Abstract
1. Single latent pacemaker cells were isolated from cat right atrium, and studied in a whole-cell configuration using a nystatin-perforated patch recording method. The nystatin method avoids alterations in intracellular Ca2+, cellular constituents and run-down of ionic currents. 2. Depolarizing voltage clamp pulses from -40 mV elicited L-type Ca2+ current (ICa) that exhibited an initial rapid phase of inactivation followed by a secondary slower inward current component that decayed over about 100 ms. The secondary inward component appeared as a slowly decaying inward tail current following short (10-40 ms) depolarizing clamp steps. 3. Slowly decaying inward currents were abolished by internally dialysing pacemaker cells with 2 mM EGTA using a ruptured patch recording method. Inward tail currents were also abolished by exposure to 1 microM ryanodine and significantly decreased by replacing 85% of external Na+ with lithium, without effect on peak ICa. These findings identify a Na(+)-Ca2+ exchange current (INa-Ca) that is mediated by sarcoplasmic reticulum (SR) Ca2+ release. 4. Properties of INa-Ca and ICa differed significantly: (i) ICa exhibited a bell-shaped voltage dependence that peaked at 0 mV and decreased at more positive voltages. INa-Ca was maximal at -10 mV and remained relatively constant at more positive voltages; (ii) a paired pulse protocol showed that the time course of INa-Ca recovery (5 s) was significantly longer than that of ICa (2 s); (iii) cadmium (50 microM) induced an inhibition of ICa that did not correlate in time with changes in INa-Ca. 5. The duration of depolarizing steps between 10 and 120 ms had no effect on the time course of INa-Ca tail currents. 6. Isoprenaline > or = 5 x 10(-8) M significantly increased peak ICa amplitude, peak INa-Ca amplitude, accelerated INa-Ca rate of decay and decreased the absolute time of INa-Ca decay. 7. Free-running pacemaker action potentials were clamped during diastole at either -40 or -70 mV (maximum diastolic potential) for variable periods of time. At times between 0.2 and 1 s, INa-Ca exhibited a voltage-dependent increase in amplitude over time, i.e. INa-Ca recovered more rapidly from -70 mV than from -40 mV. At times > 2 s, INa-Ca exhibited a voltage-dependent decline in amplitude over time, i.e. from -40 mV INa-Ca decreased by 10% of maximum whereas from -70 mV INa-Ca decreased by 60% of maximum.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








