Abstract
1. To determine whether conductive pathways contribute to the H+ efflux from granulocytes, we used the whole-cell patch-clamp technique combined with microfluorimetric determinations of cytosolic pH (pHi) in single, dimethylsulphoxide-differentiated HL-60 cells. 2. In voltage-clamp mode, depolarization of the cell from the resting potential (around -60 mV) to +60 mV caused an increase in pHi that was accompanied by a sizeable outward current. 3. Ion substitution experiments and analysis of the reversal potential of tail currents indicated that the outward current is carried largely by H+ ions. 4. Full activation of the H+ current occurred within 1-2s after depolarization and deactivation within 100-200 ms upon repolarization. 5. This H+ conductance was strongly dependent on pHi, being larger at acidic pH. In addition, at low pHi the threshold for voltage activation of the H+ conductance was shifted to more negative values. 6. Addition of millimolar concentrations of Cd2+ and Zn2+ to the bath solution reduced the maximum H+ conductance and shifted the voltage dependence of the H+ conductance to more positive potentials. The effects were reversible. 7. In conclusion, our results demonstrate that granulocytic HL-60 cells possess a voltage-gated and pHi-sensitive H+ conductance. Because both a depolarization and a cytosolic acidification occur during the activation of granulocytes, this conductance may play a role in pHi homeostasis of granulocytes during microbial killing.
