Abstract
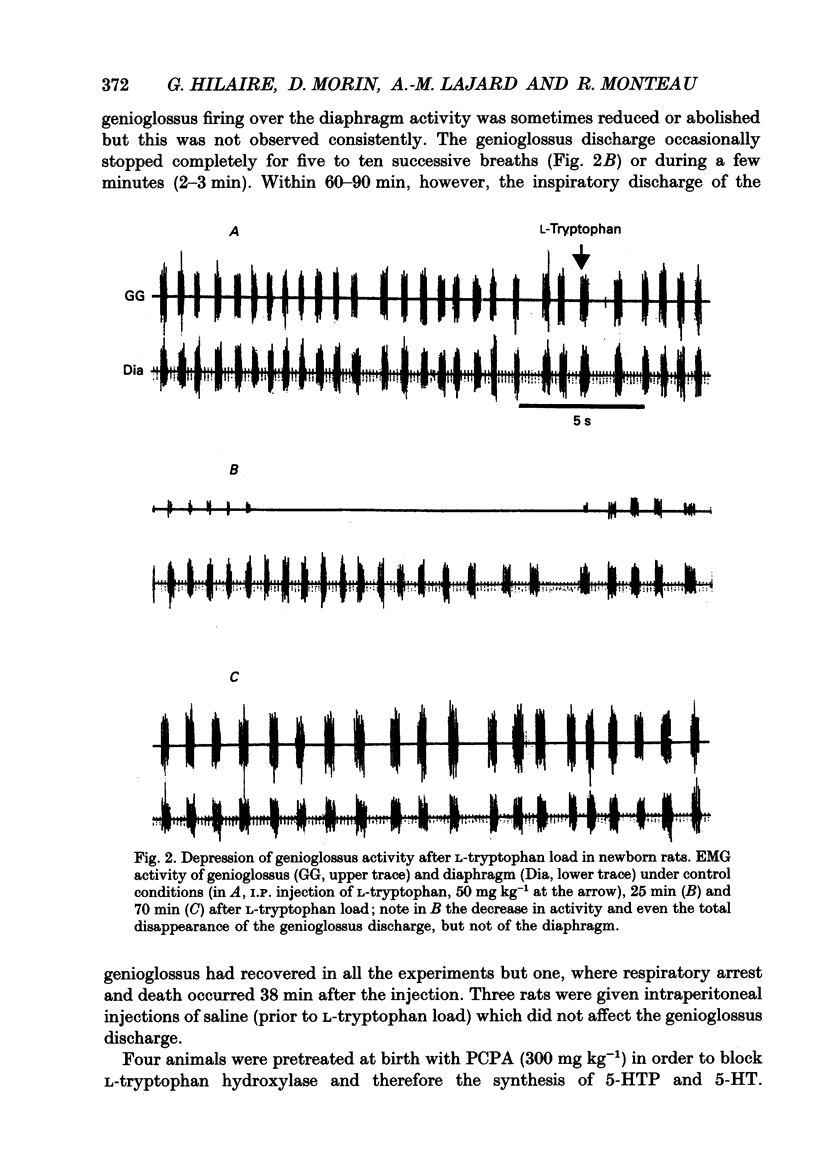
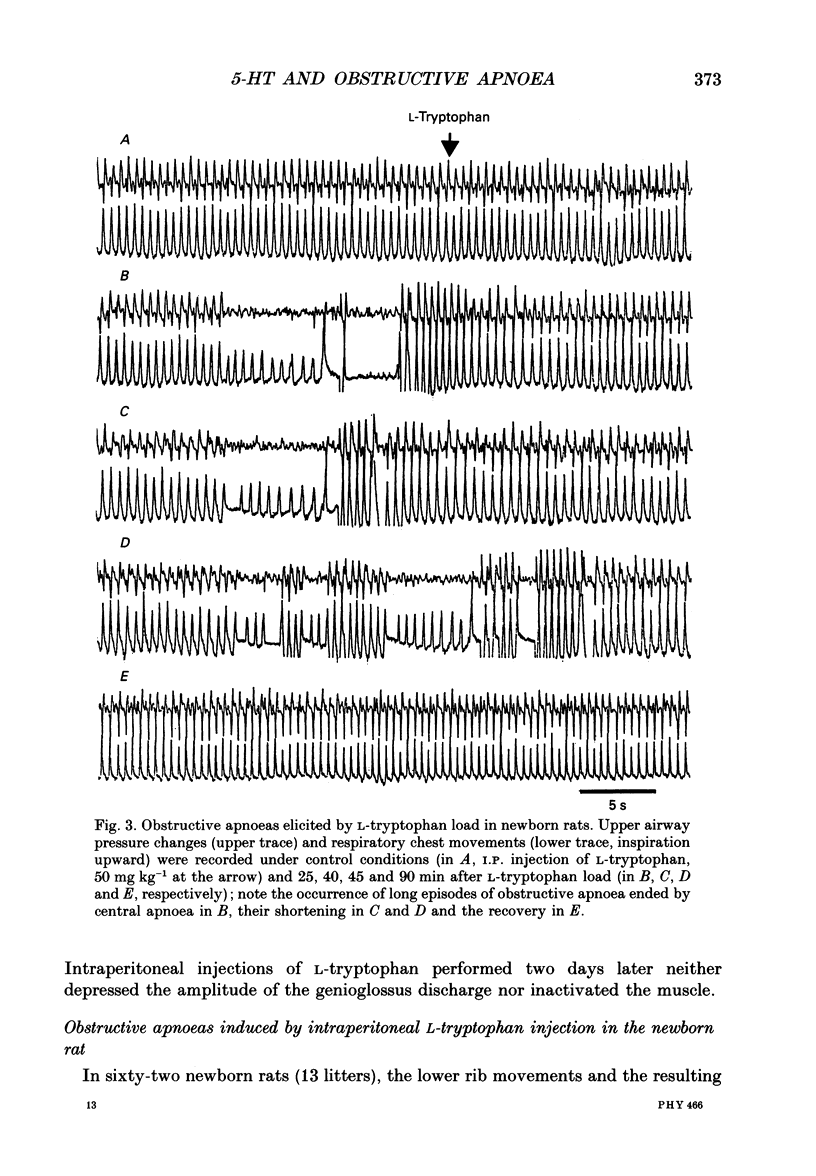
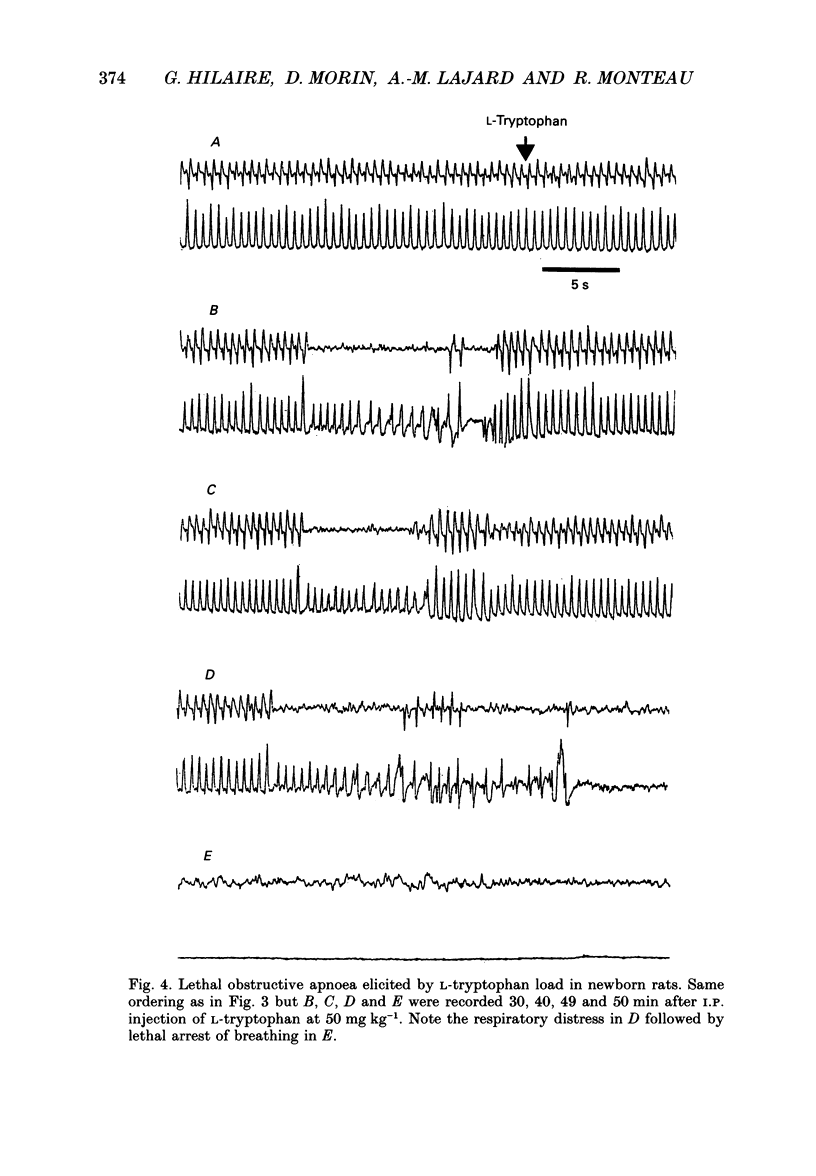
1. Experiments were performed on anaesthetized newborn rats (aged 3-10 days) to know whether an increase in central serotonin levels might favour the occurrence of obstructive apnoeas as previously suggested by in vitro results from our group. 2. The levels of serotonin (5-HT), its precursor 5-hydroxytryptophan (5-HTP) and its metabolite, 5-hydroxyindole-acetic acid (5-HIAA), were analysed in cerebrospinal fluid samples collected at the level of the obex prior to and after intraperitoneal injection of L-tryptophan (50 mg kg-1) in sixty-eight anaesthetized newborn rats (control rats prior to injection and injected rats 15, 30 and 45 min after the injection). A significant increase in 5-HT and 5-HTP levels occurred 30 min after the injection, attesting to the activation of 5-HT biosynthesis. 3. The EMG activity of both the genioglossus and the diaphragm was recorded before and after L-tryptophan load (50 mg kg-1) in twenty-two newborn rats. After the injection of L-tryptophan, the amplitude of the integrated genioglossus activity decreased, or was even abolished, either during a few respiratory cycles or for long periods in twenty-one out of twenty-two animals. Recovery of the genioglossus activity occurred within 45-60 min. 4. The thoracic respiratory movements and the resulting upper airway pressure changes were recorded before and after L-tryptophan injection (50 mg kg-1) in sixty-two animals. In some litters (n = 7), most of the animals (21/25) displayed, within 20-40 min of the injection, recurrent episodes of obstructive apnoea often followed by central ones. These respiratory difficulties became severe and drastic, and led in two instances