Abstract
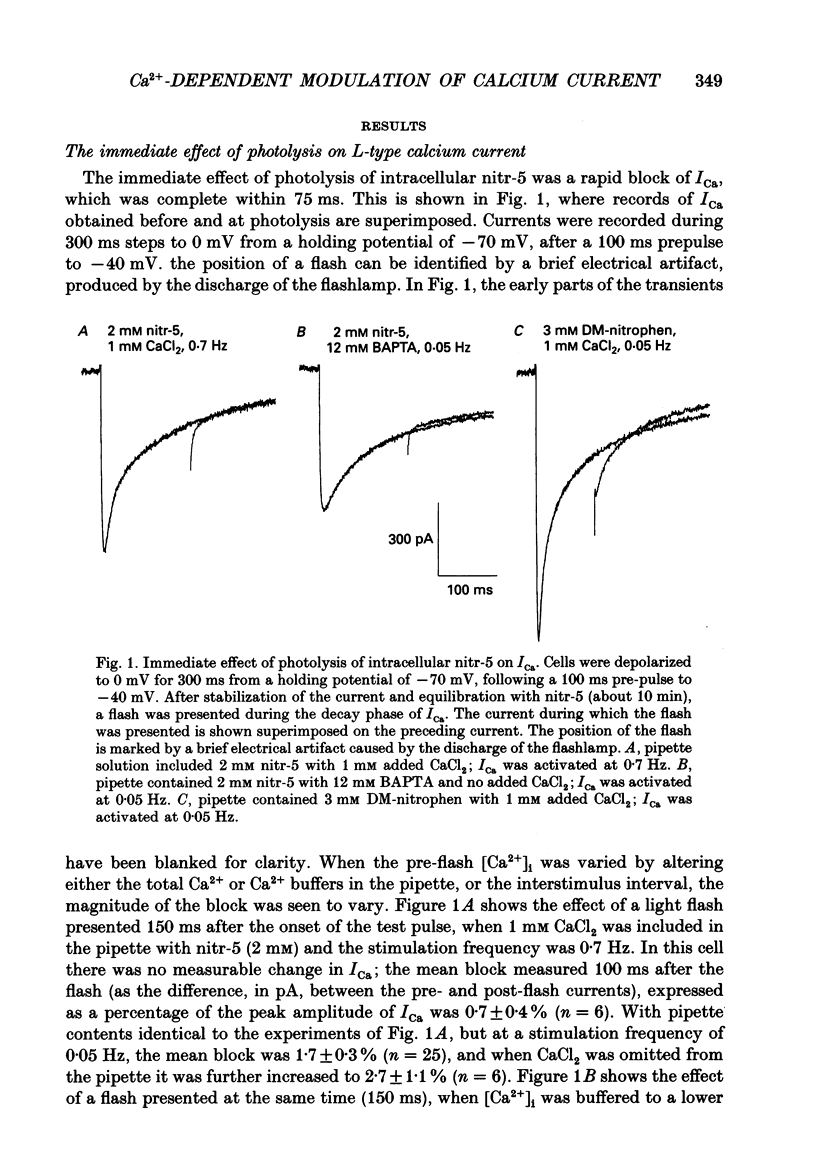
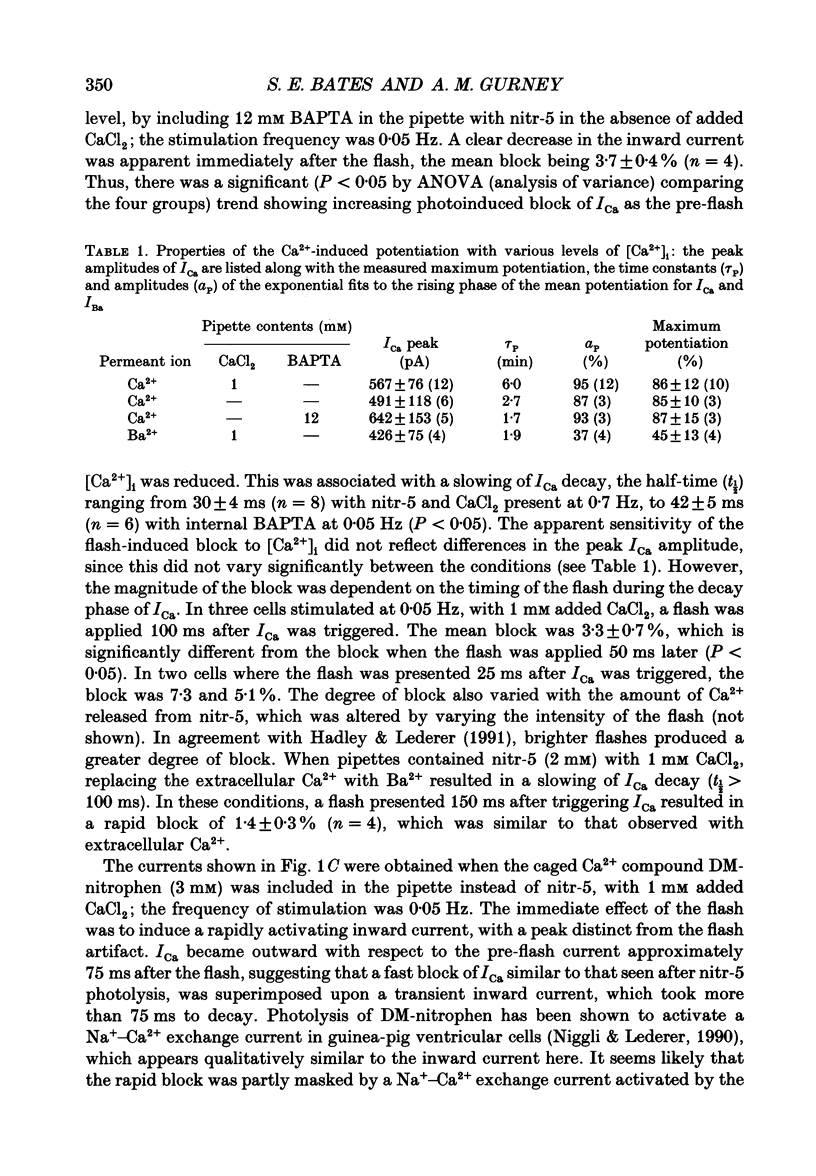
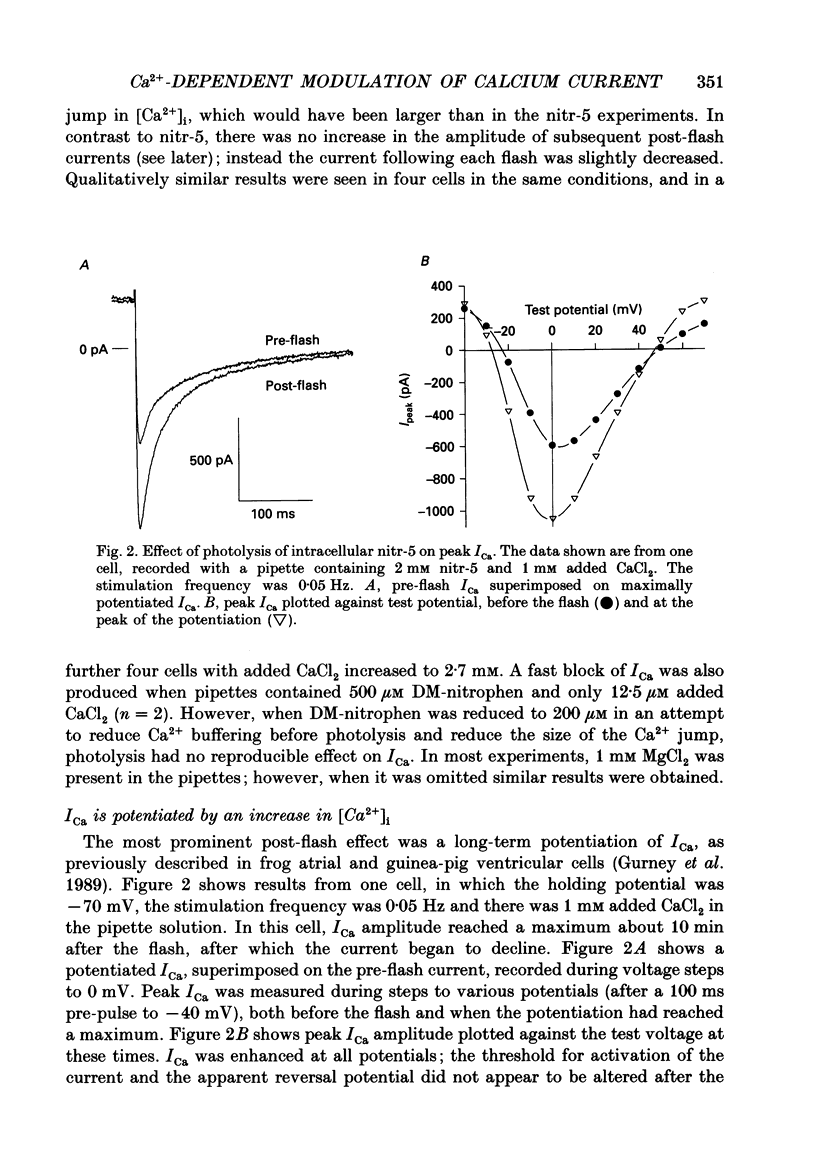
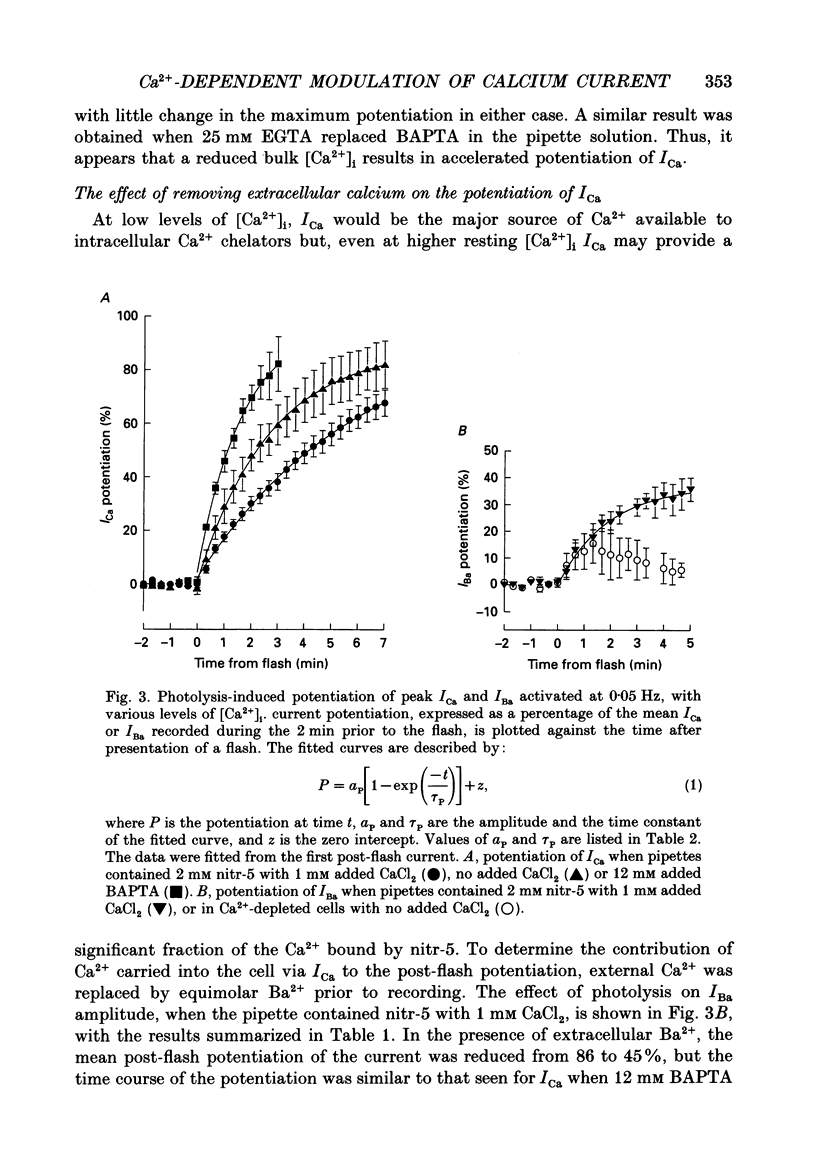
1. The caged calcium compound nitr-5 has been used to investigate the response of the L-type calcium current (ICa) of guinea-pig ventricular cells to a rapid increase in the free intracellular calcium concentration ([Ca2+]i). 2. When 2 mM nitr-5 or 3 mM DM-nitrophen was loaded into cells via a patch pipette and photolysed during the decay phase of ICa, a partial block of the current developed within 75 ms. The block was reduced by increasing the pre-flash [Ca2+]i and enhanced by adding high concentrations of Ca2+ chelators to the pipette-filling solution. 3. The photolysis-induced block was not suppressed in the presence of isoprenaline, suggesting a direct action of Ca2+ on the channels rather than a mechanism involving channel phosphorylation. 4. The most prominent effect of nitr-5 photolysis was a slow potentiation of ICa. When ICa was activated at frequencies between 0.05 and 0.7 Hz with various levels of pre-flash [Ca2+]i, peak ICa was approximately doubled in amplitude following photolysis. 