Full text
PDF


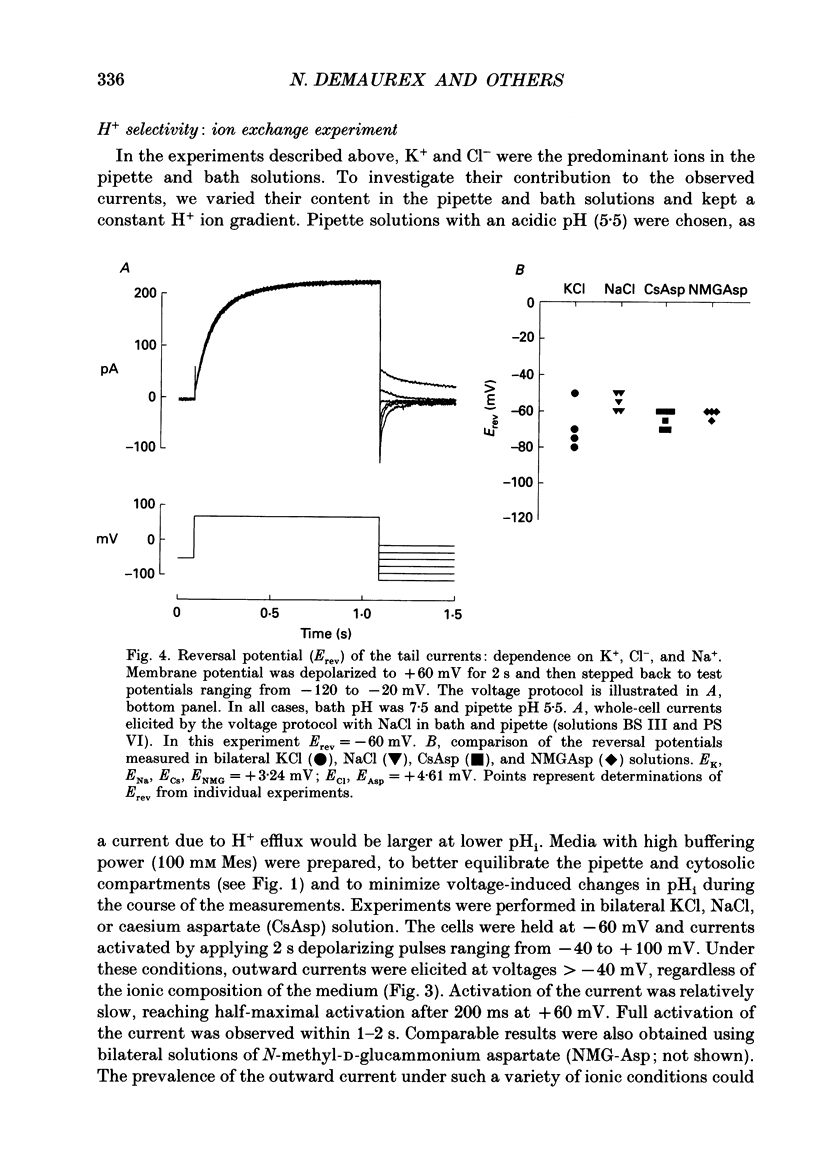
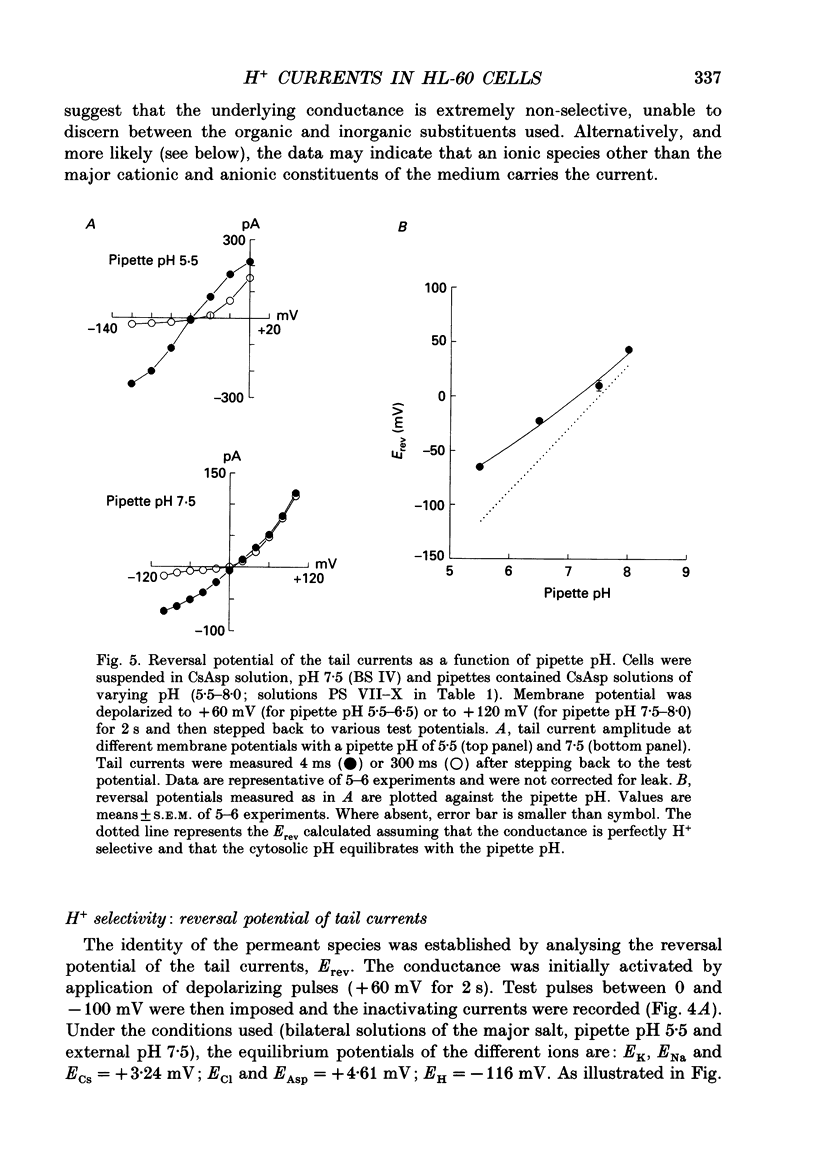
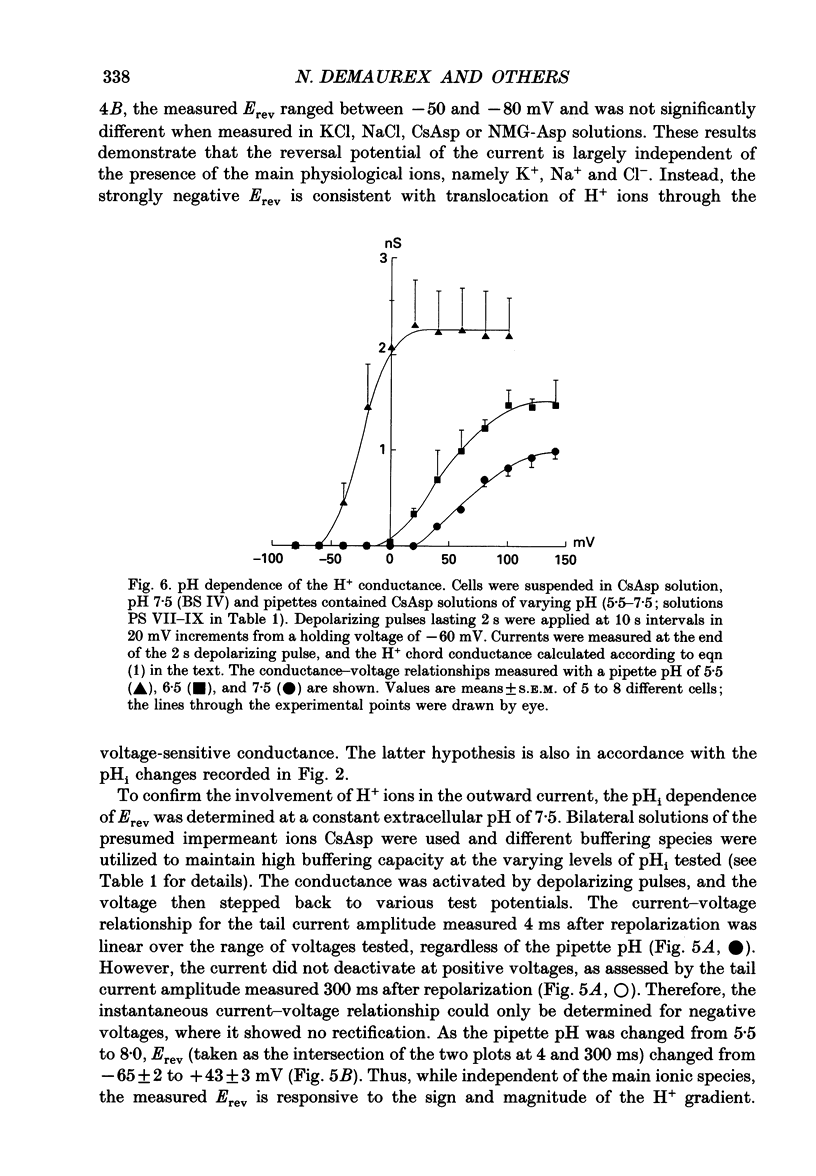
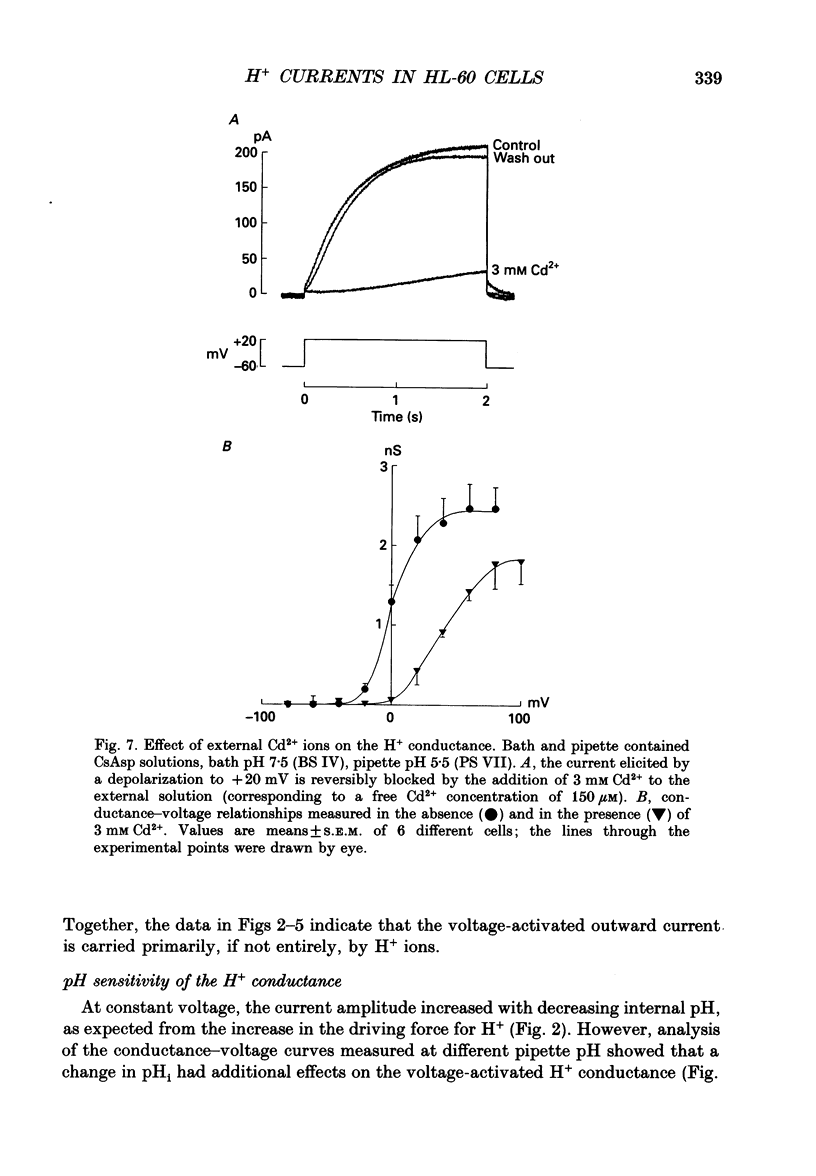





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Barish M. E., Baud C. A voltage-gated hydrogen ion current in the oocyte membrane of the axolotl, Ambystoma. J Physiol. 1984 Jul;352:243–263. doi: 10.1113/jphysiol.1984.sp015289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S., Reinisch L., Beebe D. C. Intracellular pH measurement using single excitation-dual emission fluorescence ratios. Am J Physiol. 1990 Jan;258(1 Pt 1):C171–C178. doi: 10.1152/ajpcell.1990.258.1.C171. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Schwartz J. H., Tauber A. I. Proton secretion by stimulated neutrophils. Significance of hexose monophosphate shunt activity as source of electrons and protons for the respiratory burst. J Clin Invest. 1984 Aug;74(2):455–459. doi: 10.1172/JCI111442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Tauber A. I. Subcellular localization of the human neutrophil NADPH oxidase. b-Cytochrome and associated flavoprotein. J Biol Chem. 1984 Jan 10;259(1):47–52. [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflugers Arch. 1990 Oct;417(2):234–239. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- Byerly L., Meech R., Moody W., Jr Rapidly activating hydrogen ion currents in perfused neurones of the snail, Lymnaea stagnalis. J Physiol. 