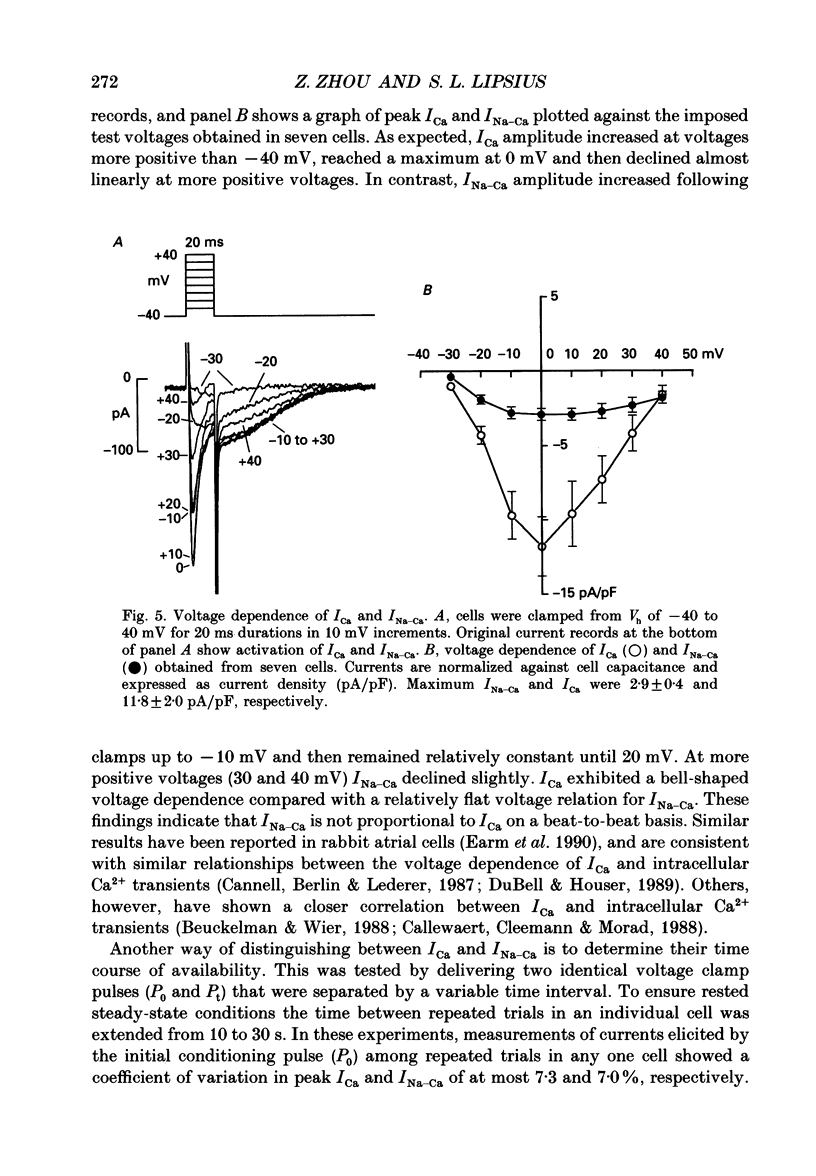



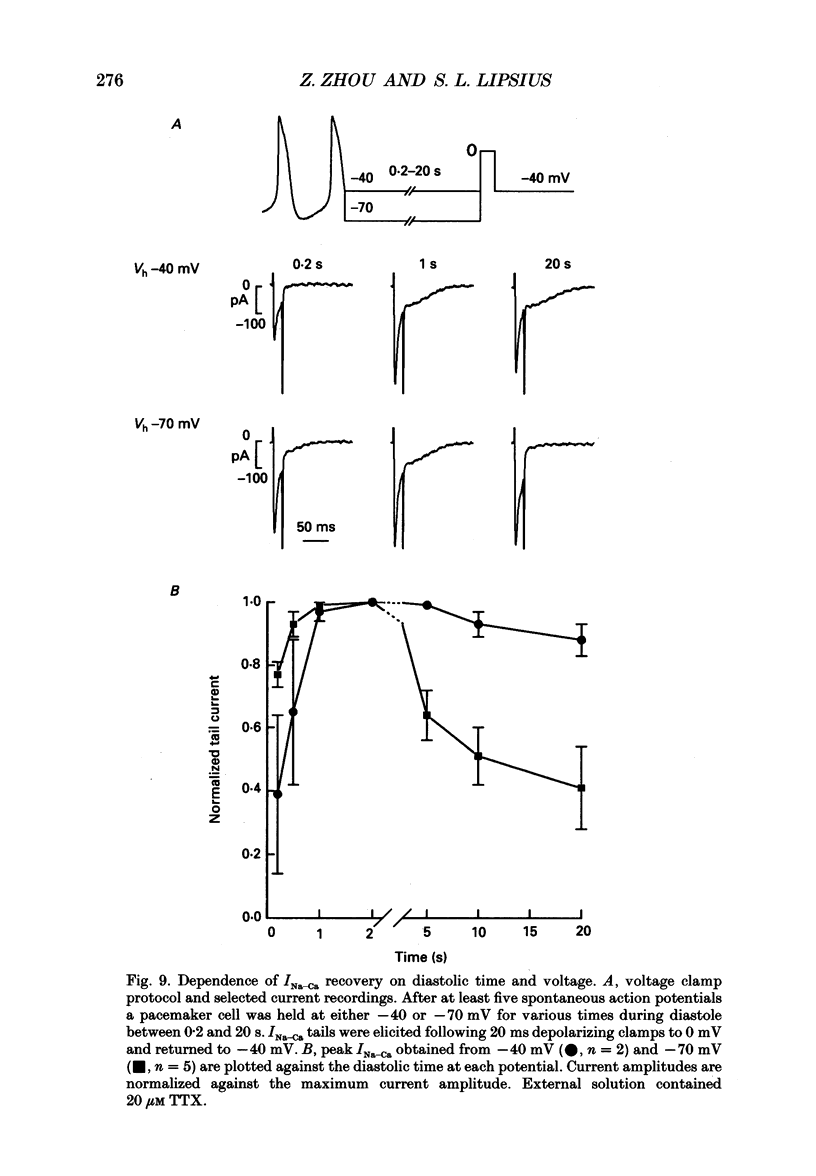









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barcenas-Ruiz L., Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in heart: membrane currents and changes in [Ca2+]i. Science. 1987 Dec 18;238(4834):1720–1722. doi: 10.1126/science.3686010. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Bridge J. H. Relaxation of rabbit ventricular muscle by Na-Ca exchange and sarcoplasmic reticulum calcium pump. Ryanodine and voltage sensitivity. Circ Res. 1989 Aug;65(2):334–342. doi: 10.1161/01.res.65.2.334. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in guinea-pig cardiac cells: exchange current and changes in intracellular Ca2+. J Physiol. 1989 Jul;414:499–520. doi: 10.1113/jphysiol.1989.sp017700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Kimura J., Noble D., Noble S. J., Taupignon A. The ionic currents underlying pacemaker activity in rabbit sino-atrial node: experimental results and computer simulations. Proc R Soc Lond B Biol Sci. 1984 Sep 22;222(1228):329–347. doi: 10.1098/rspb.1984.0067. [DOI] [PubMed] [Google Scholar]
- Brown H. F., Kimura J., Noble D., Noble S. J., Taupignon A. The slow inward current, isi, in the rabbit sino-atrial node investigated by voltage clamp and computer simulation. Proc R Soc Lond B Biol Sci. 1984 Sep 22;222(1228):305–328. doi: 10.1098/rspb.1984.0066. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Cleemann L., Morad M. Epinephrine enhances Ca2+ current-regulated Ca2+ release and Ca2+ reuptake in rat ventricular myocytes. Proc Natl Acad Sci U S A. 1988 Mar;85(6):2009–2013. doi: 10.1073/pnas.85.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Crespo L. M., Grantham C. J., Cannell M. B. Kinetics, stoichiometry and role of the Na-Ca exchange mechanism in isolated cardiac myocytes. Nature. 1990 Jun 14;345(6276):618–621. doi: 10.1038/345618a0. [DOI] [PubMed] [Google Scholar]
- Earm Y. E., Ho W. K., So I. S. Inward current generated by Na-Ca exchange during the action potential in single atrial cells of the rabbit. Proc R Soc Lond B Biol Sci. 1990 May 22;240(1297):61–81. doi: 10.1098/rspb.1990.0027. [DOI] [PubMed] [Google Scholar]
