Abstract
Tumor dissemination to the central nervous system (CNS) is almost a rule in the treatment journey of advanced HER2+ breast cancer (BC). Recent results demonstrated high intracranial efficacy with Trastuzumab Deruxtecan (T-DXd). However, a real-world evidence is lacking in literature. We conducted a multicenter, observational, retrospective real-world analysis on 39 cases collected at 12 Italian Oncological Units. Patients with brain metastases (BMs) from HER2 + BC treated with T-DXd in various treatment lines were enrolled. Primary endpoint was the intracranial overall response rate (iORR). Secondary endpoints were intra- and global progression free survival (iPFS - gPFS); other secondary objectives were the intracranial disease control rate (iDCR), duration of response (iDoR), clinical benefit rate at 6 and 12 months (iCBr), overall survival, and safety. iORR was 59%, iPFS was 15.6 months, gPFS was 11.8 months. iDCR was 94.9%, iDoR was 11.9 months, and iCBr at 6 and 12 months were 69.2% and 59%, respectively. OS was not reached, with an overall rate of 77.9% of patients alive at 12 months. This study confirmed the high intracranial efficacy and manageable safety profile of T-DXd in this first-ever real world analysis.
Subject terms: Breast cancer, Targeted therapies, Outcomes research
Introduction
Almost 30–50% of patients with human epidermal growth factor (HER2)-positive breast cancer (BC) will develop brain metastases (BMs) in its treatment journey1. This is both related to an improvement in overall survival (OS) with new HER2-targeted agents and to an increased detection rate with the latest imaging techniques2. Despite impressive results achieved for extracranial disease, the central nervous system (CNS) compartment remains highly challenging, because the blood-brain barrier (BBB) limits penetration of many compounds, including chemotherapy and targeted agents3,4. This reflects the poor prognosis of patients with BMs1.
Traditionally, local approaches such as whole-brain radiotherapy (WBRT), stereotactic radiotherapy (SRT), stereotactic radiosurgery (SRS), and neurosurgery have been and are still today a mainstay in BMs treatment5. Indeed, those therapeutical approaches must be associated with a prompt and adequate systemic intervention.
There are growing evidences that support the effectiveness of antibody-drug conjugates (ADCs) for BC BMs in terms of intracranial (IC) response. Ado-trastuzumab emtansine (TDM-1), the first-in class ADC incorporating the HER2-targeted antitumor properties of trastuzumab with the cytotoxic activity of the microtubule-inhibitory agent DM1, demonstrated clinical activity in patients with breast cancer BMs6–8. Trastuzumab Deruxtecan (T-DXd), an ADC consisting of a humanized HER-2 directed monoclonal antibody and deruxtecan, a topoisomerase-I inhibitor, recently obtained impressive results both in heavily pretreated patients that previously received TDM-19 and in the directly comparative DESTINY BREAST03 phase III trial10. In a dedicated subgroup analysis of patients with clinically inactive/asymptomatic BMs of the DB-03 trial, T-DXd demonstrated a clear improvement over TDM-1 in terms of Intracranial Response Rate (IRR, 65.7% versus 34.3%) and global progression-free survival (PFS, 15 [95% confidence interval (CI) 12.5–22.2] versus 3 [95% CI 2.8–5.8] months)11. Although there are some evidences for patients with stable BMs in clinical trials, those with active CNS involvement are often excluded and BM-related outcomes are not often included among study endpoints12,13. This clearly represents an urgent clinical unmet need.
The HER2CLIMB trial paved the way for studies that included patients with active BMs14, demonstrating a clinically and statistically meaningful benefit with the addition of tucatinib, an anti-HER2 tyrosine kinase inhibitor, to trastuzumab and capecitabine: in patients with BMs, progression-free survival (PFS) at 1 year was 24.9% in the tucatinib-combination group and 0% in the placebo-combination group (hazard ratio, 0.48; 95% CI, 0.34 to 0.69; P < 0.001). To date, this is the preferred treatment regimen for patients with active BMs, as intracranial activity of T-DXd is less defined15.
Recently the ROSET-BM trial, that investigated the role of T-DXd in patients with active BMs and leptomeningeal disease (LMD), showed a meaningful activity in this delicate setting, witnessing the drug efficacy regardless of the type of CNS involvement16.
Preliminary data from the DEBBRAH, a non-randomized single-arm design trial, indicated encouraging intra- and extracranial activity of T-DXd in different cohort of pretreated patients: 1, with stable BMs after surgery, SRS/SRT, and/or WBRT; 2, with asymptomatic untreated BMs; 3, with progressing BMs after surgery, SRS/SRT, and/or WBRT17.
Moreover, the TUXEDO trial, an open-label, single-arm, phase 2 study, met its primary endpoint with an intracranial response rate (IRR) of 73.3% in patients with HER2-positive BC and newly diagnosed untreated or progressing after previous local therapy BMs, previous exposure to trastuzumab and pertuzumab and no indication for immediate local therapy18.
Despite encouraging results, these studies are characterized by a small sample size (21 and 15 patients, respectively), and are more useful to pave the way for larger randomized clinical trials rather than to make definitive conclusions.
To date, a real-world evidence focused on intracranial activity for patients with BMs treated with T-DXd is still lacking. We have recently reported the results of an Italian large retrospective analysis (De-Real study), which evaluated the effectiveness and safety of T-DXd in a ‘field-practice’ scenario19.
