Abstract
Hepatocyte growth factor (HGF) is a substance that stimulates the proliferation of hepatocytes which promote healing. We developed a macrophage membrane-encapsulated nanosphere drug delivery system containing HGF for the study of burn wound healing. Twenty-seven Sprague–Dawley rats were randomly divided into three groups: a saline control (NS) group, an engineered macrophage membrane-encapsulated nanospheres (ETMM@NPS) group, and an engineered macrophage membrane-encapsulated nanospheres treatment with HGF-loaded gene (HGF@ETMM@NPS) group.The wound tissue sections were examined histologically using hematoxylin and eosin (H&E) and Masson trichrome staining. Immunohistochemistry and Western blotting were performed to determine the expression of relevant proteins. The wound-healing, blood flow and complete epithelialization rates were significantly better in the HGF@ETMM@NPS group compared to the NS and ETMM@NPS groups. Expression of B-cell lymphoma 2-associated X-protein was significantly lower, and B-cell lymphoma 2, cluster of differentiation 31, HGF, alpha smooth muscle actin, and PCNA expression was significantly higher in the HGF@ETMM@NPS group compared with the other two groups. PCNA and HGF expression was significantly up-regulated in the HGF@ETMM@NPS group. The HGF@ETMM@NPS complex drug delivery system used in this research promoted wound healing via effective delivery of HGF to burn wounds, thereby accelerating skin cell growth and migration.
Keywords: Deep second-degree burns, Trabecular repair, Targeted drug delivery system, PLGA nanospheres, Engineered macrophage membranes
Subject terms: Skin manifestations, Trauma, Acute inflammation, Inflammasome
Introduction
The burn healing process is highly integrated and overlapping, comprising phases such as inflammatory response, cell recruitment, matrix deposition, epithelialization, and tissue remodeling1–4. Second-degree burn wound healing includes inflammation, epithelial regeneration, granulation, neovascularization, and wound contraction phases5–8. Although there are several treatments available to accelerate wound healing, there is still a need for further research into more optimal treatment options9–12.
The control of the ongoing inflammatory response, the management of the differentiation of fibroblasts to myofibroblasts, and the control of the concentration of different growth factors (GFs) are major factors that influence the rate of burn wound healing11,12. Once a blood vessel is injured, it initiates a rapid hemostatic response and shifts to an inflammatory phase13,14. Two inflammatory cytokines, transforming growth factor-1 and platelet-derived growth factor (PDGF), induce fibroblasts to migrate and proliferate towards the wound site, undergoing phenotypic changes that transform fibroblasts into myofibroblasts15. A number of growth factors, including epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and PDGF, have been employed to stimulate endothelial cell activation and to facilitate neovascularization, with the objective of increasing GF concentrations16. In particular, VEGF and PDGF have been utilized to induce endothelial cell activation and neovascularization17. The fundamental benefit of incorporating GFs into materials is that they protect the molecules from being denatured or cleared by the effects of unfavorable physiological environments and prevent side effects due to sudden bursts or off-target releases by maintaining localized GF concentrations at the site of burns or trauma18–20.
While GFs play an essential role in normal and healthy physiological functions, their use in tissue engineering is constrained by several limitations17. The biological half-life of GFs is notably brief due to their rapid breakdown or loss of activity in the physiological environment. Genes for GFs can be injected into injured tissues to gradually and slowly increase the concentration of different GFs in the tissues. However, if direct injection of the genes is not successful in transfecting the cells, appropriate gene delivery vectors are required18. Consequently, non-viral vectors have attracted greater attention due to their superior biocompatibility and safety. Given that the principal components of nanoparticles (NPs) are biodegradable polymers with good biocompatibility and a gradual release, NPs have been employed as vectors for the treatment of various diseases in recent years21–23.
Fibroblasts play an important role in the wound-healing process. Dermal fibroblasts, in particular, are involved in a number of key activities, including the dissolution of fibrin clots, the creation of new extracellular matrix (ECM) and collagen structures, and the assistance of other cells in effective wound healing and wound contraction18. Fibroblasts are regarded as sentinels of the inflammatory response during tissue repair, exhibiting a multitude of evolving functions. These include early pro-inflammatory, leukocyte recruitment and activation of an anti-migratory phenotype, which subsequently evolves into a proliferative and anti-migratory phenotype19. A fibrotic and pro-angiogenic phenotype, and finally an anti-angiogenic homogeneous myofibroblast phenotype, may also be observed. However, when there is an aberrant inflammatory response to wounds or when wounds are oversized, the over-contraction and continued differentiation may lead to scar formation. therefore, the most critical role in preventing scar formation is to appropriately control the inflammatory response and the differentiation of fibroblasts to myofibroblasts20,24.
Hepatic stellate cells (HSCs), vascular endothelial cells (ECs), and Kupffer cells (KCs) are just a few of the many cell types that express HGF25,26. It was demonstrated that HGF exerts its effects on a diverse range of cells, including epithelial and vascular ECs. HGF is a multifunctional factor that regulates the growth, migration, and morphogenesis of a multitude of cells27,28. HGF is also crucial for wound healing, angiogenesis, and tissue and organ regeneration. HGF stimulates cell proliferation by binding to c-Met receptors on target cells and activating the downstream rat sarcosa/mitogen activated protein kinase, phosphoinositide-3-kinase/AKK, and phosphatidylinositol-3-kinase/protein kinase B (PI3K/AKT) pathways. Cell survival is mediated by downstream PI3K/AKT, which facilitates ECM breakdown in a disordered state and promotes cell migration and wound healing.Hepatocyte growth factor activator (HGFA), coagulation factors XII and XI, metalloproteinases, heparin, tissue-type plasminogen activator, plasma calcium kinase, and protein hydrolase (matriptase) have been identified as activators of HGF29,30.
Inflammation-related cell membrane-encapsulated or camouflaged NPs are frequently utilized as an essential drug delivery tool21,22. This biomimetic technology, known as cell membrane-encapsulated nanoparticles (NPs), aims to create a therapeutic system with a core of NPs encapsulated in the membranes of natural cells, including bacterial cells, cancer cells, stem cells, platelets, or red blood cells. They offer considerable potential for therapeutic and diagnostic applications, including drug delivery, immunomodulation, immunization, and detoxification23,31. Leukocytes exhibit a number of exploitable properties, including ease of crossing biological barriers, long blood circulation time, trans-endothelial migratory properties, natural tumor tropism, and recognition of diseased or inflamed areas.Macrophage membrane-encapsulated NPs can accurately deliver drugs to target liver tissues with ischemia–reperfusion injury, thereby reducing damage by neutralizing endotoxins32,33.
However, there is a paucity of studies investigating the utilization of gene-carrying membrane-mimicking nanospheres in burns treatment34,35. Current literature is limited to findings regarding specific inflammatory cells that migrate to the site of inflammation and those that aggregate in high numbers36. Therefore, the objective of this study was to engineer macrophages to express integrin α4/α5/β1 on their cell membranes, extract their cell membranes to encapsulate HGF-loaded gene nanospheres, and analyze the ability of this drug-carrying system to target burn wounds and promote repair of deep second-degree burns in cellular and animal models. The second objective of this study was to determine the mode of action of this drug delivery method for the treatment of deep second-degree burns.
Materials and methods
Materials and instruments
Animals
Thirty SPF-grade Sprague–Dawley rats (8 weeks old; male; body weight 250–300 g) were provided by the Animal Experiment Centre of Nantong University.
