Abstract
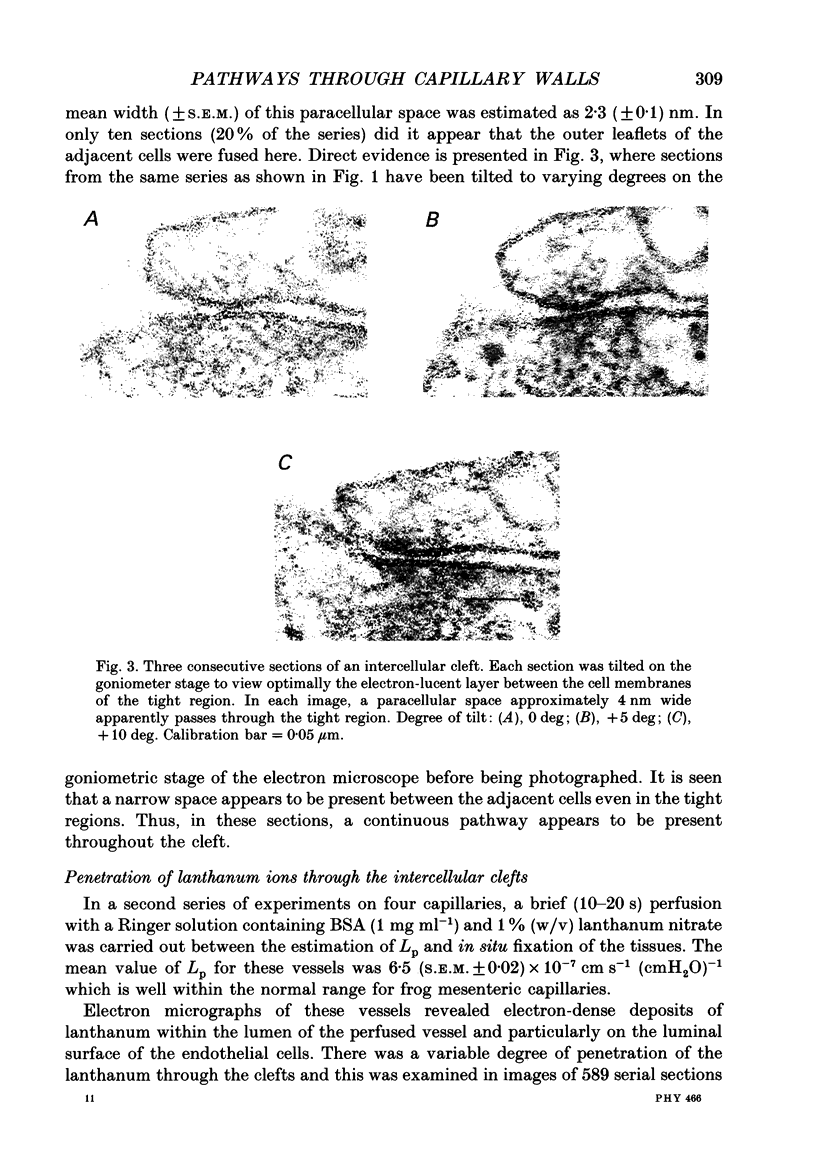
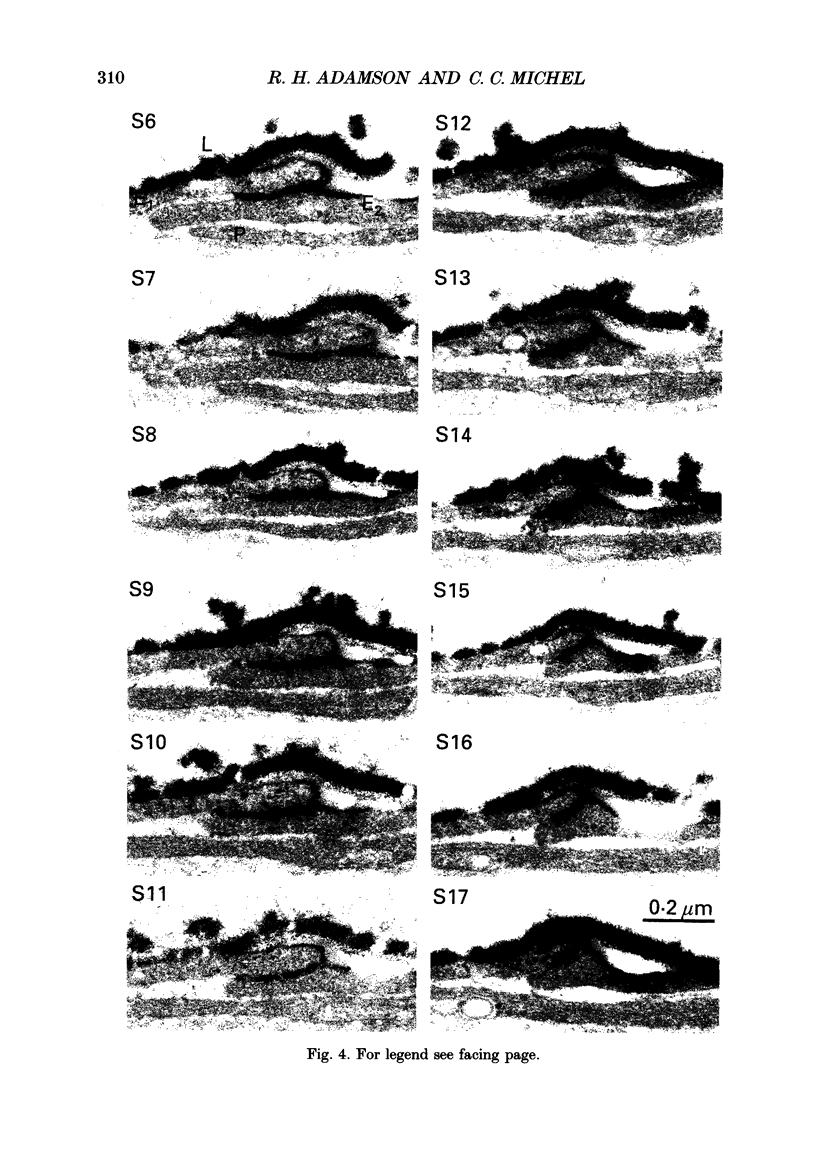
1. The three-dimensional ultrastructure of endothelial intercellular clefts of frog mesenteric capillaries of known hydraulic permeability (Lp) has been investigated in the absence and presence of lanthanum ions as tracers of extracellular solute. 2. Experiments were carried out on the exposed mesenteries of pithed frogs and Lp of a chosen microvessel perfused with a Ringer solution containing serum albumin (10-40 mg ml-1) was determined. In some experiments the mesentery was fixed in situ with 2.5% glutaraldehyde immediately after Lp had been measured. In other experiments, measurement of Lp was followed by brief microperfusion (10-20 s) with a second Ringer solution containing 1% lanthanum nitrate as a tracer before in situ fixation of the tissue. The tissue was prepared for electron microscopy using standard techniques. The perfused capillary was identified in the block and serial transverse sections were cut along its length over regions where Lp had been measured. 3. In six capillaries where the tissues were fixed immediately after measurement of Lp, Lp had a mean value (+/- S.E.M.) of 4 (+/- 0.5) x 10(-7) cm s-1 (cmH2O)-1. Serial (30-40 nm) sections of these vessels revealed that a single short narrow region of the intercellular clefts ran almost continuously from section to section. Additional tight regions were regularly seen, but they usually extended for relatively few sections. In 13.36 microns of reconstructed cleft, there were three interruptions of the tight region of 0.14, 0.14 and 0.17 microns respectively. In the region of these discontinuities, the wide region was uninterrupted from luminal to abluminal surface. 4. Examination of the tight junction on a tilting stage revealed that the outer leaflets of the adjacent cells were not fused, but separated by a gap of mean width (+/- S.E.M.) 2.3 (+/- 0.1) nm. 5. In four capillaries perfused with lanthanum nitrate before fixation, mean Lp (+/- S.E.M.) was 6.5 (+/- 0.02) x 10(-7) cm s-1 (cmH2O)-1. Segments of intercellular clefts, totalling 23.56 microns in