5. At a stimulation frequency of 0.05 Hz, when nitr-5 was the only chelator present in the pipette, the time course of the potentiation was fitted by a single exponential with a time constant (tau P) of 2.7 min. When 1 mM CaCl2 was added to the pipette-filling solution, the time course of the potentiation was slowed (tau P = 6 min), although its amplitude was unchanged. With 12 mM BAPTA (a calcium chelator) added instead of CaCl2, the response was accelerated (tau P = 1.7 min). 6. Equimolar substitution of extracellular Ca2+ with Ba2+ significantly suppressed the flash-induced potentiation. The time course of the potentiation of the barium current, IBa (tau P = 1.9 min) was similar to that of ICa with BAPTA in the pipette. Potentiation of IBa was largely blocked in Ca(2+)-depleted cells when CaCl2 was omitted from the pipette. 7. When ICa was activated at frequencies of > or = 0.1 Hz, with 1 mM CaCl2 added to the nitr-5 (2 mM) in the pipette, the onset of the flash-induced potentiation was best fitted by two exponentials; one was similar to the single component seen at 0.05 Hz and the other was approximately one order of magnitude faster. The contribution of the faster component was positively correlated to the stimulation frequency. 8. The flash-induced potentiation of ICa was suppressed in the presence of a supramaximal concentration of the beta-adrenergic agonist isoprenaline.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
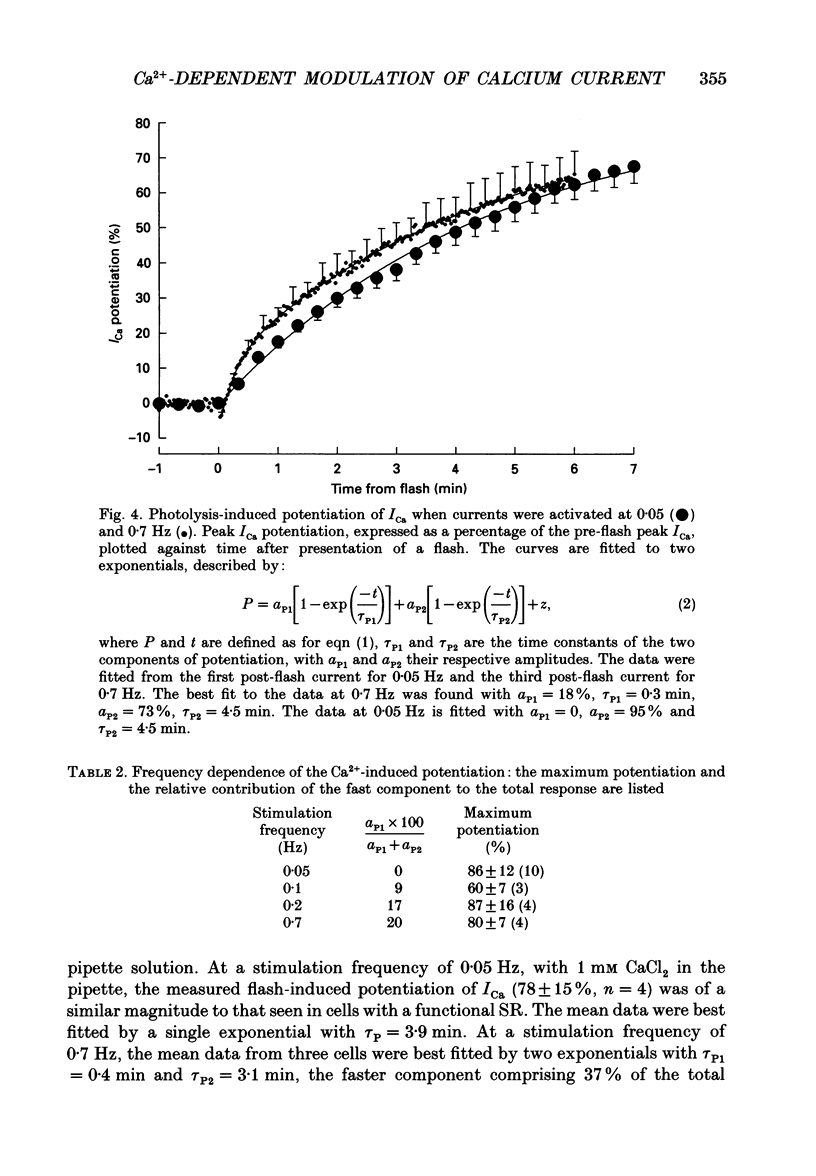
- Bates S. E., Gurney A. M. Modulation of L-type calcium current in mammalian ventricular myocytes by photolysis of caged calcium. Adv Exp Med Biol. 1992;311:385–386. doi: 10.1007/978-1-4615-3362-7_46. [DOI] [PubMed] [Google Scholar]
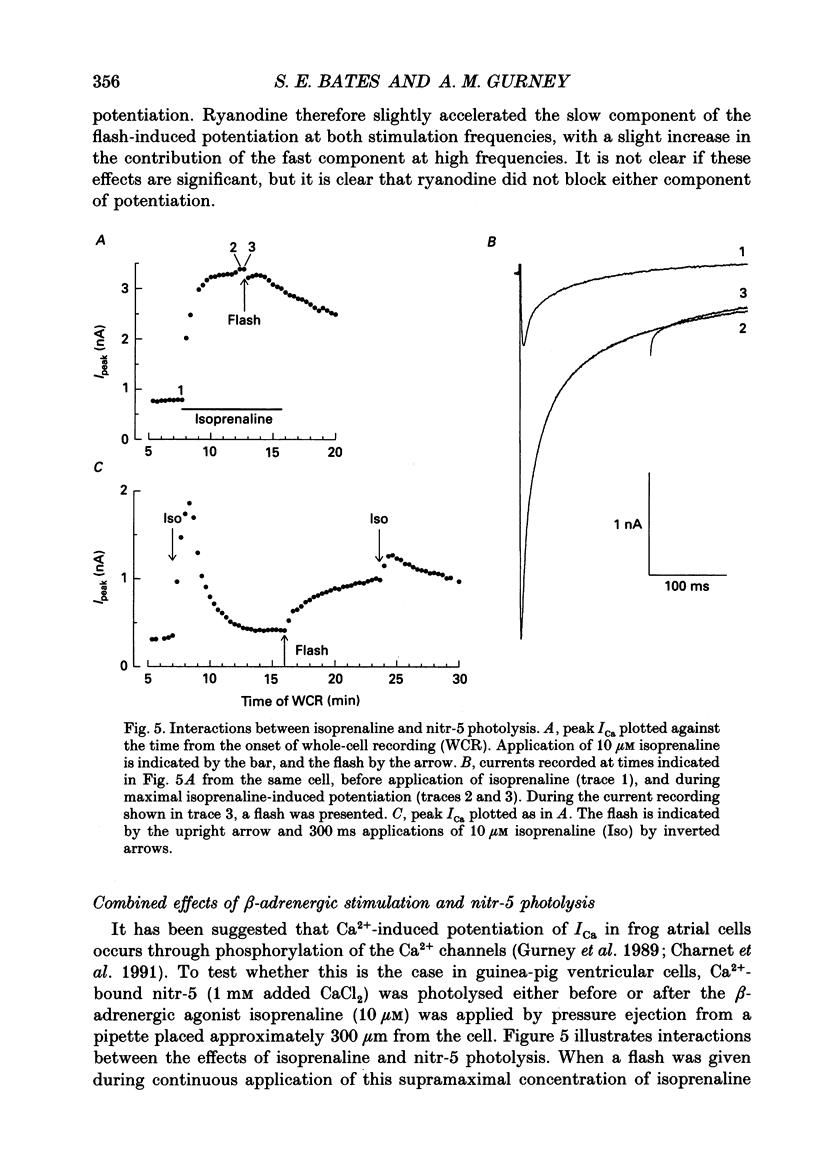
- Bechem M., Pott L. Removal of Ca current inactivation in dialysed guinea-pig atrial cardioballs by Ca chelators. Pflugers Arch. 1985 May;404(1):10–20. doi: 10.1007/BF00581485. [DOI] [PubMed] [Google Scholar]