- Earm Y. E., Noble D. A model of the single atrial cell: relation between calcium current and calcium release. Proc R Soc Lond B Biol Sci. 1990 May 22;240(1297):83–96. doi: 10.1098/rspb.1990.0028. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Spindler A. J., Twist V. W. Sodium-calcium exchange during the action potential in guinea-pig ventricular cells. J Physiol. 1989 Apr;411:639–661. doi: 10.1113/jphysiol.1989.sp017596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehara T., Noma A., Ono K. Calcium-activated non-selective cation channel in ventricular cells isolated from adult guinea-pig hearts. J Physiol. 1988 Sep;403:117–133. doi: 10.1113/jphysiol.1988.sp017242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Noble D., Shimoni Y., Spindler A. J. Inward current related to contraction in guinea-pig ventricular myocytes. J Physiol. 1987 Apr;385:565–589. doi: 10.1113/jphysiol.1987.sp016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W., Shimoni Y. Slow inward tail currents in rabbit cardiac cells. J Physiol. 1989 Oct;417:447–463. doi: 10.1113/jphysiol.1989.sp017812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryshko L. V., Stiffel V., Bers D. M. Rapid cooling contractures as an index of sarcoplasmic reticulum calcium content in rabbit ventricular myocytes. Am J Physiol. 1989 Nov;257(5 Pt 2):H1369–H1377. doi: 10.1152/ajpheart.1989.257.5.H1369. [DOI] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. J., Horn R. Influence of sodium-calcium exchange on calcium current rundown and the duration of calcium-dependent chloride currents in pituitary cells, studied with whole cell and perforated patch recording. J Gen Physiol. 1989 Nov;94(5):789–812. doi: 10.1085/jgp.94.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F. A., Erickson H., Block B. A., Meissner G. Evidence for a junctional feet-ryanodine receptor complex from sarcoplasmic reticulum. Biochem Biophys Res Commun. 1987 Mar 13;143(2):704–709. doi: 10.1016/0006-291x(87)91411-2. [DOI] [PubMed] [Google Scholar]