to respiratory distress and death. Lower doses of L-tryptophan (10 mg kg-1) did not induce any respiratory disorders unless these were potentiated by pargyline treatment (50 mg kg-1, n = 7). The obstructive apnoeas liable to occur after an L-tryptophan load (50 mg kg-1) were prevented by blocking the 5-HT receptor with methysergide (50 mg kg-1, n = 5) or by blocking the 5-HT biosynthesis by applying p-chlorophenylalanine (PCPA) pretreatment at birth (300 mg kg-1, n = 7). In other litters (n = 6), none of the eighteen newborn rats tested were affected by L-tryptophan, however, In five young adult rats, L-tryptophan again had no effect.4+ ĕ
Full text
PDF



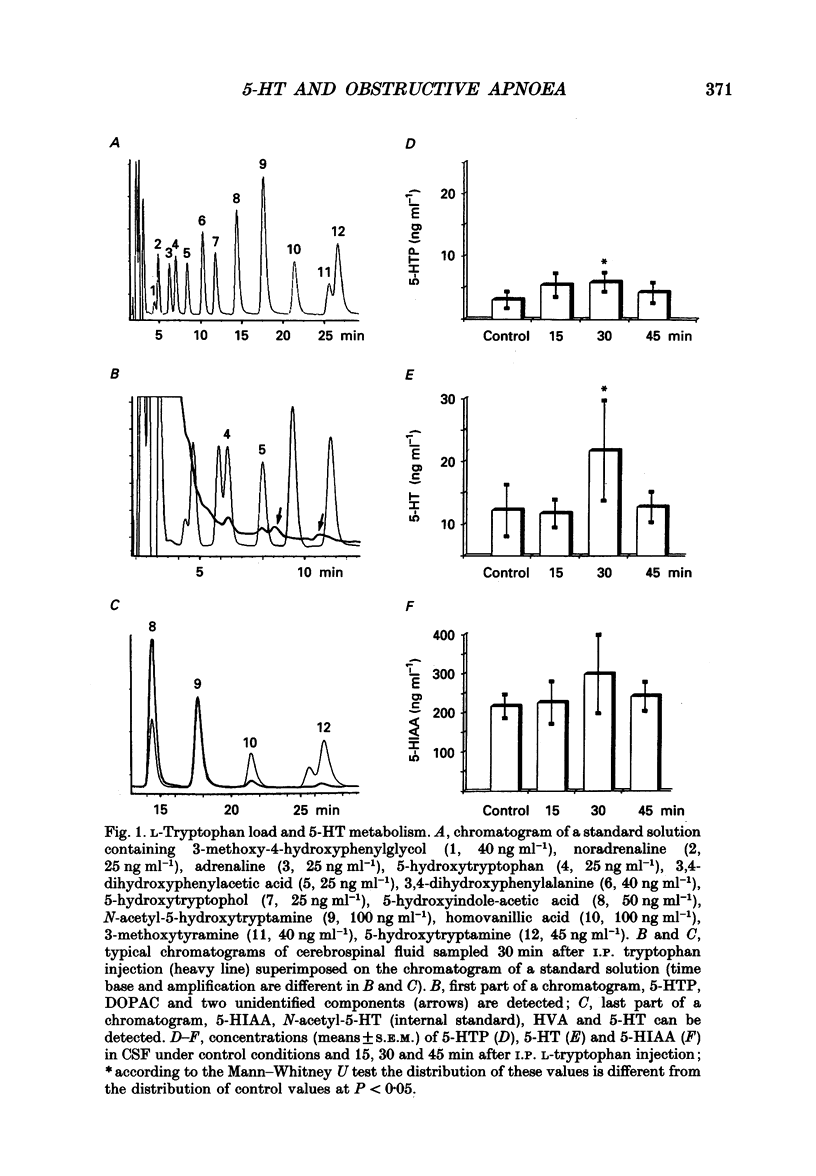







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldes L. D., Marco L. A., Chronister R. B. Serotonin-containing axon terminals in the hypoglossal nucleus of the rat. An immuno-electronmicroscopic study. Brain Res Bull. 1989 Sep;23(3):249–256. doi: 10.1016/0361-9230(89)90154-8. [DOI] [PubMed] [Google Scholar]
- Bartlett D., Jr Respiratory functions of the larynx. Physiol Rev. 1989 Jan;69(1):33–57. doi: 10.1152/physrev.1989.69.1.33. [DOI] [PubMed] [Google Scholar]
- Bourgoin S., Artaud F., Enjalbert A., Héry F., Glowinski J., Hamon M. Acute changes in central serotonin metabolism induced by the blockade or stimulation of serotoninergic receptors during ontogenesis in the rat. J Pharmacol Exp Ther. 1977 Sep;202(3):519–531. [PubMed] [Google Scholar]
- Bourgoin S., Faivre-Bauman A., Benda P., Glowinski J., Hamon M. Plasma tryptophan and 5-HT metabolism in the CNS of the newborn rat. J Neurochem. 1974 Aug;23(2):319–327. doi: 10.1111/j.1471-4159.1974.tb04361.x. [DOI] [PubMed] [Google Scholar]