- Belles B., Malécot C. O., Hescheler J., Trautwein W. "Run-down" of the Ca current during long whole-cell recordings in guinea pig heart cells: role of phosphorylation and intracellular calcium. Pflugers Arch. 1988 Apr;411(4):353–360. doi: 10.1007/BF00587713. [DOI] [PubMed] [Google Scholar]
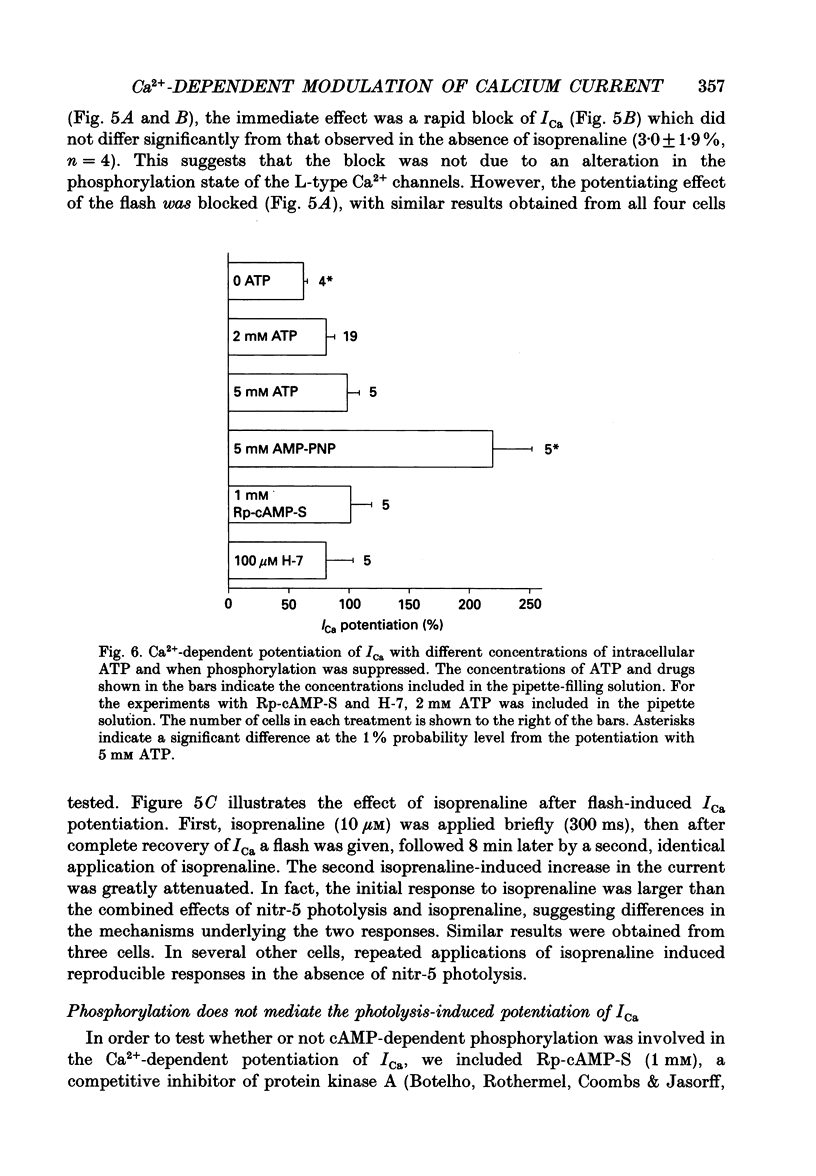
- Botelho L. H., Rothermel J. D., Coombs R. V., Jastorff B. cAMP analog antagonists of cAMP action. Methods Enzymol. 1988;159:159–172. doi: 10.1016/0076-6879(88)59017-1. [DOI] [PubMed] [Google Scholar]
- Charnet P., Richard S., Gurney A. M., Ouadid H., Tiaho F., Nargeot J. Modulation of Ca currents in isolated frog atrial cells studied with photosensitive probes. Regulation by cAMP and Ca2+: a common pathway? J Mol Cell Cardiol. 1991 Mar;23(3):343–356. doi: 10.1016/0022-2828(91)90070-3. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Noble D., Spindler A. J. Mechanism of the use dependence of Ca2+ current in guinea-pig myocytes. J Physiol. 1988 Nov;405:461–475. doi: 10.1113/jphysiol.1988.sp017342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Noble D., Spindler A. J. Use-dependent reduction and facilitation of Ca2+ current in guinea-pig myocytes. J Physiol. 