1984 Jun;351:199–216. doi: 10.1113/jphysiol.1984.sp015241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A. The human neutrophil respiratory burst oxidase. J Infect Dis. 1990 Jun;161(6):1140–1147. doi: 10.1093/infdis/161.6.1140. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986 Jul 1;237(1):111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T. E. Hydrogen ion currents in rat alveolar epithelial cells. Biophys J. 1991 Nov;60(5):1243–1253. doi: 10.1016/S0006-3495(91)82158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N., Schlegel W., Varnai P., Mayr G., Lew D. P., Krause K. H. Regulation of Ca2+ influx in myeloid cells. Role of plasma membrane potential, inositol phosphates, cytosolic free [Ca2+], and filling state of intracellular Ca2+ stores. J Clin Invest. 1992 Sep;90(3):830–839. doi: 10.1172/JCI115958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. A., Cross A. R., Jones O. T. Studies on the electron-transfer mechanism of the human neutrophil NADPH oxidase. Biochem J. 1989 Sep 1;262(2):575–579. doi: 10.1042/bj2620575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W., Biggar W. D. Cytoplasmic pH regulation in normal and abnormal neutrophils. Role of superoxide generation and Na+/H+ exchange. J Biol Chem. 1986 Jan 15;261(2):512–514. [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Characterization of the amiloride-sensitive Na+-H+ antiport of human neutrophils. Am J Physiol. 