- Cameron W. E., Jodkowski J. S., Fang H., Guthrie R. D. Electrophysiological properties of developing phrenic motoneurons in the cat. J Neurophysiol. 1991 Mar;65(3):671–679. doi: 10.1152/jn.1991.65.3.671. [DOI] [PubMed] [Google Scholar]
- Card J. P., Rinaman L., Schwaber J. S., Miselis R. R., Whealy M. E., Robbins A. K., Enquist L. W. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990 Jun;10(6):1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff J., Girin E., Alix D., Cann-Moisan C., Sizun J., Barthelemy L. Neurotransmission et mort subite du nourrisson. Etude du liquide céphalo-rachidien. C R Acad Sci III. 1992;314(10):451–454. [PubMed] [Google Scholar]
- Cramer H., Warter J. M., Renaud B., Krieger J., Marescaux C. H., Hammers R. Cerebrospinal fluid adenosine 3',5'-monophosphate, 5-hydroxyindoleacetic acid and homovanillic acid in patients with sleep apnoea syndrome. J Neurol Neurosurg Psychiatry. 1981 Dec;44(12):1165–1167. doi: 10.1136/jnnp.44.12.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeoğlu A., Fisher L. A. Central nervous actions of serotonin and a serotonin1A receptor agonist: cardiovascular excitation at low doses. J Pharmacol Exp Ther. 1991 Apr;257(1):425–432. [PubMed] [Google Scholar]
- Garpestad E., Basner R. C., Ringler J., Lilly J., Schwartzstein R., Weinberger S. E., Weiss J. W. Phenylephrine-induced hypertension acutely decreases genioglossus EMG activity in awake humans. J Appl Physiol (1985) 1992 Jan;72(1):110–115. doi: 10.1152/jappl.1992.72.1.110. [DOI] [PubMed] [Google Scholar]
- Guilleminault C., Hill M. W., Simmons F. B., Dement W. C. Obstructive sleep apnea: electromyographic and fiberoptic studies. Exp Neurol. 1978 Oct;62(1):48–67. doi: 10.1016/0014-4886(78)90040-7. [DOI] [PubMed] [Google Scholar]
- Herbst J. J., Book L. S., Bray P. F. Gastroesophageal reflux in the "near miss" sudden infant death syndrome. J Pediatr. 1978 Jan;92(1):73–75. doi: 10.1016/s0022-3476(78)80074-2. [DOI] [PubMed] [Google Scholar]