1988 Nov;405:439–460. doi: 10.1113/jphysiol.1988.sp017341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson A. L., Zucker R. S. Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys J. 1985 Dec;48(6):1003–1017. doi: 10.1016/S0006-3495(85)83863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A. M., Tsien R. Y., Lester H. A. Activation of a potassium current by rapid photochemically generated step increases of intracellular calcium in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 May;84(10):3496–3500. doi: 10.1073/pnas.84.10.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. W., Lederer W. J. Ca2+ and voltage inactivate Ca2+ channels in guinea-pig ventricular myocytes through independent mechanisms. J Physiol. 1991 Dec;444:257–268. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S. E., O'Gara P., Jones S. M., Brown L. A., Vescovo G., Poole-Wilson P. A. Species dependence of contraction velocity in single isolated cardiac myocytes. Cardioscience. 1990 Mar;1(1):49–53. [PubMed] [Google Scholar]
- Hartzell H. C., Méry P. F., Fischmeister R., Szabo G. Sympathetic regulation of cardiac calcium current is due exclusively to cAMP-dependent phosphorylation. Nature. 1991 Jun 13;351(6327):573–576. doi: 10.1038/351573a0. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibres: the slow inward current component under the influence of modified [Ca2+]i. Pflugers Arch. 1977 Oct 19;371(1-2):61–69. doi: 10.1007/BF00580773. [DOI] [PubMed] [Google Scholar]
- Jackson A. P., Timmerman M. P., Bagshaw C. R., Ashley C. C. The kinetics of calcium binding to fura-2 and indo-1. FEBS Lett. 1987 May 25;216(1):35–39. doi: 10.1016/0014-5793(87)80752-4. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H., Ellis-Davies G. C. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S. Potentiation of the calcium-channel currents of internally perfused mammalian heart cells by repetitive depolarization. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3941–3945. doi: 10.1073/pnas.84.11.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban E., Tsien R. W. Enhancement of calcium current during digitalis inotropy in mammalian heart: positive feed-back regulation by intracellular calcium? J Physiol. 