Major instruments
The major instruments used were:
PeriCam PSI System blood perfusion imager (Perimed AB, Järfälla, Stockholm, Sweden)
General purpose small-animal anesthesia machine (R500; RWD Life Science Co., Ltd., Shenzhen, China)
Scanning Electron Microscope (SEM) (S-3400 N; Hitachi, Tokyo, Japan)
Mastersizer 3000 laser particle size analyzer (Malvern Instruments Ltd., Malvern, UK)
Laser Particle Sizer Nano ZS (Malvern Instruments Ltd., Malvern, UK)
Fully automated orthogonal fluorescence microscope Axio Imager M2 (CARL ZEISS, Jena, Germany)
Small-animal live imaging system, Tanon ABL X5 (Tanon Science & Technology Co. Ltd, Shanghai, China)
Biological microscope (Leica DMR 3000; Leica, Bensheim, Germany)
Sonoplus HD 2070 ultrasonic homogenizer (Bandelin Electronic, Berlin, Germany)
Dynamic laser light scatterer (DLS)
Transmission electron microscope (TEM)
Reagent consumables
The reagent consumables used were:
RIPA lysis buffer containing protease inhibitor (Servicebio, Wuhan, Hubei, China)
Omni-Easy™ one-step PAGE gel preparation kit (Shanghai Yase Biomedical Technology Co., Ltd., Shanghai, China)
3% pentobarbital sodium: comprising 3 g of pentobarbital sodium dissolved in 100 mL of water in a 37 °C temperature box until transparent, and then refrigerated at 4 °C
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (DiR) solution (Sigma Aldrich, St Louis, MO, USA)
Hypotonic lysis solution: 1 × 10–3 mol potassium-chloride (KCl), 1.5 × 10–3 mol magnesium chloride (MgCl2) and 1 × 10–3 mol benzenesulfonyl fluoride added to 10 × 10–3 mol Tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl) (pH = 8.0) and refrigerated at 4 °C
Polylactic-co-glycolic acid (PLGA) (Mw = 40,000–75,000) (Sigma Aldrich, Saint Louis, MO, USA)
Polyvinyl alcohol (PVA) (7% [w/v]) (Sigma Aldrich, St. Louis, MO, USA)
Polyvinylidene membrane (Millipore Corp., Bedford, MA, USA)
Goat Anti-Mouse IgG H&L (HRP) (Abcam, Cambridge, UK)
Experimental procedures and methods
Preparation and characterization of the engineered macrophage membrane-encapsulated gene-carrying nanosphere drug delivery system
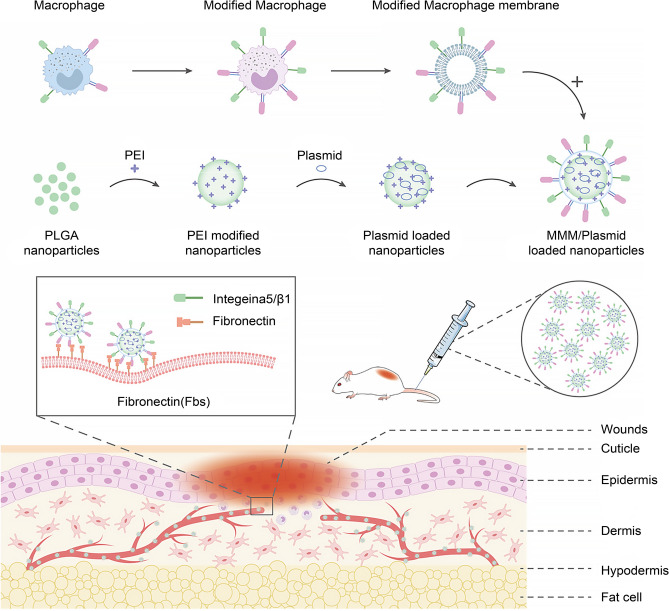
Nanospheres were prepared using the compound emulsion method, and polyetherimide-modified nanospheres were loaded with HGF to produce gene-loaded nanospheres. Macrophages were engineered and modified to express high levels of integrin α4/α5. The α5/β1 integrin receptors were expressed on the cell membranes, which were extracted to encapsulate the drug-loaded nanospheres. The membrane-encapsulated drug-loaded nanospheres were prepared to provide a drug delivery system (Fig. 1). The expression of integrin α4/α5/β1 on the engineered cell membranes was quantified. The particle size, morphology, zeta potential, drug release profile (Fig. 3), and the cellular uptake rate and transfection efficiency of integrins were determined for drug-loaded nanospheres and membrane-encapsulated nanospheres (Fig. 4).
Fig. 1.
Schematic diagram of the mechanism of the engineered macrophage membrane-encapsulated gene-carrying nanosphere drug delivery system to promote skin burn repair. Abbreviations: PLGA, polylactic-co-glycolic acid; PEI, polyetherimide; MMM, modified macrophage membrane (engineered macrophage membrane).
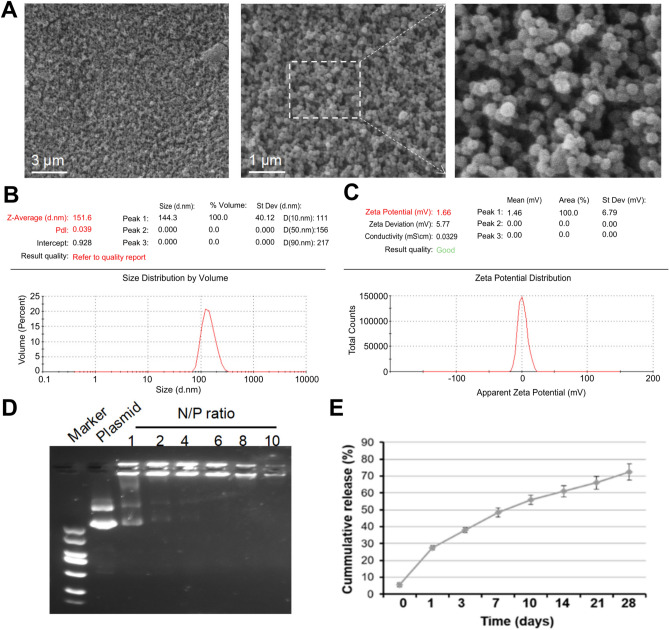
Fig. 3.
Relevant features of the gene-carrying nanospheres. (A) Scanning electron microscope image of gene-carrying nanospheres. (B, C) Particle size analysis graph of gene-carrying nanospheres. (D) Gel blocking assay. (E) In vitro release of gene-carrying nanospheres. Abbreviation: N/P ratio: ratio of ammonia root of polyetherimide to the phosphate group of plasmid.
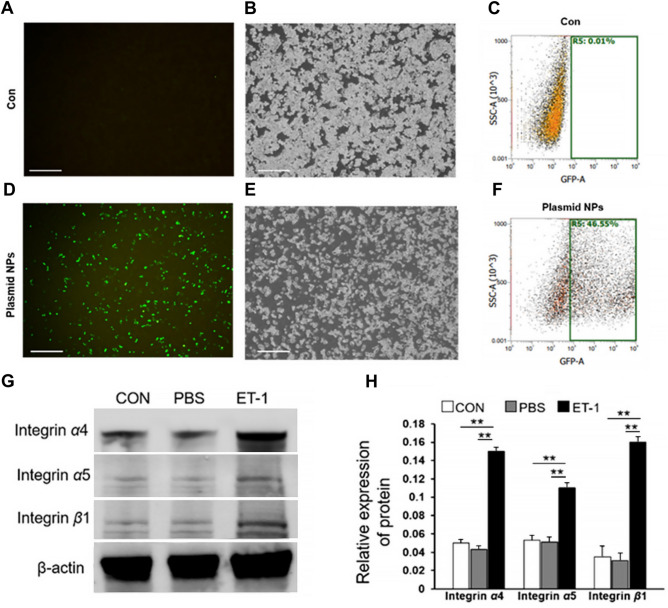
Fig. 4.
Up-regulation of target protein expression by pEGFP-N1-loaded nanospheres. (A-F) Transfection of macrophages with pEGFP-N1-loaded nanospheres effectively transfected plasmids into macrophages. (G, H) Integrin expression in macrophages. Abbreviations: pEGFP-N1, plasmid enhanced green fluorescent protein-N1; CON, control; PBS, phosphate-buffered saline; ET-1, endothelin. **P < 0.01.
Preparation of gene-carrying nanospheres
A plasmid encoding HGF was constructed. The open reading frames of the HGF gene were inserted into the plasmid enhanced green fluorescent protein (pEGFP)-N1 vector, and the insertion sequence was confirmed by sequencing. The pEGFP-N1 vector carries a fragment of the EGFP gene, which can be used to detect transfection efficiency (Fig. 4A–F).