In an effort to add further information on the clinical activity and safety of T-DXd in both naïve and previously treated patients with BMs, we performed a sub-analysis from our database.
Results
The clinical characteristics of the 39 patients are shown in Table 1. Median age was 55 years (35–72). Thirty-six (92.3%) patients had estrogen and/or progesterone receptor positive tumors, with 23 (59.0%) previously treated with anti-hormonal treatment in adjuvant setting.
Table 1.
Baseline characteristics
| Patients | (n = 39) N (%) |
|---|---|
| Age median (range) | 55 (35–72) |
| Performance Status ECOG (n.; %) | |
| 0 | 12 (30.8) |
| 1 | 20 (51.3) |
| 2 | 7 (17.9) |
| Hormonal Receptor status (n.; %) | |
| Estrogen Positive | 25 (64.1) |
| Progesterone Positive | 21 (53.8) |
| HER2 Status (IHC) (n.; %) | |
| HER2 2 + SISH/FISH Amplification | 6 (15.4) |
| HER2 3+ | 33 (84.6) |
| Previous Neo-adjuvant therapies (n.; %) | 20 (51.3) |
| Trastuzumab with or without Pertuzumab regimens | 10 (25.6) |
| Anthracycline-taxanes regimens | 10 (25.6) |
| Anthracycline-sparing regimens | 0 |
| Previous adjuvant therapies (n.; %) | 24 (61.5) |
| Trastuzumab with or without Pertuzumab regimens | 21 (53.8) |
| Anthracycline-taxanes regimens | 11 (28.2) |
| No anthracycline regimen | 13 (33.3) |
| Previous Hormonal adjuvant therapies (n.; %) | 23 (59) |
| Median lines for metastatic disease (range) | 2 (0–5) |
| Previous anti-HER2 lines for metastatic disease (n.; %) | |
| 0 | 2 (5.1) |
| 1 | 15 (38.5) |
| 2 | 10 (25.6) |
| 3 | 8 (20.5) |
| >4 | 4 (10.3) |
| Previous anti-HER2 therapies for metastatic disease (n.; %) | 37 (94.8) |
| Pertuzumab/trastuzumab/taxane | 33 (84.6) |
| T-DM1 | 21 (53.8) |
| Lapatinib/capecitabine | 12 (30.8) |
| Tucatinib/Lapatinib/capecitabine | 1 (2.6) |
| Trastuzumab + chemotherapy: | 9 (23.1) |
| Paclitaxel | 3 (7.7) |
| Vinorelbine | 3 (7.7) |
| Docetaxel + vinorelbine | 3 (7.7) |
| Trastuzumab + hormonal therapy | 1 (2.6) |
| Median Disease Free Interval from BMs (months, range) | |
| Since initial diagnosis | 46 (0–199) |
| Since metastatic diagnosis | 14 (0–96) |
| No. of brain metastases (n.; %) | |
| 1 | 6 (15.4) |
| 2 | 2 (5.1) |
| 3 | 5 (12.8) |
| 4 | 23 (59.0) |
| Not reported | 3 (7.7) |
| No. of patients with neurological symptoms (n.; %) | |
| Yes | 18 (46.1) |
| No | 21 (53.9) |
| Previous BM local therapy (n.; %) | |
| No therapy | 5 (12.8) |
| SRS | 16 (41.0) |
| WBRT | 13 (33.3) |
| Surgery + SRS | 3 (7.7) |
| Surgery + WBRT | 1 (2.6) |
| WBRT + SRS + surgery | 1 (2.6) |
| BM local therapy during T-DXd (n.; %) | |
| No therapy | 5 (12.8) |
| SRS | 8 (20.5) |
| WBRT | 0 |
| Surgery + SRS | 1 (2.5) |
| Surgery + WBRT | 0 |
| WBRT + SRS + surgery | 0 |
| Extracranial metastatic sites (n.; %) | |
| 0 | 6 (15.3) |
| 1 | 7 (17.9) |
| 2 | 12 (30.8) |
| 3 | 5 (12.8) |
| > =4 | 9 (23.1) |
| Dominant metastatic sites (n.; %) | |
| Liver | 14 (35.9) |
| Lung | 7 (17.9) |
| Bone | 7 (17.9) |
| Soft tissues | 2 (5.1) |
| Brain | 9 (23.1) |
| Median progression Free Survival of previous I line (months and range) | 10 (4–96) |
| Median progression Free Survival of previous II lines (months and range) | 8 (2–29) |
| Time from the end of local brain treatments to T-DXd (median, range) | 7 (0–27) |
| T-DXd line | |
| 1 | 2 (5.1) |
| 2 | 12 (30.8) |
| 3 | 8 (20.5) |
| 4 | 8 (20.5) |
| > =5 | 9 (23.2) |
IHC Immunoistochemistry, SISH/FISH Silver in situ hybridization/Fluorescent in situ hybridization. SRS Stereotactic Radiosurgery, WBRT Whole Brain Radiotherapy.