- Herbst J. J., Minton S. D., Book L. S. Gastroesophageal reflux causing respiratory distress and apnea in newborn infants. J Pediatr. 1979 Nov;95(5 Pt 1):763–768. doi: 10.1016/s0022-3476(79)80733-7. [DOI] [PubMed] [Google Scholar]
- Hernández J., Manjarréz G. G., Chagoya G. Newborn humans and rats malnourished in utero: free plasma L-tryptophan, neutral amino acids and brain serotonin synthesis. Brain Res. 1989 May 29;488(1-2):1–13. doi: 10.1016/0006-8993(89)90687-2. [DOI] [PubMed] [Google Scholar]
- Hutson P. H., Sarna G. S., Kantamaneni B. D., Curzon G. Monitoring the effect of a tryptophan load on brain indole metabolism in freely moving rats by simultaneous cerebrospinal fluid sampling and brain dialysis. J Neurochem. 1985 Apr;44(4):1266–1273. doi: 10.1111/j.1471-4159.1985.tb08753.x. [DOI] [PubMed] [Google Scholar]
- Jansen A. H., Chernick V. Development of respiratory control. Physiol Rev. 1983 Apr;63(2):437–483. doi: 10.1152/physrev.1983.63.2.437. [DOI] [PubMed] [Google Scholar]
- Kinney H. C., Filiano J. J., Harper R. M. The neuropathology of the sudden infant death syndrome. A review. J Neuropathol Exp Neurol. 1992 Mar;51(2):115–126. doi: 10.1097/00005072-199203000-00001. [DOI] [PubMed] [Google Scholar]
- Kurtz D., Krieger J., Stierle J. C. EMG activity of cricothyroid and chin muscles during wakefulness and sleeping in the sleep apnea syndrome. Electroencephalogr Clin Neurophysiol. 1978 Dec;45(6):777–784. doi: 10.1016/0013-4694(78)90145-1. [DOI] [PubMed] [Google Scholar]
- McCall R. B., Aghajanian G. K. Serotonergic facilitation of facial motoneuron excitation. Brain Res. 1979 Jun 15;169(1):11–27. doi: 10.1016/0006-8993(79)90370-6. [DOI] [PubMed] [Google Scholar]