Preparation of poly(lactic-co-glycolic acid) (PLGA)-loaded nanospheres
The preparation of the complex emulsion was conducted using the following procedure. Two hundred milligrams of PLGA (Mw = 40,000–75,000) (Sigma, Saint Louis, MO, USA) was dissolved in 2 mL of dichloromethane (DCM), followed by the addition of 6 mL of PVA (7% [w/v]) (Sigma Aldrich, St. Louis, MO, USA). The primary emulsion was homogenized for 1 min with a Sonoplus HD 2070 ultrasonic homogenizer (Bandelin Electronic, Berlin, Germany). The primary emulsion was added to 100 mL of aqueous PVA solution (1%, w/v), sonicated again for 3 min, and then stirred for 24 h at room temperature to volatilize the residual DCM. The nanosphere suspension was then centrifuged (13,000 rpm, 5 min) at 4 °C to isolate the PLGA nanospheres. The isolated PLGA nanospheres were washed twice in deionized water and finally resuspended in deionized water for use.
Preparation of PLGA nanoparticle/plasmid complexes
The plasmids and PLGA nanospheres were combined to form complexes. The following steps were employed. A solution of PLGA nanospheres (10 μg/μL) was combined with 200 μg of polyetherimide (PEI) (100 μg/μL) in deionized water to render the nanospheres positively charged. The PEI-modified nanosphere solution was then added to the plasmid solution at varying N/P ratios (ratio of ammonia root of PEI to the phosphate group of plasmid). Subsequently, the solution was gently agitated and incubated at room temperature for 20 min to facilitate the formation of PLGA nanosphere/plasmid complexes.
Preparation of engineered macrophages
A pharmacological stimulation approach was employed to up-regulate specific proteins on the surface of RAW 264.7. To modify integrin α4/α5/β1, endothelin (ET-1) was added to stimulate RAW 264.7 to up-regulate integrin α4/α5/β1 protein expression. This was verified by Western blot analysis of the protein level expression of integrin α4/α5/β1 (Fig. 4).
Extraction of engineered macrophage membranes
To obtain bioactive membranes, macrophages were lysed using hypotonic lysis. The hypotonic lysis solution, comprising 1 × 10–3 mol KCl, 1.5 × 10–3 mol MgCl2 and 1 × 10–3 mol benzenesulfonyl fluoride, was first configured by adding 10 × 10–3 mol Tris–HCl (pH = 8.0). The engineered RAW 264.7 cells were collected and centrifuged at 1,000 rpm for 5 min at 4 °C. The cells were then resuspended in pre-cooled phosphate-buffered saline and washed and centrifuged three times. Two milliliters of pre-cooled hypotonic lysate were added to resuspend the cells, and an ice bath was used for 15 min. The cell suspension was then subjected to a freeze–thaw cycle 10 times to achieve complete cell rupture. The suspension was subsequently centrifuged at 850 × g for 10 min at 4 °C, and the supernatant was collected and centrifuged at 15,000 × g for 30 min. The resulting precipitate, comprising engineered RAW 264.7 cell membrane fragments, was collected and resuspended in an appropriate amount of hypotonic lysate.
Preparation of engineered macrophage membrane-encapsulated gene-carrying nanospheres
Macrophage membrane vesicles were harvested by subjecting the extracted membranes to sonication for 20 min and subsequent extrusion through a membrane filter with a pore size of 400 nm. The macrophage vesicles were then mixed with gene-carrying nanospheres at a ratio of 1:1 (mass ratio of membrane proteins to nanospheres), sonicated, and extruded through a membrane filter with a pore size of 200 nm.
Characterization of membrane-coated gene-carrying nanospheres correlation
The particle size distribution and zeta potential of nanospheres and membrane-coated nanospheres were quantified using DLS. TEM was employed to determine the morphology (Fig. 5A; B).
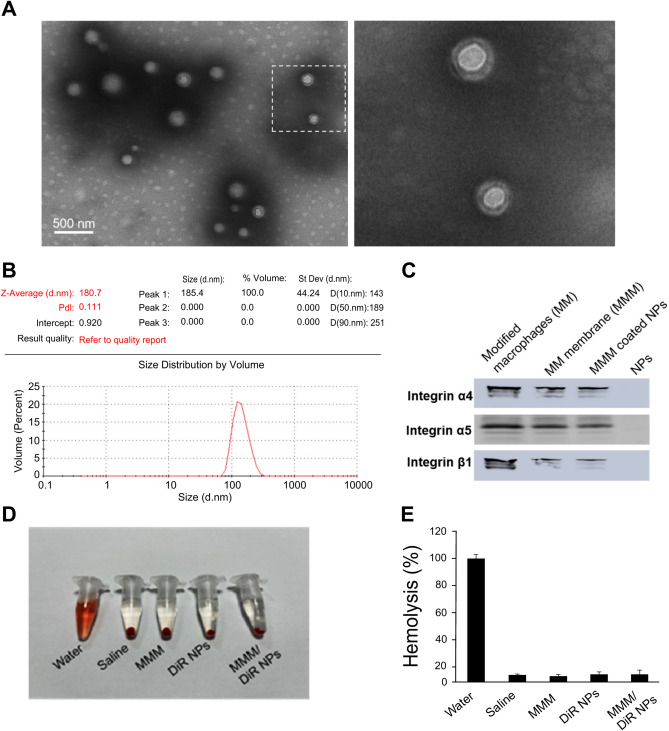
Fig. 5.
Membrane-coated DiR-loaded nanospheres electron micrographs and related features. (A) Transmission electron micrograph of modified macrophage membrane-coated DiR-loaded nanospheres. (B) Particle size map of membrane-coated DiR-loaded nanospheres. (C) MMM/DiR-NPS carrying integrins. (D, E) MMM/DiR-NPS have good hemocompatibility. Abbreviations: DiR, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide; MMM/DiR-NPS, modified macrophage membrane-coated DiR-loaded nanospheres.
Preparation of engineered membrane-coated DiR-loaded nanospheres and detection of their targeting to burn sites and in vivo distribution
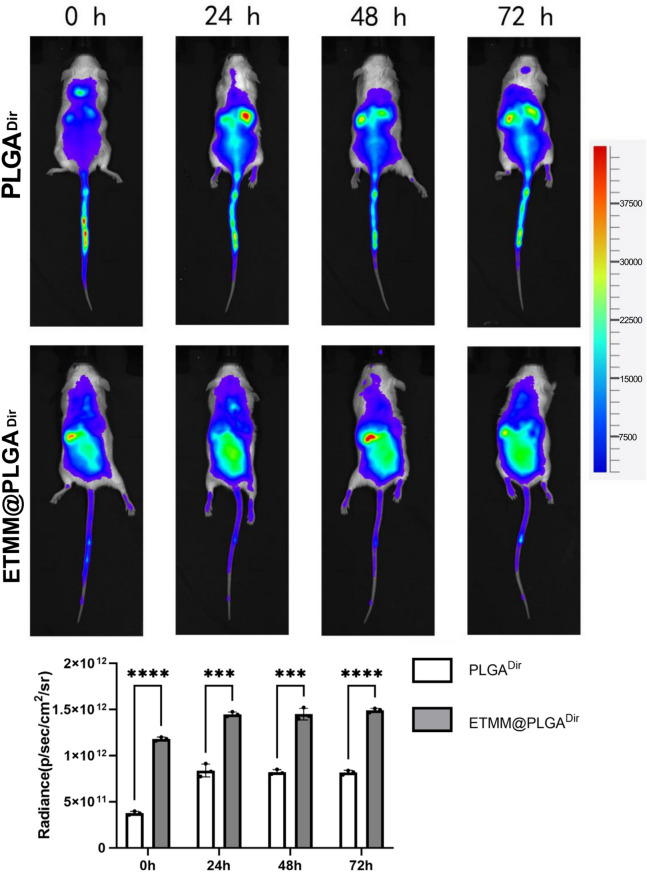
DiR was selected as the fluorescent dye, and DiR-loaded nanospheres were prepared using the complex emulsion method and coated with engineered membranes to produce membrane-coated DiR-loaded nanospheres. A rat burn model was prepared, and the membrane-coated DiR-loaded nanospheres were injected into the tail vein. The distribution of the nanospheres in the body was observed at different time points, with a particular focus on whether they aggregated at the burn site and the degree of aggregation (Fig. 6).