In the curative setting, 10 (25.6%) patients received both trastuzumab +/− pertuzumab and anthracycline +/− taxanes based regimens as a neoadjuvant treatment, while 24 (61.5%) patients received adjuvant therapy: 21 (53.8%) trastuzumab +/− pertuzumab of which 11 (28.2%) combined to anthracycline and taxanes based-regimens and 13 (33.3%) combined to anthracycline-sparing therapy. In the advanced setting, 37 (94.8%) patients received previous anti-HER2 treatments: 33 (84.6%) trastuzumab + pertuzumab and taxane (docetaxel/paclitaxel), 21 (53.8%) T-DM1, 12 (30.8%) Lapatinib plus capecitabine, and 9 (23.1%) trastuzumab plus chemotherapy regimens. Only 1 (2.6%) patient received trastuzumab plus anti-hormonal therapy.
All patients in the sample size received standard therapy, none of them was involved in a clinical trial before or after receiving T-DXd.
Before administering T-DXd, median PFS (mPFS) of first line treatments was 10 months (4–96), while mPFS of second line treatments was 8 months (2–29). Median previous treatment lines for HER2+ metastatic disease was 2 (0–5); T-DXd was administered as a first line treatment for advanced disease in 2 patients (5.1%), as a second line in 15 patients (38.5%), as a third, fourth, fifth or later line in 10 patients (25.6%), 8 patients (20.5%) and 4 patients (10.3%), respectively.
Six (15.4%) out of 36 patients had only one BM, 2 (5.1%) 5 (12.8%) patients had two and three BMs, respectively, while most patients (23, 59.0%) had four or more brain lesions. The exact number of BMs was not reported for three (7.7%) patients. Eighteen (46.1%) patients reported neurological symptoms while starting T-DXd.
Thirty-four (87.2%) patients received one or more local approach for intracranial control of brain lesions, with most of them receiving Stereotactic Radiosurgery [SRS (16 (41%) and WBRT (15, 38.4%)]. Five (12,8%) had no previous locoregional treatment before T-DXd administration. During T-DXd, three of these five patients received SRS for active lesions, while in two patients, after careful evaluation with the radiotherapist, the choice was to defer local treatment at neurological symptoms onset. Among treated patients, there was no delay in T-DXd administrations as RT was performed inter-cycles, with no added toxicities. Among already treated patients, nine (23%) had new or progressive BMs not subjected to CNS directed therapy since documented progression, for a total of fourteen (35,8%) patients with untreated/active BMs. Five out of nine were treated with new SRS, one with radiosurgery and three were discussed at the multidisciplinary meeting with a decision to defer local treatment.
The median time from the end of local brain treatments to the first administration of T-DXd was 7 months (range 0–27). Six (15.3%) patients had no evidence of extracranial involvement, whilst 7 (17.9%), 12 (30.8%), 5 (12.8%), and 9 (23.0%) patients had one, two, three, and four or more extracranial metastatic sites, respectively.
Intracranial efficacy
Regarding the single outcomes, one patient (2.6%) achieved a complete response (CR), twenty-two (56.4%) a partial response (PR, for an overall response rate of 59.0%), fourteen patients (35.9%) had a stable disease (SD), while two showed (5.1%) an intracranial progressive disease (PD) (Table 2). When considering the treatment line, there were one PR and one SD in first line, eleven PR and one SD in second line, one CR, five PR, and two SD in third line, three PR, four SD, and one PD in fourth line, two PR, six SD, and one PD in fifth or later lines (Table 6).
Table 2.
Intracranial and global best responses with T-DxD (39 patients)
| Response to T-Dxd | Intracranial Best Response n. (%) | Global Best Response n. (%) |
|---|---|---|
| Complete Response | 1 (2.6) | 0 |
| Partial Response | 22 (56.4) | 27 (69.2) |
| Overall Response rate | 23 (59) | 27 (69.2) |
| Stable Disease | 14 (35.9) | 10 (25.6) |
| Progressive disease | 2 (5.1) | 2 (5.1) |
Table 6.
Intracranial Efficacy of T-DxD based on treatment line
| Response to T-Dxd | N. of treatment line | ||||
|---|---|---|---|---|---|
| I line (n = 2) | II line (n = 12) | III line (n = 8) | IV line (n = 8) | >IV line (n = 9) | |
| Complete Response | 0 | 0 | 1 | 0 | 0 |
| Partial Response | 1 | 11 | 5 | 3 | 2 |
| Stable Disease | 1 | 1 | 2 | 4 | 6 |
| Progressive disease | 0 | 0 | 0 | 1 | 1 |
| Median icPFS (range) | NE | 14.2 (NE) | 15.5 (10.1–21.1) | 11.1 (6.8–15.6) | 16.5 (NE) |
icPFS intracranial progression free survival, NE not evaluable.
At a median follow up of 12 months, intracranial mPFS was 15.6 months (10.5–20.8). iDCR was 94.9% (87.9–100.0), iDoR was 11.9 months (10.1–13.7), and iCBr at 6 and 12 months were 69.2% and 59%, respectively. OS was not reached, with an overall rate of 77.9% of patients alive at 12 months.
When dividing patients according to the intracranial responses (CR/PR vs SD/PD), a statistically significant difference both in global mPFS [15.5 (13.7–17.3) vs 7.9 (6.4–9.5), p = < 0.001)] and icPFS [15.8 (14.1–17.5) vs 11.2 (3.2–19.2), p = 0.01] was found (Fig. 1a and 1b, respectively).