length, were reconstructed from serial sections and throughout these, electron-dense deposits of lanthanum were observed to fill the luminal parts of the intercellular clefts up to the tight region. Lanthanum deposits filled the entire cleft to the abluminal surface at eleven sites, which accounted for a length of 2.52 microns out of the 23.56 microns. Only five of these regions were delimited within a continuous series of sections and their mean length (+/- S.E.M.) was 0.16 (+/- 0.063) microns.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson R. H. Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. J Physiol. 1990 Sep;428:1–13. doi: 10.1113/jphysiol.1990.sp018197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard M., Frøkjaer-Jensen J. Functional aspects of the ultrastructure of terminal blood vessels: a qualitative study on consecutive segments of the frog mesenteric microvasculature. Microvasc Res. 1982 Jan;23(1):1–30. doi: 10.1016/0026-2862(82)90028-0. [DOI] [PubMed] [Google Scholar]
- Bundgaard M. The three-dimensional organization of tight junctions in a capillary endothelium revealed by serial-section electron microscopy. J Ultrastruct Res. 1984 Jul;88(1):1–17. doi: 10.1016/s0022-5320(84)90177-1. [DOI] [PubMed] [Google Scholar]
- Clough G., Michel C. C. Quantitative comparisons of hydraulic permeability and endothelial intercellular cleft dimensions in single frog capillaries. J Physiol. 1988 Nov;405:563–576. doi: 10.1113/jphysiol.1988.sp017348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough G., Michel C. C. The effects of chemical fixation on the permeability of frog mesenteric capillaries. J Physiol. 1987 Nov;392:463–474. doi: 10.1113/jphysiol.1987.sp016791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry F. E., Michel C. C. A fiber matrix model of capillary permeability. Microvasc Res. 1980 Jul;20(1):96–99. doi: 10.1016/0026-2862(80)90024-2. [DOI] [PubMed] [Google Scholar]