Fig. 6.
Modified macrophage membrane-encapsulated loaded DiR nanospheres targeting burn site.***P < 0.001, ****P < 0.0001. DiR, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide; MMM/DiR-NPS, modified macrophage membrane-coated DiR-loaded nanospheres; M/DiR-NPS, membrane-coated DiR-loaded nanospheres.
Preparation of membrane-coated DiR-loaded nanospheres
The same complex emulsion method as described in "Preparation of poly(lactic-co-glycolic acid) (PLGA)-loaded nanospheres" was employed for preparation, except 100 mg of DiR was dissolved in the DCM with the PLGA.
Second-degree burn model
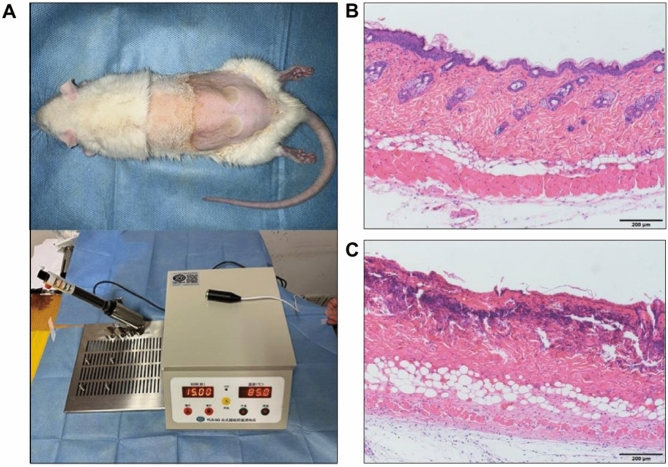
All animal experiments were conducted in accordance with the Guidelines for the Protection and Use of Laboratory Animals of Nantong University and approved by the Ethical Review Committee of the Affiliated Hospital of Nantong University. Eight-week-old male Sprague–Dawley rats were anaesthetized by intraperitoneal injection of 3% sodium pentobarbital (30 mg/kg), and their backs were shaved 1 day in advance. Following anesthesia, a temperature controller (YLS-5Q; BeijingZhongShiDiChuang Technology Development Company, Beijing, China) was employed to create the burn model. The temperature of the instrument was set at 85 °C, and the scalding head of the instrument with an area of 1 cm2 was placed on the back of the rat for 15 s (the pressure was the self-weight of the iron bar) to form a deep burn wound. The rats were euthanized 48 h after burn wounding, and the depth of the skin injury was assessed by histological examination using hematoxylin and eosin (H&E) staining (Fig. 2).
Fig. 2.
Establishment of a rat model of deep second-degree burns. (A) Schematic representation of the rat model. (B) H&E micrograph of normal rat skin tissue. (C) H&E-micrograph of burnt rat skin tissue. Abbreviation: H&E, hematoxylin and eosin.
Targeting of burn sites by membrane-coated DiR-loaded nanospheres and in vivo distribution
A single intravenous injection of membrane-coated DiR-loaded nanospheres was administered to anesthetized rats with a high inflammatory response 6 h after burn injury. The distribution of nanospheres was observed at different time points after injection, with a particular focus on the site of the burn injury, using a small-animal live imaging system, Tanon ABL X5 (Tanon Science & Technology Co. Ltd, Shanghai, China). In addition, various organs were tested to observe the systemic distribution of the drug.
Detection of hemocompatibility of engineered membrane-coated gene-carrying nanospheres
A 1-mL sample of rat blood was diluted with 1.25 mL of 0.9% sodium chloride solution. Subsequently, 0.1 mL of the diluted whole blood was added to the gene-carrying nanospheres or membrane-encapsulated gene-carrying nanosphere solution (5 mL, 1 mg/mL) and incubated for 1 h at 37 °C. Following this incubation period, centrifugation at 3000 rpm for 5 min was performed. The absorbance of the supernatant was measured at 540 nm to determine the hemoglobin released from the lysed erythrocytes (Fig. 5D; E).
Therapeutic efficacy of engineered membrane-coated gene-carrying nanospheres for burn wound repair
A model of rat deep second-degree burn wounds was constructed in order to compare the epithelialization rate of wounds in different treatment groups (Fig. 2). The blood flow of wounds was analyzed using a blood perfusion meter in order to compare the prognosis of wounds in different treatment groups. The production of skin tissue appendages on wounds was observed using H&E staining.
Grouping and treatment of rats
Following their introduction to the rearing room, the experimental animals were permitted to acclimatize for a period of 4 days. During this period, they were fed and watered as normal, but were not permitted to consume food or water for a period of 12 h prior to the commencement of the experiment. Four hours prior to the start of the experiment, they were also prohibited from consuming water. Throughout this period, the animals were observed and reared in accordance with standard practice. At the conclusion of this period, the animals were weighed to confirm that they were within the required weight range.
Eight-week-old male Sprague–Dawley rats were randomly divided into three groups: a saline control group (NS group), a membrane-coated nanospheres treatment group (ETMM@NPS group), and a membrane-coated gene-carrying nanospheres treatment group (HGF@ETMM@NPS group). On the day of the experiment, all rats were shaved on the back and the floating hairs were removed with a hair removal cream. The rats were then washed with warm water and dried. All rats were anesthetized by intraperitoneal injection of 3% sodium pentobarbital (30 mg/kg). Following anesthesia, a temperature controller (YLS-5Q; (BeijingZhongShiDiChuang Technology Development Company, Beijing, China) was employed to create a rat dorsal skin deep second-degree burn model. Rats in the HGF@ETMM@NPS group were administered membrane-coated gene-carrying nanospheres (0.2 mL) via the tail vein, while rats in the ETMM@NPS group were administered membrane-coated nanospheres (0.2 mL) via the tail vein, and rats in the NS group were injected with 0.2 mL of saline via the tail vein.
Prognosis of a membrane-coated gene-loaded nanosphere drug delivery system in the treatment of rat burn wounds
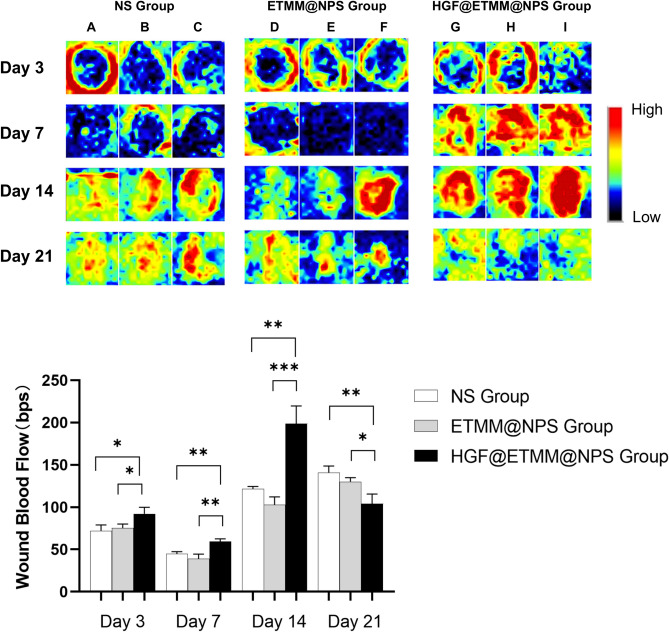
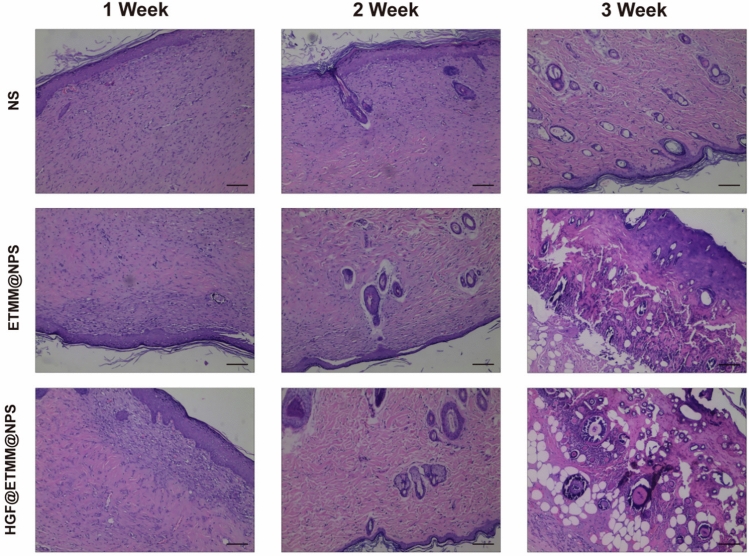
The three groups of rats were photographed daily to observe wound healing and compare the epithelialization rate of the wounds in the three groups. Three rats were taken from each of the three groups at 3, 7, 14, and 21 days after burn injury, and scanned with PeriScan PIM3 (PeriMed, Järfälla, Sweden) to measure the blood flow density of the wounds (Fig. 8). The rats were scanned three times, with all results recorded to one decimal place. This enabled the wounds to be compared with the different conditions under treatment. Subsequently, three rats were randomly selected from each of the three groups and euthanized. The wounds were then subjected to H&E staining, which enabled the skin tissue appendages to be compared in the three groups (Fig. 9).