Fig. 1. Survival outcome of patients treated with Trastuzumab Deruxtecan according to treatment response.
a Global progression free survival of intracranial responders vs no responders (Kaplan–Meier curve). Responders : patients achieving intracranial complete or partial response (total 23). Non-Responders: patients with an intracranial stable disease or progression (total 16). b Intracranial Progression Free Survival of Intracranial Responders vs no Responders (Kaplan-Meier Curve). Responders : patients achieving intracranial complete or partial response (total 23). Non-Responders: patients with an intracranial stable disease or progression (total 16).
No difference (p = 0.75) were observed in mPFS between patients that did [15.6 (10.8–20.4)] or did not [15.8 (n.e.)] receive a local approach.
When dividing patients for treated/stable (25) or untreated/active (14), BMs, there were 1 (4%) and 0 CRs, 12 (48%) and 10 (71.4%) PRs, 12 (48%) and 2 (14.3%) SDs, 0 and 2 (14.3%) PDs, respectively. In these two groups of patients, iDCR was 100% and 85.7%, iDoR was 11.9 months (NE) and 14.0 months (9.6–18.4), iCBr at 6 and 12 months were 68% and 71.4%, and 68%, 42.8% respectively.
Data regarding global intracranial activity and according to BMs status are summarized in Tables 3–5, respectively.
Table 4.
Intracranial Efficacy of T-Dxd in treated/stable BMs
| Outcome measure | |
|---|---|
| mPFS (months) | 15.6 (95% CI: 10.6–20.7) |
| Disease Control Rate (%) | 100 |
| Duration of Response (months) | 11.9 (N.E.) |
| Clinical Benefit n. (%) | |
| 6 months | 17 (68.0) |
| 12 months | 17 (68.0) |
| Overall Survival at 12 months (%) | 84.0 |
Table 3.
Intracranial Efficacy of T-Dxd
| Outcome measure | |
|---|---|
| mPFS (months) | 15.6 (95% CI: 10.5–20.8) |
| Disease Control Rate (%) | 94.9 (95% CI: 87.9–100.0) |
| Duration of Response (months) | 11.9 (95% CI: 10.1–13.7) |
| Clinical Benefit (%) | |
| 6 months | 27 (69.2) |
| 12 months | 23 (59.0) |
| Overall Survival at 12 months (%) | 76.6 |
Table 5.
Intracranial Efficacy of T-Dxd in untreated/active BMs
| Outcome measure | |
|---|---|
| mPFS (months) | 14.2 (95% CI: 8.0–20.5) |
| Disease Control Rate (%) | 85.7 (95% CI: 67.4–100) |
| Duration of Response (months) | 14.0 (95% CI: 9.6–18.4) |
| Clinical Benefit n. (%) | |
| 6 months | 10 (71.4) |
| 12 months | 6 (42.9) |
| Overall Survival at 12 months (%) | 62.9 |
Global (intra + extracranial) activity
For global disease activity, no CR was observed, while 27 (69.2%) PR, 10 (25.6%) SD, and 2 (5.1%) PD were documented. Results of the best intra and global responses are summarized in Table 3. Data between the two groups were substantially balanced in terms of responses.
When considering global activity, there were 2 PR in first line, 10 PR and 2 SD in second line, 7 PR and 1 SD in third line, 4 PR, 3 SD, and 1 PD in fourth line, 4 PR, 4 SD, and 1 PD in fifth or later lines. Intra- and global activity of T-DXd according to treatment line is available at Tables 6 and 7.
Table 7.
Global Efficacy of T-DxD based on treatment line
| Response to T-Dxd | N. of treatment line | ||||
|---|---|---|---|---|---|
| I line (n = 2) | II line (n = 12) | III line (n = 8) | IV line (n = 8) | >IV line (n = 9) | |
| Complete Response | 0 | 0 | 0 | 0 | 0 |
| Partial Response | 2 | 10 | 7 | 4 | 4 |
| Stable Disease | 0 | 2 | 1 | 3 | 4 |
| Progressive disease | 0 | 0 | 0 | 1 | 1 |
| Median PFS (range) | NE | 14.1 (6.8–21.3) | 15.5 (8.5–22.4) | 11.1 (6.9–15.4) | 7.9 (5.8–10.6) |
PFS progression free survival, NE not evaluable.
Considering all treatment lines together, global mPFS was 11.8 months (8.5–15.0).
Safety
Median treatment duration with T-DXd was 9.5 months (range: 1.5–16.5).
Thirty-two patients (82%) experienced an any grade toxicity from treatment with T-DXd.
The main grade 1/2 hematological toxicities were neutropenia (35.9%) and anemia (23.1%). Grade 1/2 non-hematological adverse events (AEs) that occurred in more than 10% of the patients were alopecia (59%), fatigue (53.8%), nausea (46.1%), constipation (30.7%), diarrhea (28.2%), and vomiting (10.2%).
Grade 3/4 hematological toxicities consisted of neutropenia (15.3%), anemia (5.1%) and thrombocytopenia (2.5%). Grade 3/4 non-hematological AEs were fatigue (18%), diarrhea (10.2%), nausea (7.7%), mucositis (5.1%), vomiting (2.5%), pneumonitis (2.5%), and increase in liver transaminase (2.5%).
With regard to AEs of special interest, grade 1 drop of LVEF was observed in one patient (2.5%).
