the cat with special reference to orientation specificity. J Physiol. 1992 Apr;449:457–478. doi: 10.1113/jphysiol.1992.sp019096. [DOI] [PMC free article] [PubMed] [Google Scholar]