1986 Feb;250(2 Pt 1):C283–C291. doi: 10.1152/ajpcell.1986.250.2.C283. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Furuya W., Rotstein O. D. Intracellular pH regulation in neutrophils: properties and distribution of the Na+/H+ antiporter. Soc Gen Physiol Ser. 1988;43:303–314. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henderson L. M., Chappell J. B., Jones O. T. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem J. 1988 Oct 1;255(1):285–290. [PMC free article] [PubMed] [Google Scholar]
- Henderson L. M., Chappell J. B., Jones O. T. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem J. 1987 Sep 1;246(2):325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. M., Chappell J. B. The NADPH-oxidase-associated H+ channel is opened by arachidonate. Biochem J. 1992 Apr 1;283(Pt 1):171–175. doi: 10.1042/bj2830171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapus A., Szászi K., Ligeti E. Phorbol 12-myristate 13-acetate activates an electrogenic H(+)-conducting pathway in the membrane of neutrophils. Biochem J. 1992 Feb 1;281(Pt 3):697–701. doi: 10.1042/bj2810697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K. H., Welsh M. J. Voltage-dependent and Ca2(+)-activated ion channels in human neutrophils. J Clin Invest. 1990 Feb;85(2):491–498. doi: 10.1172/JCI114464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariot P., Sartor P., Audin J., Dufy B. Intracellular pH in individual pituitary cells: measurement with a dual emission pH indicator. Life Sci. 1991;48(3):245–252. doi: 10.1016/0024-3205(91)90351-b. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. Voltage-dependent intracellular pH in Helix aspersa neurones. J Physiol. 1987 Sep;390:433–452. doi: 10.1113/jphysiol.1987.sp016710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A., Grinstein S. Protein kinase C activates an H+ (equivalent) conductance in the plasma membrane of human neutrophils. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10816–10820. doi: 10.1073/pnas.88.23.10816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Geisow M., Garcia R., Harper A., Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 1981 Apr 2;290(5805):406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- Sha'afi R. I., Molski T. F. Activation of the neutrophil. Prog Allergy. 1988;42:1–64. doi: 10.1159/000318681. [DOI] [PubMed] [Google Scholar]
- Simchowitz L. Chemotactic factor-induced activation of Na+/H+ exchange in human neutrophils. II. Intracellular pH changes. J Biol Chem. 1985 Oct 25;260(24):13248–13255. [PubMed] [Google Scholar]
- Thomas R. C., Meech R. W. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 1982 Oct 28;299(5886):826–828. doi: 10.1038/299826a0. [DOI] [PubMed] [Google Scholar]
- van Zwieten R., Wever R., Hamers M. N., Weening R. S., Roos D. Extracellular proton release by stimulated neutrophils. J Clin Invest. 1981 Jul;68(1):310–313. doi: 10.1172/JCI110250. [DOI] [PMC free article] [PubMed] [Google Scholar]