- Curry F. E., Michel C. C., Mason J. C. Osmotic reflextion coefficients of capillary walls to low molecular weight hydrophilic solutes measured in single perfused capillaries of the frog mesentery. J Physiol. 1976 Oct;261(2):319–336. doi: 10.1113/jphysiol.1976.sp011561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. J. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967 Oct;35(1):213–236. doi: 10.1083/jcb.35.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C. Capillary permeability and how it may change. J Physiol. 1988 Oct;404:1–29. doi: 10.1113/jphysiol.1988.sp017275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C. Filtration coefficients and osmotic reflexion coefficients of the walls of single frog mesenteric capillaries. J Physiol. 1980 Dec;309:341–355. doi: 10.1113/jphysiol.1980.sp013512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C., Mason J. C., Curry F. E., Tooke J. E., Hunter P. J. A development of the Landis technique for measuring the filtration coefficient of individual capillaries in the frog mesentery. Q J Exp Physiol Cogn Med Sci. 1974 Oct;59(4):283–309. doi: 10.1113/expphysiol.1974.sp002275. [DOI] [PubMed] [Google Scholar]
- Schneeberger E. E., Hamelin M. Interaction of serum proteins with lung endothelial glycocalyx: its effect on endothelial permeability. Am J Physiol. 1984 Aug;247(2 Pt 2):H206–H217. doi: 10.1152/ajpheart.1984.247.2.H206. [DOI] [PubMed] [Google Scholar]
- Schulze C., Firth J. A. The interendothelial junction in myocardial capillaries: evidence for the existence of regularly spaced, cleft-spanning structures. J Cell Sci. 1992 Mar;101(Pt 3):647–655. doi: 10.1242/jcs.101.3.647. [DOI] [PubMed] [Google Scholar]
- Ward B. J., Bauman K. F., Firth J. A. Interendothelial junctions of cardiac capillaries in rats: their structure and permeability properties. Cell Tissue Res. 1988 Apr;252(1):57–66. doi: 10.1007/BF00213826. [DOI] [PubMed] [Google Scholar]
- Weinbaum S., Tsay R., Curry F. E. A three-dimensional junction-pore-matrix model for capillary permeability. Microvasc Res. 1992 Jul;44(1):85–111. doi: 10.1016/0026-2862(92)90104-w. [DOI] [PubMed] [Google Scholar]
- Wissig S. L., Williams M. C. Permeability of muscle capillaries to microperoxidase. J Cell Biol. 1978 Feb;76(2):341–359. doi: 10.1083/jcb.76.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]