Fig. 8.
Blood flow perfusion of burn wounds. Black areas indicate low or no blood flow and blue, yellow, green, and red areas indicate increased blood flow, in that order. A, B, and C represent the three rats in the NS group, D, E, and F represent the three rats in the ETMM@NPS group, and G, H, and I represent the three rats in the HGF@ETMM@NPS group. Abbreviations: HGF, hepatocyte growth factor; NS, normal saline; ETMM@NPS, engineered macrophage membrane-encapsulated nanospheres; HGF@ETMM@NPS, engineered macrophage membrane-encapsulated nanospheres with HGF-loaded gene; bps, beats per second. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 9.
H&E staining of skin burn wounds at 7 d, 14 d, and 21 d post-injury.
Mechanism of membrane-encapsulated gene-loaded nanosphere drug delivery system for wound repair
To ascertain the impact of inflammatory response and cells on the wounds of different treatment groups, the infiltration of inflammatory cells in the wounds of rats was evaluated using immunohistochemistry. Additionally, the expression of relevant proteins, including HGF, proliferating cell nuclear antigen (PCNA) (Fig. 11), B-cell lymphoma 2 (Bcl-2), Bcl-2 associated X-protein (Bax), cluster of differentiation 31 (CD31), alpha smooth muscle actin (α-SMA), collagen I, collagen III and others (Fig. 10), was assessed using Western blot and immunohistochemistry.
Fig. 11.
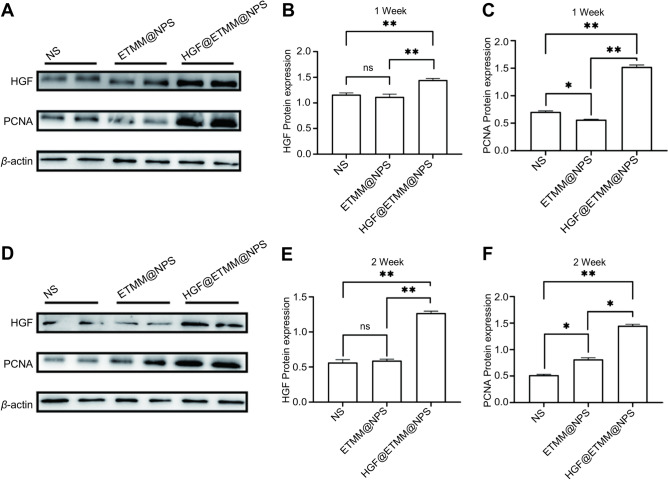
Immunoblot analysis of HGF and PCNA protein expression in rat wounds in the three groups. (A) Western blot detection of HGF and PCNA protein expression in the NS, ETMM@NPS, and HGF@ETMM@NPS groups. (B) The expression of HGF and PCNA proteins in the NS, ETMM@NPS, and HGF@ETMM@NPS groups at 7 days after burn injury. (C) Relative expression of PCNA proteins in the NS, ETMM@NPS, and HGF@ETMM@NPS groups at 7 days after burn injury. (D) Western blot detection of the expression of HGF and PCNA proteins in the NS, ETMM@NPS, and HGF@ETMM@NPS groups at 14 days after burn injury. (E) Expression of HGF and PCNA proteins in the NS, ETMM@NPS, and HGF@ETMM@NPS groups at 14 days after burn injury. (F) Relative expression of PCNA proteins in the NS, ETMM@NPS, and HGF@ETMM@NPS groups 14 days after burn injury. Abbreviations: HGF, hepatocyte growth factor; PCNA, proliferating cell nuclear antigen; NS, normal saline; ETMM@NPS, engineered macrophage membrane-encapsulated nanospheres; HGF@ETMM@NPS, engineered macrophage membrane-encapsulated nanospheres HGF-loaded.
Fig. 10.
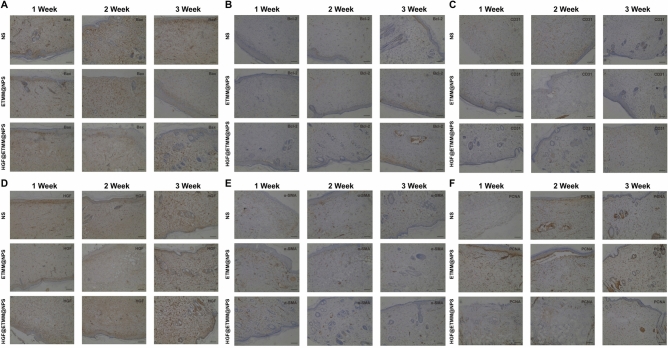
(A) Results of immunohistochemical staining with Bax antibody. (B) Results of immunohistochemical staining with Bcl-2 antibody. (C) Results of immunohistochemical staining with CD31 antibody. (D) Results of immunohistochemical staining with HGF antibody. (E) Results of immunohistochemical staining with α-SMA antibody. (F) Results of immunohistochemical staining with PCNA antibody. Abbreviations: HGF, hepatocyte growth factor; PCNA, proliferating cell nuclear antigen; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X-protein; CD31, cluster of differentiation 31; α-SMA, alpha smooth muscle actin.
Statistical analysis
The digital images of rat trauma were processed using Adobe Photoshop (San Jose, CA, USA) and ImageJ software (NIH, Bethesda, MA, USA) to quantify the degree of healing. The results were retained to one decimal place. GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA) was used to perform a one-way analysis of variance (ANOVA) of rat trauma blood flow density between groups. All results are expressed as mean ± standard deviation (SD). The statistical significance of differences between the two groups was evaluated using a Student’s t-test, while the statistical significance of differences between the three groups was analyzed using an ANOVA using SPSS statistical software (IBM Corp., Armonk, NY, USA). A P-value of less than 0.05 was considered statistically significant.
Results
Establishment of a rat model of deep second-degree burns
The rat model showing marked edema and whitening of the base of the trauma after burn injury(Fig. 2A). Normal rat skin tissue with normal hair follicle sweat glands (Fig. 2B). Burnt rat skin tissue with different degrees of damage to hair follicles and sweat glands (Fig. 2C).
Characteristics associated with gene-carrying nanospheres
Scanning electron microscope image of gene-carrying nanospheres (Fig. 3A).Gene-carrying nanospheres with a particle size of approximately 151.6 nm (Fig. 3B,C).Gel blocking assay showing that the nanospheres and plasmid can fully bind at an ratio of ammonia root of polyetherimide to the phosphate group of plasmid of 6 (Fig. 3D).In vitro release of gene-carrying nanospheres,continuously and slowly release over a period of 28 days (Fig. 3E).
pEGFP-N1-loaded nanospheres up-regulate target protein expression
Transfection of macrophages with pEGFP-N1-loaded nanospheres effectively transfected plasmids into macrophages with a transfection efficiency of up to 46.55% (Fig. 4A-F).ET-1 effectively stimulates integrin expression in macrophages (Fig. 4G,H).