- McCann M. J., Hermann G. E., Rogers R. C. Nucleus raphe obscurus (nRO) influences vagal control of gastric motility in rats. Brain Res. 1989 May 1;486(1):181–184. doi: 10.1016/0006-8993(89)91292-4. [DOI] [PubMed] [Google Scholar]
- Mengod G., Pompeiano M., Martínez-Mir M. I., Palacios J. M. Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry. Correlation with the distribution of receptor sites. Brain Res. 1990 Jul 30;524(1):139–143. doi: 10.1016/0006-8993(90)90502-3. [DOI] [PubMed] [Google Scholar]
- Monteau R., Morin D., Hennequin S., Hilaire G. Differential effects of serotonin on respiratory activity of hypoglossal and cervical motoneurons: an in vitro study on the newborn rat. Neurosci Lett. 1990 Mar 26;111(1-2):127–132. doi: 10.1016/0304-3940(90)90356-e. [DOI] [PubMed] [Google Scholar]
- Morin D., Hennequin S., Monteau R., Hilaire G. Depressant effect of raphe stimulation on inspiratory activity of the hypoglossal nerve: in vitro study in the newborn rat. Neurosci Lett. 1990 Aug 24;116(3):299–303. doi: 10.1016/0304-3940(90)90090-v. [DOI] [PubMed] [Google Scholar]
- Morin D., Monteau R., Hilaire G. Compared effects of serotonin on cervical and hypoglossal inspiratory activities: an in vitro study in the newborn rat. J Physiol. 1992;451:605–629. doi: 10.1113/jphysiol.1992.sp019181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrochi J. J., McBride P. T., Yates A. J. Brainstem immaturity in sudden infant death syndrome: a quantitative rapid Golgi study of dendritic spines in 95 infants. Brain Res. 1985 Jan 28;325(1-2):39–48. doi: 10.1016/0006-8993(85)90300-2. [DOI] [PubMed] [Google Scholar]
- Rahilly P. M. Pneumographic studies: predictors of future apnoeas but not sudden infant death in asymptomatic infants. Aust Paediatr J. 1989 Aug;25(4):211–214. doi: 10.1111/j.1440-1754.1989.tb01458.x. [DOI] [PubMed] [Google Scholar]