1982 Aug;329:589–614. doi: 10.1113/jphysiol.1982.sp014321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion N. V., Zucker R. S., Marsh S. J., Adams P. R. Modulation of M-current by intracellular Ca2+. Neuron. 1991 Apr;6(4):533–545. doi: 10.1016/0896-6273(91)90056-6. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Ca channel gating during cardiac action potentials. Biophys J. 1990 Oct;58(4):1059–1065. doi: 10.1016/S0006-3495(90)82448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli E., Lederer W. J. Voltage-independent calcium release in heart muscle. Science. 1990 Oct 26;250(4980):565–568. doi: 10.1126/science.2173135. [DOI] [PubMed] [Google Scholar]
- Noel J., Capiod T. Photolytic release of cAMP activates Ca2(+)-dependent K+ permeability in guinea-pig liver cells. Pflugers Arch. 1991 Jan;417(5):546–548. doi: 10.1007/BF00370954. [DOI] [PubMed] [Google Scholar]
- Näbauer M., Morad M. Ca2(+)-induced Ca2+ release as examined by photolysis of caged Ca2+ in single ventricular myocytes. Am J Physiol. 1990 Jan;258(1 Pt 1):C189–C193. doi: 10.1152/ajpcell.1990.258.1.C189. [DOI] [PubMed] [Google Scholar]
- O'Rourke B., Backx P. H., Marban E. Phosphorylation-independent modulation of L-type calcium channels by magnesium-nucleotide complexes. Science. 1992 Jul 10;257(5067):245–248. doi: 10.1126/science.1321495. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 1992 Mar;13(3):183–192. doi: 10.1016/0143-4160(92)90046-u. [DOI] [PubMed] [Google Scholar]
- Valdeolmillos M., O'Neill S. C., Smith G. L., Eisner D. A. Calcium-induced calcium release activates contraction in intact cardiac cells. Pflugers Arch. 1989 Apr;413(6):676–678. doi: 10.1007/BF00581820. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Backx P. H., Imredy J. P. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 1990 Dec 21;250(4988):1735–1738. doi: 10.1126/science.2176745. [DOI] [PubMed] [Google Scholar]
- Zygmunt A. C., Maylie J. Stimulation-dependent facilitation of the high threshold calcium current in guinea-pig ventricular myocytes. J Physiol. 1990 Sep;428:653–671. doi: 10.1113/jphysiol.1990.sp018233. [DOI] [PMC free article] [PubMed] [Google Scholar]