Toxicities are available in the supplementary material (Table 1 suppl.).
Discussion
T-DXd has revolutionized the treatment algorithm of HER2-positive advanced BC, and there are growing evidences that support its use even in patients with intracranial disease. To date, there was no evidence of outcome in HER2 positive advanced BC patients with BMs in a real world population. A recent retrospective exploratory pooled analysis of DESTINY BREAST 01, 02, and 03 trials20 showed superior rates in terms of IC response over comparator in patients with treated/stable and untreated/active BMs, with a particularly favourable trend in iDoR and imPFS for the latter ones. This data strengthen our beliefs that T-DXd will become a mainstay in our clinical practice even in these crucial subset of patients.
It is important to note that in the DESTINY BREAST 02 and 03 trials patients with previously untreated and asymptomatic BM were eligible but, after protocol amendments, only patients with treated, asymptomatic BMs were allowed10,21. Therefore, the population of patients with baseline BMs consists of a mix of treated/stable and untreated/active metastases.
This pooled analysis showed that patients in both groups achieved an overall response rate of 45.2% and 45.5% for treated/stable and untreated/active BMs cohorts, respectively. Intracranial CR rates were 16.3% and 15.9%, while iPR rates were 28.8% and 29.5%, respectively20.In our study, we registered significantly lower rates of intracranial CR (4% and 0) and higher rates of PR (48% and 71.4%), respectively; data for SD were substantially in line with Hurvitz et al. results (48% in our study and 46.2% in the pooled analysis) in patients with treated/stable lesions, but quite differ in those with untreated/active metastases (14.3% vs 34.1%,). These differences may rely on the unselected cohort and global lower number of patients in our real life study, and probably also on an overall good clinical performance status with lower local suffering of brain tissue (53.9% absence of neurological symptoms), rather than in a true difference in the drug activity for the different cohorts.
Data on iDCR and iDoR were substantially similar between our study and the pooled analysis, endorsing the high intracranial activity of T-DXd, with a mPFS of over 15 months.
Moreover, compared to the pooled analysis, we also provided data on CBR and OS.
It is important to note that outcomes were independent from treatment line, and sustained responses documented that the drug was globally safe in terms of timing and dosing.
In Table 8 a cautios comparison between real life and trial disease outcomes is listed.
Table 8.
Comparison between intracranial outcomes in DE-REAL and Destiny Breast 01-02-03 pooled analysis
| Outcome measure (n. of patients) | DE -REAL real life (n.39) | DE-REAL treated/stable BM (n.25) | DE-REAL untreated/active BM (n.14) | DB pooled analysis, treated/stable BMs (n.104) | DB pooled analysis, non-treated/unstable BMs (n.44) |
|---|---|---|---|---|---|
| ORR, n (%) | 23 (59.0) | 13 (52.0) | 10 (71.4) | 47 (45.2) | 20 (45.5) |
| Complete response, n (%) | 1 (2.6) | 1 (4.0) | 0 | 17 (16.3) | 7 (15.9) |
| Partial response, n (%) | 22 (56.4) | 12 (48.0) | 10 (71.4) | 30 (28.8) | 13 (29.5) |
| Stable disease, n (%) | 14 (35.9) | 12 (48.0) | 2 (14.3) | 48 (46.2) | 15 (34.1) |
| Progressive disease, n (%) | 2 (5.1) | 0 | 2 (14.3) | 3 (2.9) | 1 (2.3) |
| Duration of response (months), median (95% CI) | 11.9 (10.1–13.7) | 11.9 (N.E.) | 14.0 (9.6–18.4) | 12.3 (9.7–17.9) | 17.5 (13.6–31.6) |
| PFS (months), median (95% CI) | 15.6 (10.5–20.8) | 15.6 (10.6–20.7) | 14.2 (8.0–20.5) | 12.3 (11.1–13.8) | 18.5 (13.6–23.3) |
| Disease control rate (%,95% CI) | 94.9 (87.9–100.0) | 100 | 85.7 (67.4–100) | 91.3 (NA) | 79.6 (NA) |
| Clinical benefit (%) | |||||
| 6 months | 27 (69.2) | 17 (68.0) | 10 (71.4) | NA | NA |
| 12 months | 23 (59.0) | 17 (68.0) | 6 (42.9) | NA | NA |
| Overall Survival at 12 months (%) | 76.6 | 84.0 | 62.9 | NA | NA |
BMs Brain Metastases, DB Destiny Breast, NA Not available, ORR Overall Response Rate, PFS Progression-free survival.
When comparing intracranial responders (CR/PR) to non-responders (SD/PD), a clear improvement not only in global mPFS [15.5 (13.7–17.3) vs 7.9 (6.4–9.5), p = < 0.001)] but also in icPFS [15.8 (14.1–17.5) vs 11.2 (3.2–19.2), p = 0.01] was achieved. This witnesses and supports the strong prognostic value of intracranial response, regardless of treatment line. It is clear that, with only 2 patients that experienced intracranial PD, no correct comparison can be made with all other patients that had at least a SD.
Interestingly, no difference in mPFS was found for patients that did or did not receive a local approach for BMs (15.8 vs 15.6 months p = 0.45). This probably reflects the fact that there are resistance mechanisms related to pharmacological pressure, with intratumoral heterogeneity and number of previous anti-HER2 lines received that certainly play a role, along with the efficacy of T-DXd in this district.