- Remmers J. E. Obstructive sleep apnea. A common disorder exacerbated by alcohol. Am Rev Respir Dis. 1984 Aug;130(2):153–155. doi: 10.1164/arrd.1984.130.2.153. [DOI] [PubMed] [Google Scholar]
- Remmers J. E., deGroot W. J., Sauerland E. K., Anch A. M. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jun;44(6):931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Roth B. L., Hamblin M. W., Ciaranello R. D. Developmental regulation of 5-HT2 and 5-HT1c mRNA and receptor levels. Brain Res Dev Brain Res. 1991 Jan 15;58(1):51–58. doi: 10.1016/0165-3806(91)90236-c. [DOI] [PubMed] [Google Scholar]
- Sauerland E. K., Harper R. M. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976 Apr;51(1):160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Schechtman V. L., Harper R. M., Wilson A. J., Southall D. P. Sleep apnea in infants who succumb to the sudden infant death syndrome. Pediatrics. 1991 Jun;87(6):841–846. [PubMed] [Google Scholar]
- Stanley M., Traskman-Bendz L., Dorovini-Zis K. Correlations between aminergic metabolites simultaneously obtained from human CSF and brain. Life Sci. 1985 Oct 7;37(14):1279–1286. doi: 10.1016/0024-3205(85)90242-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Berger A. J. Direct excitation of rat spinal motoneurones by serotonin. J Physiol. 1990 Apr;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S., Becker L. E. Developmental abnormalities of medullary "respiratory centers" in sudden infant death syndrome. Exp Neurol. 1985 Dec;90(3):580–587. doi: 10.1016/0014-4886(85)90155-4. [DOI] [PubMed] [Google Scholar]
- Valdes-Dapena M. The pathologist and the sudden infant death syndrome. Am J Pathol. 1982 Jan;106(1):118–131. [PMC free article] [PubMed] [Google Scholar]