Detection of membrane-coated DiR-loaded nanospheres and their associated features
Transmission electron micrograph of modified macrophage membrane-coated DiR-loaded nanospheres showing a membrane-like structure at the periphery of the nanospheres (Fig. 5A). Particle size map of membrane-coated DiR-loaded nanospheres with a particle size of 180.7 nm (Fig. 5B).MMM/DiR-NPS carrying integrins (Fig. 5C). MMM/DiR-NPS have good hemocompatibility (Fig. 5D,E).
Modified macrophage membrane-coated DiR-loaded nanospheres targeting the burn site
Modified macrophage membrane-encapsulated loaded DiR nanospheres targeting burn site.Compared with M/DiR-NPS, MMM/DiR-NPS have stronger ability to target the burn site (Fig. 6).
Burn wound healing
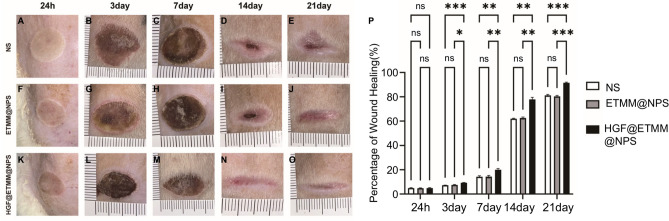
To assess the rate of wound healing, the morphology of the wounds was photographed at 24 h and on days 3, 7, 14, and 21 post-injury (Fig. 7). At 24 h post-injury, the base of the wound was whitish and basically free of exudation. At 3 days post-injury, the wound was covered by a crust and there was no obvious exudation. At 7 days post-injury, the burned area of all rats was reduced and crusted. At 14 days post-injury, the crust was reduced to the center and some of the crusts had sloughed off to form a new epithelium. At 21 days post-injury, the wounds in all groups were basically healed, and the neoplastic skin was slightly reddish. At day 7 (P < 0.01) and day 14 (P < 0.05), the wound healing rate of the HGF@ETMM@NPS group was significantly higher than that of the other groups. The wounds in all groups were essentially healed by day 21, with the scars in the HGF@ETMM@NPS group being significantly smaller and lighter in color than those in the other two groups.
Fig. 7.
Comparison of wound healing of deep second-grade burns in rats. Abbreviations: NS, normal saline; ETMM@NPS, engineered macrophage membrane-encapsulated nanospheres; HGF@ETMM@NPS, engineered macrophage membrane-encapsulated nanospheres with HGF-loaded gene; bps, beats per second. *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significant.
Analysis of trabecular blood flow
Trabecular blood flow was assessed on day 3 and at 1, 2, and 3 weeks after burn injury (Fig. 8). Blood flow in the wound area was lower than in the peri-wound area in all three groups, and zones of high blood flow were found at the edges of the wounds. The differences in wound blood flow between the three groups were small (P > 0.05), and the area of low blood flow in the center of the wounds was significantly smaller in all three groups at week 2 after burn injury compared with week 1. The area of low blood flow at the edge of the wounds was significantly attenuated in the control rats, and the blood flow at the wounds and edges of the wounds was significantly increased in the HGF@ETMM@NPS treatment rats. By the 3rd week after burning, the areas of low blood flow on the traumatic surfaces of the rat skin in the three groups had disappeared, indicating that the traumatized skin surfaces of the rats had completely healed, whereas the HGF@ETMM@NPS treatment group obviously had no areas of high blood flow and the density of blood flow tended to be normal.
H&E staining analysis
There are three main overlapping processes involved in wound healing, i.e. inflammation, proliferation, and alteration. To observe the changes in the epidermis, sebaceous glands, hair follicles and other tissues of the wound at the microscopic level, the rats were euthanized at 7, 14, and 21 days after wounding and the skin of the wound was collected for H&E staining. The sections were examined under the microscope (Fig. 9). On day 14, the eschar gradually fell off, the epidermal morphology improved, the new collagen fibers were disorganized, and there were fewer inflammatory cells in the HGF@ETMM@NPS treatment group than in the other groups (Fig. 9). By day 21, the wound had basically healed and the epidermis had basically returned to normal morphology (Fig. 9). The HGF@ETMM@NPS treatment group had more new sebaceous glands and hair follicles than the other groups (Fig. 9). In addition, the skin tissue in the HGF@ETMM@NPS group showed better healing ability and better re-epithelialization (Fig. 9).
Immunohistochemical analysis
The results of immunohistochemical staining of the traumatic tissues with antibodies against Bax, Bcl-2, CD31, HGF, PCNA, and α-SMA at 7, 14, and 21 days after burn injury showed that the expression of Bax (Fig. 10A) in the traumatic tissues of the HGF@ETMM@NPS treatment group was significantly lower than that of the other groups. The expression of Bcl-2 (Fig. 10B), CD31 (Fig. 10C), HGF (Fig. 10D), α-SMA (Fig. 10E), and PCNA (Fig. 10F) was significantly higher in the HGF@ETMM@NPS treatment group than in the other groups and showed strong positive signals.
Effect of HGF@ETMM@NPS N/P complex on expression of related proteins
Western blotting was used to detect the expression of HGF and PCNA in the skin tissues of three groups of rats. Proteins were isolated from wounds at 1 and 2 weeks after burn injury. These proteins were co-incubated with anti-HGF antigen antibody and anti-PCNA antigen antibody. There was a statistically significant difference in PCNA expression among the three groups of rats at 1 week after burn injury (Fig. 11A, C), and PCNA expression was significantly up-regulated in the tissues of the HGF@ETMM@NPS treatment group at 2 weeks after treatment (Fig. 11D, F). PCNA can be used as an indicator to assess the proliferation status of traumatized cells. HGF was significantly up-regulated after injection of macrophage membrane-encapsulated nanoparticle/plasmid complex at 1 and 2 weeks after burn injury (Fig. 11A, B; Fig. 11D, E).
Discussion
The treatment of burn wounds and related complications present significant challenges for treating clinicians. Deep burn wounds are particularly difficult to treat, requiring a lengthy recovery period13,14. For deep second-degree burns, early debridement of necrotic tissue and skin grafting remain the mainstream treatments to reduce the risk of infection and minimize scarring. However, the long treatment cycle of traditional burn dressing replacement materials places a heavy physical and psychological burden on patients. In standard clinical treatments, GF drugs are administered subcutaneously in a soluble form around the wound. However, this mode of administration may result in peri-wound infections, and the drugs are structurally unstable with a short half-life37,38.
Currently, novel and efficient biomaterials are receiving increasing attention in the field of wound healing, with the aim of effectively promoting healing by controlling the inflammatory response, reducing the risk of infection, and minimizing scar formation. The expression of genes in ribonucleic acid (RNA) has been linked to various diseases. RNA delivery, a novel approach to gene therapy, is constrained by limitations such as localized delivery, impaired RNA degradation, and non-targeted effects37,38. The use of nanoparticle materials has emerged as a promising research area with diverse applications in drug delivery and diagnostics. These materials offer advantages such as controlled release and enhanced biodegradability. Previous studies have demonstrated the efficacy of NPs as a drug delivery system for the treatment of burn wounds, thereby underscoring the pressing need to advance the use of nanodrug conjugates39,40.
HGF exerts its effects on a diverse range of cells, including vascular endothelial cells and epithelial cells. It is a multifunctional factor that regulates the growth, migration, and morphogenesis of a multitude of cells30,41. HGF can also regulate epithelial-mesenchymal interactions through paracrine and autocrine mechanisms, and it plays an important role in angiogenesis, trauma healing, tissue and organ regeneration, and morphogenesis. Therefore, we selected NPs to bind to HGF. First, the nanospheres were prepared using the complex emulsion method, and the gene-loaded nanospheres were prepared by modifying the nanospheres with PEI to load the HGF gene nanospheres. To enhance the targeting capacity of NPs to aggregate at the site of inflammation and to improve their delivery efficiency, a novel drug targeting system was constructed in this study. Macrophage membranes were selected to encapsulate the NPs, and recombinant ET-1 was used to promote the expression of integrin α4/α5/β1 in the macrophage cells42. Engineered and modified macrophage membranes were then extracted to encapsulate the HGF-loaded nanosphere particles, further enhancing the delivery function of the NPs. Subsequently, the complex was injected into the rat tail vein. The HGF-loaded nanosphere particles were delivered to the wound, where they were released at a controlled rate. This process promoted angiogenesis, accelerated wound healing, effectively controlled the burn-induced inflammatory response, and inhibited fibroblast differentiation to myofibroblasts generated during wound scar formation.