- Lansman J. B., Hess P., Tsien R. W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J Gen Physiol. 1986 Sep;88(3):321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Calcium-activated inward current and contraction in rat and guinea-pig ventricular myocytes. J Physiol. 1987 Oct;391:545–560. doi: 10.1113/jphysiol.1987.sp016755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Rubenstein D. S., Fox L. M., McNulty J. A., Lipsius S. L. Electrophysiology and ultrastructure of eustachian ridge from cat right atrium: a comparison with SA node. J Mol Cell Cardiol. 1987 Oct;19(10):965–976. doi: 10.1016/s0022-2828(87)80569-2. [DOI] [PubMed] [Google Scholar]
- Rubenstein D. S., Lipsius S. L. Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circ Res. 1989 Apr;64(4):648–657. doi: 10.1161/01.res.64.4.648. [DOI] [PubMed] [Google Scholar]
- Shattock M. J., Bers D. M. Rat vs. rabbit ventricle: Ca flux and intracellular Na assessed by ion-selective microelectrodes. Am J Physiol. 1989 Apr;256(4 Pt 1):C813–C822. doi: 10.1152/ajpcell.1989.256.4.C813. [DOI] [PubMed] [Google Scholar]
- Tseng G. N. Calcium current restitution in mammalian ventricular myocytes is modulated by intracellular calcium. Circ Res. 1988 Aug;63(2):468–482. doi: 10.1161/01.res.63.2.468. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Vereecke J., Carmeliet E., Lipsius S. L. Ionic currents activated during hyperpolarization of single right atrial myocytes from cat heart. Circ Res. 1991 Apr;68(4):1059–1069. doi: 10.1161/01.res.68.4.1059. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Lipsius S. L. Properties of the pacemaker current (If) in latent pacemaker cells isolated from cat right atrium. J Physiol. 1992;453:503–523. doi: 10.1113/jphysiol.1992.sp019242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Calcium-activated chloride current in rabbit ventricular myocytes. Circ Res. 1991 Feb;68(2):424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]
- duBell W. H., Boyett M. R., Spurgeon H. A., Talo A., Stern M. D., Lakatta E. G. The cytosolic calcium transient modulates the action potential of rat ventricular myocytes. J Physiol. 1991 May;436:347–369. doi: 10.1113/jphysiol.1991.sp018554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- duBell W. H., Houser S. R. Voltage and beat dependence of Ca2+ transient in feline ventricular myocytes. Am J Physiol. 1989 Sep;257(3 Pt 2):H746–H759. doi: 10.1152/ajpheart.1989.257.3.H746. [DOI] [PubMed] [Google Scholar]