The recent findings of phase 3b/4 single arm Destiny-Breast12 trial22 confirmed the activity and efficacy of T-Dxd in the largest prospective BC population with stable/active (263) and without (241) BMs and treated with one or more prior anti-HER2-based regimens. Most of BMs patients received previous loco-regional therapies. In the BMs cohort, 12-month PFS was 61.6% (95% confidence interval (CI): 54.9–67.6), and 12-month CNS PFS was 58.9% (95% CI: 51.9–65.3). In this cohort icRR was 72%, at 12 months icPFS and overall survival were 59% and 90%, respectively. It is important to note that a direct comparison between Destiny-Breast12 and our findings is not methodologically correct due to the nature of the studies (prospective vs retrospective), the total number of patients and the differences in the data analysis.
Our findings seem to endorse the concept that we are moving to a “paradigm shift”, in which a RT deferral may be a valid option for certain patients with BMs who are eligible for a systemic therapy with a strong intracranial activity23. We obviously must consider that only 5 patients did not receive a local approach for BMs in our study before T-DXd administration, therefore these data needs careful and proper evaluation, but we need to point out that in three patients that were already treated the choice was to defer local approach, with subsequent positive outcomes. In the TUXEDO and DEBBRAH trials there were no comparison between patient that did or did not receive local treatment in terms of survival, therefore we do not have reliable data.
Toxicities were substantially in line with what was previously reported with the use of T-DXd in various settings. After growing experience with the drug, it is clear that there is an improvement in the support care management for signs and symptoms related to T-DXd, in particular nausea/vomiting and neutropenia. Also the incidence of lung infections were poorly detected (moderate/severe grade: 2.5%), even in this at-risk population (disease spreading, both systemic and locoregional previous treatments). It is important to highlight that median treatment duration was 9.5 months, that is substantially in line with what was previously reported for heavily pretreated patients in the DESTINY BREAST-04 trial (8.2 months)24. Due to the multi-line scenarios described in our dataset, a more detailed description of the single toxicity for the single treatment line was too complex (and essentially not so useful) to perform. Neurological symptoms were present in nearly half of the patients (18) at T-DXd start, but there were no new neurological safety signals during treatment.
This real world analysis have its limitations. First of all, real world data were retrospectively collected from clinical databases that were originally not designed to collect data for research. The retrospective design presents threats to both internal and external validity. Moreover, due to its nature there was no Blinded Independent Central Review (BICR) to determine disease progression; for a better definition of the intracranial activity following RANO Criteria, we decided to perform a central revision of the brain MRI. This could be one of the reasons why there were differences between our results and the already cited pool analysis performed by Hurvitz et al.
It is also important to highlight that in our database there was only one patient with leptomeningeal disease (LMD) that was potentially includible but never received any administration of T-DXd (lost at follow up). Therefore, we did not include the patient in this analysis. Data on LMD patients are dramatically needed to support the use of effective drugs even in this specific clinical scenario.
Also, this analysis lacks central pathology review, but we have to highlight that all the italian centers involved in the data collection are high-expertise certified hubs for pathology units. Indeed, differences in interpretation of the single sample could obviously exist in this context.
However, there are several strenghts. To our knowledge, this is the first real world analysis that investigates intracranial outcomes with the use of T-DXd, with a larger sample size of patients than the already cited DEBBRAH and TUXEDO trials. Moreover, patients were properly evaluated with brain MRI to perform a correct evaluation of the intracranial compartment, and this should be emphasized as in routinary clinical practice the whole body TC scan is the mainstay for practical reasons and MRI is often used solely to deepen the TC results. Furthermore, as for some real life evidences, more outcomes measures are investigated, such as the duration of response, the disease control rate and the clinical benefit other than progression free survival.
In conclusion, due to the intrinsic therapeutic power of this drug, we are witnessing an intracranial benefit from a systemic therapy; this is important as it not only delays loco-regional treatment in some cases, but also improves quality of life in patients with intracranial responses. Data from the DEBBRAH and TUXEDO-1 trials documented a preserved QoL and maintened neurocognitive functions in patients treated with T-DXd, both in standard questionnaires and dedicated neurocognitive tests18,19,25. Although we cannot make definitive conclusions in our study as we did not systematically collect data on QoL, a preserved neurocognitive function with no new safety signals endorse the concept that T-DXd helps maintaining and improving QoL.
The oncological community is waiting for the results of T-DXd in the early setting [Destiny BREAST-011 (NCT202100060321), and SHAMROCK (NCT05710666) trials in neoadjuvant setting; Destiny BREAST-05 (NCT04622319) trial in adjuvant setting] that will also provide information on the potential ability of the drug to prevent or delay CNS spreading in HER2 positive BC.
In the context of a real-world study, our data supports the use of T-DXd for both patients with treated/stable and untreated/active BMs.
Methods
Data retrieval
This study was a national multicenter, observational, and retrospective analysis (De-Real study n. 0181/2022) conducted on cases collected at 12 Italian Oncological Units, reviewed to identify HER2-positive BC and BMs patients treated with T-DXd between April 2021 and February 2023 (updated data compared to the first publication). The search rendered a total of 39 out of 153 patients (Fig. 2). Eligible patients were required to have a diagnosis of HER2-positive tumor, determined locally and defined as 3+ immunohistochemical (IHC) staining (HercepTest; Dako A/S, Glostrup, Denmark) or 2 + IHC staining and HER2/Vep17 Ratio > 2 at fluorescence in situ hybridization (FISH) (PathVision HER2 DNA probe kit; Vysis Inc., Downers Grove, IL).