HGF has been purified and molecularly cloned as a potent mitogen for mature hepatocytes and is a potent regenerative factor involved in wound healing. Mesenchymal stromal cells are capable of producing HGF, which stimulates epithelial cells in a paracrine manner29. Furthermore, HGF is detected in the culture supernatant of keratin-forming cells, which is similar to recombinant HGF, and in conditioned cultures, which have a high affinity for dextran sulphate and albumin and bind to the same epitopes through interactions with the c-Met receptor.
A close relationship exists between epithelialization, collagen deposition, and fibroblast proliferation, a process that underlies burn wound recovery. During healing, collagen synthesis commences on day 3, reaches a peak at 3–6 weeks and then undergoes remodeling35. Type III collagen is initially synthesized and deposited, while it is gradually replaced by type I collagen as the scar tissue matures, and this can be observed microscopically in the collagen zone. Within 7 days, the density of type I and type III collagen was approximately equal in all treatment groups. However, within 21 days, the HGF@ETMM@NPS group demonstrated superior performance in terms of type III collagen deposition, suggesting superior wound healing24. In the presence of the engineered macrophage membrane-encapsulated gene-carrying nanorods complex, the deposition of type I collagen was also more effective compared with the control group, suggesting a more mature scar in the presence of the complex. This may be attributed to the stimulation of dermal fibroblast migration, which induces type I collagen secretion, accelerates granulation tissue formation, increases collagen deposition, and promotes fibroblast proliferation and activity through direct mechanisms such as differentiation of muscle fibroblasts, and paracrine effects, such as GF production43. Fibroblasts are the predominant cell type in granulation tissue, and they secrete type III collagen and elastin, which along with type I collagen, constitute essential components of the ECM, playing a pivotal role in the healing process.
Our experiments demonstrated that NPs continuously and slowly released plasmids over a period of 28 days. The assembly process of the macrophage membrane and NPs did not affect the activity of key proteins of the macrophage membrane. Immunofluorescence imaging in the first week after injury showed significantly more EGFP-positive cells at the trauma site than in normal skin tissue, confirming the successful transfectability of the complex in vivo. In vivo and in vitro imaging revealed that the DiR signal intensity was higher in the ETMM@NPS group than in the PLGA/DiR group, suggesting that macrophage membranes successfully transported the complex to the target site and that simple PLGA/DiR would be dispersed in various metabolic organs. The wounds exhibited significant healing in the third week following the burn injury, with the HGF@ETMM@NPS treatment group demonstrating a significantly higher wound-healing rate than the other groups in the first 2 weeks. These results indicate that the HGF@ETMM@NPS complex has a positive effect on the healing of burn wounds and a targeting effect on burn wounds44. This targeting effect plays a role in regulating local inflammation, stimulating immune responses, maintaining vascular morphology, and ultimately promoting wound healing. These findings suggest that engineered macrophage membrane membranes are of value for research in the treatment of burns.
The blood perfusion results demonstrated that the traumatic blood flow of rats in the HGF@ETMM@NPS treatment group was superior 1, 2, and 3 weeks after burn injury. This evidence substantiated the hypothesis that the HGF@ETMM@NPS complex had a reparative effect on damaged blood vessels and facilitated the reconstruction of traumatic hematopoiesis, which is a pivotal factor in the healing of burn wounds. Histological examination of the traumatized tissue revealed a reduction in inflammatory cell infiltration and an increase in skin appendages in the HGF@ETMM@NPS treatment group, possibly attributed to the anti-inflammatory effect of macrophages. The immunohistochemistry results demonstrated the presence of HGF expression in the burned tissues of the HGF@ETMM@NPS treatment group. The NP toxicity test and the comparison between the tail vein injection of HGF@ETMM@NPS complex and saline revealed no significant change in organ morphology under the microscope, and the results of the blood analysis were normal, indicating that the HGF@ETMM@NPS complex was safe.
Another issue of interest is the in vitro transfection rate of the complex. We tested the transfection rate to study the potential role of the complex in wound healing. The results demonstrated that the fluorescence expression intensity of EGFP was higher and the protein content of bFGF and VEGFA was higher in cells treated with the complex. We concluded that the HGF@ETMM@NPS complex is an effective approach for promoting wound healing and for delivering and releasing drugs to the wound. Furthermore, this complex accelerated skin cell growth, migration, blood circulation re-establishment, and wound healing.
From a clinical perspective, dressing changes often result in significant pain and discomfort for burn patients13,14. While the use of sedative and analgesic drugs and music therapy can alleviate pain, they can also induce anxiety and panic in some patients. Consequently, the study of intravenous administration to promote wound healing remains a priority, and this study has clinical significance because intravenous administration of the HGF@ETMM@NPS complex has the potential to reduce the number of administrations, alleviate the pain of dressing changes, and promote wound healing. The notable bio-targeting effects of the engineered macrophage membranes on rat burn wounds suggest its potential application in burn wound therapy.
However, this study did have one significant limitations. That is, the efficacy of this biotherapeutic strategy in humans remains to be demonstrated, given the significant interspecies differences between rats and humans.
Conclusions
In summary, The wound-healing, blood flow and complete epithelialization rates were significantly better in the HGF@ETMM@NPS group compared to the NS and ETMM@NPS groups. Expression of B-cell lymphoma 2-associated X-protein was significantly lower, and B-cell lymphoma 2, cluster of differentiation 31, HGF, alpha smooth muscle actin, and PCNA expression was significantly higher in the HGF@ETMM@NPS group compared with the other two groups. PCNA and HGF expression was significantly up-regulated in the HGF@ETMM@NPS group.Our fndings indicate the potential of the HGF@ETMM@NPS delivery system as a new treatment method for burn wound healing.
Supplementary Information
Acknowledgements
We thank the Basic Science Research of Nantong Science and Technology Plan Project (JC22022048) and the Research Center of Clinical Medicine of Affiliated Hospital of Nantong University for providing the corre- sponding experimental equipment for this study.
Author contributions
Conceptualization, Q.R.Z. And K.S.H.; methodology, Y.Z.; software, Z.H.Z. and B.L.W.; validation, Z.H.Z., X.H.Z. and S.C.M; formal analysis, D.H.Z. and S.T.Z.; investigation, Z.H.L.; resources,K.S.H. and Y.Z.; data curation, Z.H.Z. and B.L.W.; writing—original draft preparation, Z.H.Z.; writing—review and editing, K.S.H.,Q.R.Z. and Y.Z..; visualization, Z.H.Z. and S.C.M.; supervision, X.H.Z.; project administration, K.S.H.; funding acquisition, K.S.H and Q.R.Z.. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Basic Science Research of Nantong Science and Technology Plan Project (JC22022048).
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical statement
The study is reported in accordance with ARRIVE guidelines.The animal experiments of the study were reviewed and approved by the Animal Experiment Ethics Committee of Nantong University.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhihan Zhu, Xinghua Zhu, Shichen Miao have contributed equally to this paper.
Contributor Information
Yi Zhang, Email: 198zy@163.com.
Qingrong Zhang, Email: 719560340@qq.com.
Kesu Hu, Email: gc-sh@163.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-86716-2.