Fig. 2.
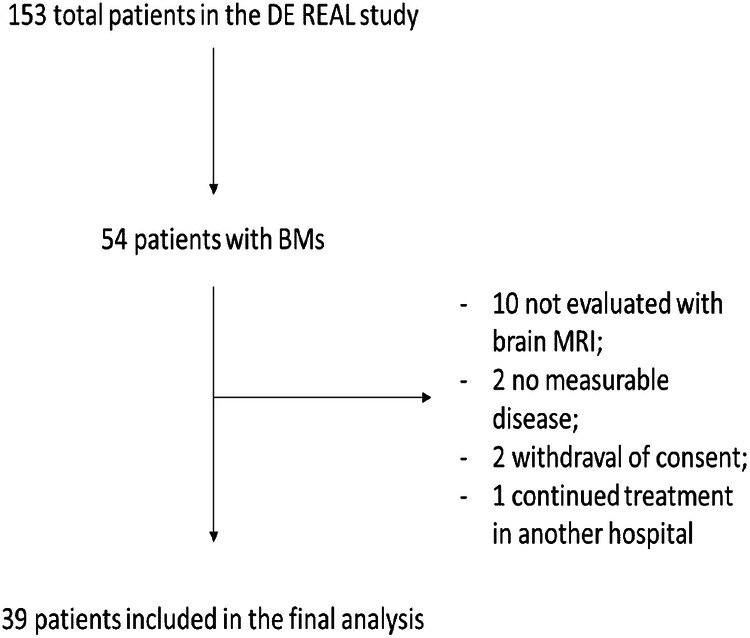
Patients selection.
All patients were age > 18 years and were treated with at least one dose of T-DXd.
Only patients with measurable and/or evaluable advanced disease were included. No restrictions were made based on previous lines of therapy or local treatment received for metastatic disease. Progressive and/or recurrent disease prior to T-DXd initiation had to be documented in all cases.
Only patients who were evaluated with brain magnetic resonance imaging (MRI) for CNS were eligible for the study. Brain MRI was used to assess brain involvement, whereas chest and abdomen tomography scans were used to assess all extracranial metastatic sites.
The BM status was determined according to the following definitions by the US FDA Clinical Trial Eligibility Criteria26: treated/stable BMs, in patients with prior CNS-directed therapy for BMs, and CNS stable disease; untreated/active BMs, in patients with new or progressive BMs not subjected to CNS directed therapy since documented progression.
Radiological restaging was performed every three cycles of T-DXd (12 weeks of therapy). Due to the retrospective nature of the study, intra- and extracranial responses were assessed based on the recommendations of the Response Criteria in Solid Tumors 1.1 (RECIST 1.1)27. For a more appropriate intracranial response assessment, a local image review was performed according to the Response Assessment in Neuro-Oncology- Brain Metastases (RANO-BM) criteria28.
This study was developed in agreement with the recent ESMO Guidance for Reporting Oncology real-World evidence (GROW) guidelines for descriptive research29.
The study was conducted in accordance with the ethical standards of the Declaration of Helsinki and its subsequent amendments and within the protocol approved by the ethics committee (Policlinico Umberto I Hospital University, examination number: 0181/2022) and all other institutions: Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Istituto Di Ricovero E Cura A Carattere Scientifico (IRCCS) Pascale, Naples, Italy; Fondazione Policlinico Universitario Campus Bio-Medico, Rome, Italy; Sandro Pertini Hospital, Rome Italy; Dario Camberlingo Hospital, Francavilla Fontana, Brindisi, Italy; Central Hospital Of Belcolle, Viterbo, Italy; Fondazione Irccs Istituto Nazionale Dei Tumori, Milan, Italy; IRCCS Istituto Romagnolo Per Lo Studio Dei Tumori “Dino Amadori” IRST, Meldola, Italy; Regina Elena National Cancer Institute Rome, Italy; Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy. All alive patients provided written informed consent to the use of medical records for research purposes.
Treatment schedule
Patients received T-DXd at the standard dose of 5.4 mg per body weight kilogram every 3 weeks, until progression, unacceptable toxicity, patient’s or physician’s request to discontinuation or patient refusal. Antiemetic and concomitant treatments that did not interfere with the drug, including the use of both G-CSF and bisphosphonates therapy, were allowed.
Study objectives and outcomes measures
The primary objective of the study was to evaluate the efficacy of T-DXd on BMs in a real-world population, in terms of intracranial responses (complete and partial responses). The main secondary endpoints were intra- and global progression free survival (iPFS - gPFS) defined as the time elapsed between the first dose of T-DXd and brain-only or intra-extracranial progression of the disease and death30, respectively.
Other secondary objectives were: the intracranial disease control rate (iDCR: the sum of complete, partial and stable diseases), intracranial duration of response (iDoR: the time from the first radiological evidence of response to the progression), intracranial clinical benefit rate at 6 and 12 months (iCBr: the sum of the complete, partial and stable disease rates over a certain time period) and the overall survival (OS, the time elapsed between the first dose of T-DXd and death from any cause or censoring at the time of last follow-up). Treatment-related adverse events (AEs) were categorized and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 5.031. AEs were collected at every clinical evaluation performed in the referral centers.