References
- 1.Costa de Oliveira Souza, C. M. et al. Nanostructured cellulose–gellan–xyloglucan–lysozyme dressing seeded with mesenchymal stem cells for deep second-degree burn treatment. Int. J. Nanomed.16, 833–850. 10.2147/ijn.S289868 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebrahimpour, N. et al. The efficacy of a traditional medicine preparation on second-degree burn wounds in rats. J. Ethnopharmacol.10.1016/j.jep.2020.112570 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Homaeigohar, S., Li, M. & Boccaccini, A. R. Bioactive glass-based fibrous wound dressings. Burns Trauma10, tkac038. 10.1093/burnst/tkac038 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norman, G. et al. Antiseptics for burns. Cochrane Database Syst. Rev.7, CD011821. 10.1002/14651858.CD011821.pub2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shao, Y. et al. Neutrophil extracellular traps contribute to myofibroblast differentiation and scar hyperplasia through the Toll-like receptor 9/Kappanuclear Kappafactor Kappa-B/interleukin-6 pathway. Burns Trauma10, tkac044. 10.1093/burnst/tkac044 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song, H., Yuan, Z., Peng, Y. & Luo, G. Extracorporeal membrane oxygenation combined with continuous renal replacement therapy for the treatment of severe burns: Current status and challenges. Burns Trauma9, tkab017. 10.1093/burnst/tkab017 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Surowiecka, A. et al. Mesenchymal stem cells in burn wound management. Int. J. Mol. Sci.10.3390/ijms232315339 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang, M., Xu, X., Lei, X., Tan, J. & Xie, H. Mesenchymal stem cell-based therapy for burn wound healing. Burns Trauma9, tkab002. 10.1093/burnst/tkab002 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan, Y. et al. Induced pluripotent stem cells-derived microvesicles accelerate deep second-degree burn wound healing in mice through miR-16-5p-mediated promotion of keratinocytes migration. Theranostics10, 9970–9983. 10.7150/thno.46639 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan, X. et al. Strategies for improving adipose-derived stem cells for tissue regeneration. Burns Trauma10, tkac028. 10.1093/burnst/tkac028 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang, C. et al. Chitosan degradation products promote healing of burn wounds of rat skin. Front. Bioeng. Biotechnol.10, 1002437. 10.3389/fbioe.2022.1002437 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao, L. et al. Protective effects of polyphenolic extracts from longan seeds promote healing of deep second-degree burn in mice. Food Funct.10, 1433–1443. 10.1039/c8fo02330a (2019). [DOI] [PubMed] [Google Scholar]
- 13.Zhao, Z. A. et al. Cellular and molecular mechanisms in vascular repair after traumatic brain injury: a narrative review. Burns Trauma11, tkad033. 10.1093/burnst/tkad033 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Żwierełło, W. et al. Burns: Classification, pathophysiology, and treatment: A review. Int. J. Mol. Sci.10.3390/ijms24043749 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeJong, H. et al. Objective quantification of burn scar stiffness using shear-wave elastography: Initial evidence of validity. Burns46, 1787–1798. 10.1016/j.burns.2020.05.009 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Chen, L. et al. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS ONE9, e96161. 10.1371/journal.pone.0096161 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eming, S. A., Murray, P. J. & Pearce, E. J. Metabolic orchestration of the wound healing response. Cell. Metab.33, 1726–1743. 10.1016/j.cmet.2021.07.017 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Goren, I. et al. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am. J. Pathol.175, 132–147. 10.2353/ajpath.2009.081002 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marconi, G. D. et al. Epithelial-Mesenchymal Transition (EMT): The type-2 EMT in wound healing, tissue regeneration and organ fibrosis. Tissue Regener. Organ Fibrosis. Cells10.3390/cells (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas, T. et al. Differential roles of macrophages in diverse phases of skin repair. J. Immunol.184, 3964–3977. 10.4049/jimmunol.0903356 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Narain, A., Asawa, S., Chhabria, V. & Patil-Sen, Y. Cell membrane coated nanoparticles: Next-generation therapeutics. Nanomedicine12, 2677–2692 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Boltnarova, B. et al. PLGA based nanospheres as a potent macrophage-specific drug delivery system. Nanomaterials10.3390/nano11030749 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen, G. et al. Engineering bifunctional calcium alendronate gene-delivery nanoneedle for synergistic chemo/immuno-therapy against HER2 positive ovarian cancer. Adv. Sci. (Weinh)10, e2204654654. 10.1002/advs.202204654 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDougall, S., Dallon, J., Sherratt, J. & Maini, P. Fibroblast migration and collagen deposition during dermal wound healing: mathematical modelling and clinical implications. Philos. Trans. A Math. Phys. Eng. Sci.364, 1385–1405 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Bussolino, F. et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell. Biol.119, 629–641 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto, K. & Nakamura, T. Roles of HGF as a pleiotropic factor in organ regeneration. EXS65, 225–249 (1993). [PubMed] [Google Scholar]
- 27.Choi, J.-S., Heang, Oh. S., Kim, Y.-M. & Lim, J.-Y. Hyaluronic acid/alginate hydrogel containing hepatocyte growth factor and promotion of vocal fold wound healing. Tissue Eng. Regener. Med.17, 651–658. 10.1007/s13770-020-00280-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czyz, M. HGF/c-MET signaling in melanocytes and melanoma. Int. J. Mol. Sci.10.3390/ijms19123844 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nayeri, F. et al. Autocrine production of biologically active hepatocyte growth factor (HGF) by injured human skin. J. Dermatol. Sci.43, 49–56 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Usunier, B. et al. HGF and TSG-6 released by mesenchymal stem cells attenuate colon radiation-induced fibrosis. Int. J. Mol. Sci.10.3390/ijms22041790 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreira, L., Costa, C., Pires, J., Teixeira, J. P. & Fraga, S. How can exposure to engineered nanomaterials influence our epigenetic code? A review of the mechanisms and molecular targets. Mutat. Res./Rev. Mut. Res.10.1016/j.mrrev.2021.108385 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Ou, Z. et al. Macrophage membrane-coated nanoparticles alleviate hepatic ischemia-reperfusion injury caused by orthotopic liver transplantation by neutralizing endotoxin. Int. J. Nanomed.15, 4125–4138. 10.2147/ijn.S253125 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pena, S. A. et al. Gene therapy for neurological disorders: challenges and recent advancements. J. Drug Target.28, 111–128. 10.1080/1061186x.2019.1630415 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Song, Y. et al. Platelet membrane-coated nanoparticle-mediated targeting delivery of Rapamycin blocks atherosclerotic plaque development and stabilizes plaque in apolipoprotein E-deficient (ApoE−/−) mice. Nanomed. Nanotechnol. Biol. Med.15, 13–24. 10.1016/j.nano.2018.08.002 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Sun, J. et al. M2 macrophage membrane-mediated biomimetic-nanoparticle carrying COX-siRNA targeted delivery for prevention of tendon adhesions by inhibiting inflammation. Small10.1002/smll.202300326 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Wang, X.-H. et al. Fibroblast-like cells promote wound healing via PD-L1-mediated inflammation resolution. Int. J. Biol. Sci.18, 4388–4399. 10.7150/ijbs.69890 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smigiel, K. S. & Parks, W. C. Macrophages, wound healing, and fibrosis: Recent insights. Curr. Rheumatol. Reports10.1007/s11926-018-0725-5 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Volk, S. W., Radu, A., Zhang, L. & Liechty, K. W. Stromal progenitor cell therapy corrects the wound-healing defect in the ischemic rabbit ear model of chronic wound repair. Wound Repair Regen15, 736–747 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Yang, L. et al. Recent advances in lipid nanoparticles for delivery of mRNA. Pharmaceutics10.3390/pharmaceutics14122682 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, M., Cheng, S., Jin, Y., Zhang, N. & Wang, Y. Membrane engineering of cell membrane biomimetic nanoparticles for nanoscale therapeutics. Clin. Transl. Med.10.1002/ctm2.292 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao, Y., Ye, W., Wang, Y.-D. & Chen, W.-D. HGF/c-Met: A key promoter in liver regeneration. Front. Pharmacol.10.3389/fphar.2022.808855 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.József, L., Khreiss, T., Fournier, A., Chan, J. S. D. & Filep, J. G. Extracellular signal-regulated kinase plays an essential role in endothelin-1-induced homotypic adhesion of human neutrophil granulocytes. Br. J. Pharmacol.135, 1167–1174 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, Q. et al. Effects of nanoparticle-mediated Co-delivery of bFGF and VEGFA genes to deep burn wounds: An in vivo study. Colloids Surfaces B: Biointerfaces10.1016/j.colsurfb.2021.112135 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Zhou, X., Cao, X., Tu, H., Zhang, Z.-R. & Deng, L. Inflammation-targeted delivery of celastrol via neutrophil membrane-coated nanoparticles in the management of acute pancreatitis. Mol. Pharmac.16, 1397–1405. 10.1021/acs.molpharmaceut.8b01342 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.