Statistical analysis
Data were summarized by absolute counts and percentages when related to categorical item and with median and range in case of quantitative variables. Survival curves were estimated using the Kaplan-Meier method and median values were reported alongside their 95% confidence intervals. Differences between survival curves were assessed by the log-rank test.
Supplementary information
Acknowledgements
This study was funded by the Italian minister of health – Ricerca Corrente 2023. We would like to thank Mrs Elisabetta Bozzoli for the data management.
Author contributions
Conceptualization, Methodology, Investigation, Writing, review, and editing. Visualization: A.F., A.R., D. G., S.P., A.B. Investigation: A.F., A.R, R.C., S.P., F.P., G.D., P.F, A.F.,C.V.,M.P.,L.C.,G.F.,E.DM., I.P., F.P.,O.G.,D.G.,M.DL., G.F., G.S., A.B. Manuscript Editing: All ; Approval to submission: All.
Data availability
The data that support the findings of this study are available from the first author, AF and corresponding author, AR, upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alessandra Fabi, Alessandro Rossi.
Supplementary information
The online version contains supplementary material available at 10.1038/s41698-025-00801-3.
References
- 1.Garcia-Alvarez, A., Papakonstantinou, A. & Oliveira, M. Brain metastases in HER2-positive breast cancer: current and novel treatment strategies. Cancers13, 2927 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venur, V. A. & Leone, J. P. Targeted therapies for brain metastases from breast cancer. Int J. Mol. Sci.17, 1543 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurvitz, S. A. et al. Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from SystHERs. Clin. Cancer Res.25, 2433–2441 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Steeg P. S. The blood-tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 18, 696–714 (2021). [DOI] [PubMed]
- 5.Soffietti, R. et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro. Oncol.19, 162–174 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartsch, R. et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin. Exp. Metastasis32, 729–737 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Jacot, W. et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res. Treat.157, 307–318 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Fabi, A. et al. T-DM1 and brain metastases: Clinical outcome in HER2-positive metastatic breast cancer. Breast41, 137–143 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med.382, 610–621 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortés, J. et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med.386, 1143–1154 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Hurvitz, S. A. et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer patients with brain metastases from the randomized DESTINY-Breast03 trial. Original Artic.9, 102924 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin, N. U., Gaspar, L. E. & Soffietti, R. Breast cancer in the central nervous system: multidisciplinary considerations and management. Am. Soc. Clin. Oncol. Educ. Book37, 45–56 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Patel, R. R. et al. Exclusion of patients with brain metastases from cancer clinical trials. Neuro Oncol.22, 577–579 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murthy, R. K. et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med382, 597–609 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Stavrou, E., Winer, E. P. & Lin, N. U. How we treat HER2-positive brain metastases. ESMO Open6, 100256 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niikura, N. et al. Treatment with trastuzumab deruxtecan in patients with HER2-positive breast cancer and brain metastases and/or leptomeningeal disease (ROSET-BM). NPJ Breast Cancer9, 82 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-García J. M., et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro. Oncol. noac144 (2022). [DOI] [PMC free article] [PubMed]
- 18.Bartsch, R. et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat. Med28, 1840–1847 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botticelli, A. et al. Real-World Outcomes of Trastuzumab Deruxtecan in Patients With HER2+ Metastatic Breast Cancer: The DE-REAL Study. Oncologist, (2023). [DOI] [PMC free article] [PubMed]
- 20.Hurvitz, S. A. et al. A pooled analysis of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-positive (HER2+) metastatic breast cancer (mBC) with brain metastases (BMs) from DESTINYBreast (DB) -01, -02, and -03. Ann. Onc. Abstr. 377O34, s335–s336 (2023). [Google Scholar]
- 21.André, F. et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet401, 1773–1785 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Harbeck N., et al. Trastuzumab deruxtecan in HER2-positive advanced breast cancer with or without brain metastases: a phase 3b/4 trial. Nat Med. 2024 Sep 13. 10.1038/s41591-024-03261-7. [DOI] [PMC free article] [PubMed]
- 23.Ippolito, E. et al. Radiotherapy for HER 2 positive brain metastases: urgent need for a paradigm shift. Cancers (Basel)14, 1514 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med387, 9–20 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starzer, A. M. et al. Quality of life and neurocognitive function in patients with active brain metastases of HER2-positive breast cancer treated with trastuzumab-deruxtecan: Secondary endpoint analysis of the prospective single-arm phase II TUXEDO-1 trial. Ann. Onc.33, s668 (2022). [Google Scholar]
- 26.-] U.S. Department of Health and Human Services – Food and Drug Administration. Cancer Clinical Trial Eligibility Criteria: Brain Metastases. 2020.
- 27.Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer45, 228–247 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Lin, N. U. et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol.16, e270–e278 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Castelo-Branco, L. et al. ESMO Guidance for Reporting Oncology real-World evidence (GROW). Ann. Oncol.34, 1097–1112 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Schwartz, L. H. et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer62, 132–137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institute of Health. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. NIH Publication (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the first author, AF and corresponding author, AR, upon reasonable request.



